Abstract
The aim of this study was to impregnate the chelating agent 3,3-bis-N,N,bis-(carboxymethyl)aminomethyl-o-cresolsulfonephthalein in chitosan and to investigate the adsorption of Cu(II) ions. The chemical modification was confirmed by FTIR spectrometry, thermogravimetric analysis (TGA) and energy dispersive x-ray spectroscopy (EDX). The adsorption studies were carried out with Cu(II) ions in a batch process and were shown to be dependent on pH. The adsorption kinetics was tested using three models: pseudo first-order, pseudo second order and intraparticle diffusion. The experimental kinetics data were best fitted with the pseudo second-order model (R² = 0.999), which provided a rate constant, k2, of 1.21 x 10-3 g mg-1 min-1. The adsorption rate depended on the concentration of Cu(II) ions on the adsorbent surface and on the quantity of Cu(II) ions adsorbed at equilibrium. The Langmuir isotherm model provided the best fit for the equilibrium data in the concentration range investigated, with the maximum adsorption capacity being 81.0 mg of Cu(II) per gram of adsorbent, as obtained from the linear equation of the isotherm. Desorption tests revealed that around 90% of the adsorbed metal was removed, using EDTA solution as the eluent. This result suggests that the polymeric matrix can be reused.
Chitosan; impregnation; adsorption; xylenol orange; Cu(II)
ARTIGO TÉCNICO CIENTÍFICO
Impregnation of chelating agent 3,3-bis-N,N bis-(carboxymethyl)aminomethyl-o-cresolsulfonephthalein in biopolymer chitosan. Adsorption equilibrium of Cu(II) in aqueous medium
Luciano Vitali; Karin C. Justi; Mauro C. M. Laranjeira; Valfredo T. Fávere
Departamento de Química, UFSC
Corresponding author Corresponding author: Valfredo T. de Fávere Departamento de Química, UFSC Campus Universitário-Trindade CEP: 88040-900, Florianópolis, SC E-mail: favere@qmc.ufsc.br
ABSTRACT
The aim of this study was to impregnate the chelating agent 3,3-bis-N,N,bis-(carboxymethyl)aminomethyl-o-cresolsulfonephthalein in chitosan and to investigate the adsorption of Cu(II) ions. The chemical modification was confirmed by FTIR spectrometry, thermogravimetric analysis (TGA) and energy dispersive x-ray spectroscopy (EDX). The adsorption studies were carried out with Cu(II) ions in a batch process and were shown to be dependent on pH. The adsorption kinetics was tested using three models: pseudo first-order, pseudo second order and intraparticle diffusion. The experimental kinetics data were best fitted with the pseudo second-order model (R2 = 0.999), which provided a rate constant, k2, of 1.21 x 10-3 g mg-1 min-1. The adsorption rate depended on the concentration of Cu(II) ions on the adsorbent surface and on the quantity of Cu(II) ions adsorbed at equilibrium. The Langmuir isotherm model provided the best fit for the equilibrium data in the concentration range investigated, with the maximum adsorption capacity being 81.0 mg of Cu(II) per gram of adsorbent, as obtained from the linear equation of the isotherm. Desorption tests revealed that around 90% of the adsorbed metal was removed, using EDTA solution as the eluent. This result suggests that the polymeric matrix can be reused.
Keywords: Chitosan, impregnation, adsorption, xylenol orange, Cu(II).
Introduction
The contamination of water particularly by metals is a serious environmental problem since they are toxic even in low concentrations, and also they are not biodegradable being capable of accumulating in living organisms causing various diseases and disorders[1]. Rapid industrialization has increased the disposal of metals in the environment. Effluent treatment is currently one of the most important goals for industry and other institutions, especially those where the effluent generated has a complex mixture of different contaminants such as organic compounds and metals[2,3].
Adsorption or extraction in the solid phase is a technique, which has been commonly used for the removal of metals from liquid effluents[4-6]. Some of the great advantages of adsorption over other techniques are the low quantity of residues generated, easy recovery of metals and the possibility to reuse the absorbent[7,8].
The development of chelating adsorbents prepared by simple impregnation of complexing organic reagents by ion exchange, physical adsorption processes or by the superposition of both phenomena, offers a great advantage due to the simplicity of the modification of the adsorbent surface.
The use of these modified adsorbents with the aim of separating and pre-concentrating trace metal ions offers innumerous advantages in relation to chemical immobilization. The chelating agent may be easily varied and, therefore, the same adsorbent can be used for different purposes, a selective complexing agent can be impregnated thus obtaining selectivity for a specific metal ion, greater flexibility in the working conditions, and high adsorption capacity for metal ions, due to the complexing agent not being bound covalently, which gives the ligand a less rigid position in the adsorbent. The immobilization by chemical binding is limited by the complexity of the synthesis, high costs for the preparation of the materials and the presence of a more rigid structure in the adsorbent, limiting considerably the coordination geometry, hindering the complexation of the metal[9].
The biopolymer chitosan is obtained from the partial deacetylation reaction of the chitin in concentrated alkaline solutions[10]. The presence of amino groups in the polymeric chain allows these groups, when protonated, to have a high density of positive charge enabling the ionic interaction with innumerous complexing agents containing anionic groups and, thus, allowing an improvement in their characteristics as an adsorbent[11-15].
Many dyes have complexing agent characteristics, among them 3,3-bis-N,N, bis-(carboxymethyl)aminomethyl-o-cresolsulfonephthalein (xylenol orange, XO) is an excellent complexometric indicator and potentiometric reagent used for the determination of many metal ions [16]. The chelating agent XO may form stable complexes with many metals due to the existence of many coordination groups, such a carboxylate and phenolate. The large-sized molecule and the presence of sulfonate groups provide a high level of interaction with the adsorbent matrix, facilitating its impregnation on the polymer surface.
The objective of this study was to chemically modify the chitosan surface with the agent XO in order to obtain a new adsorbent material and evaluate its adsorptive properties in relation to Cu(II) metal ions.
Experimental
Materials
The chitosan used for the preparation of the new chelating resin was obtained from Purifarma (SP), having a degree of deacetylation of 90.0%, water content of 8.0%, maximum ash content of 1.0% and pH between 7.0-9.0. The complexometric agent xylenol orange in the form of tetrasodium salt was acquired from the group VETEC Química Fina LTDA. A solution of 909 mg L-1 Cu(II) was prepared from dihydrated copper (II) chloride (98%) obtained from the group VETEC Química Fina LTDA. This solution was standardized with a certified copper standard, acquired from Merck. The solutions for the copper calibration were prepared from the appropriate dilutions of a stock solution of copper (II).
Impregnation of Chelating Agent Xylenol Orange in Chitosan
The new adsorbent material (CTS-XO) was obtained weighing 5g samples of chitosan (pale yellow solid) and placing them in a 1000 mg L-1 aqueous solution of xylenol orange. The pH of the solution was adjusted to around 3 with a sulfuric acid solution for the protonation of amino groups, since chitosan is insoluble in sulfuric medium. The solution was kept under shaking for 24 hours. After this stage the solution was filtered and the intense red colored solid was obtained. Next, this material was washed with distilled water and placed in a desiccator under vacuum. After the characterization by FTIR, TGA and EDX techniques the new adsorbent was then crosslinked in a 2.5% (m/v) glutaraldehyde aqueous solution and kept under shaking for 24 hours at 25 ºC in order to be used in acid medium. The adsorbent was separated from the solution by filtration and washed with distilled water to remove the excess of crosslinking agent. The Figure 1 shows the structure of chelating adsorbent CTS-XO before crosslinking.
Instrumentation
Fourier-tranformed infrared spectra of the chitosan and the new adsorbent were obtained in KBr pellets using a Perkin Elmer PC FTIR 16 spectrophotometer.
Thermogravimetric analysis of the CTS, XO and CTS-XO samples were carried out with TGA-50 Shimadzu, with a heating rate of 10 ºC min-1, under a nitrogen atmosphere.
A flame atomic absorption spectrometer (FAAS), Varian Spectra AA 50, equipped with an air-acetylene flame atomizer and Hitachi hollow cathode lamp (HLA-4S), was used for the copper determination in the adsorption experiments. As the operational parameters of the FAAS the values provided by the manufacturer were used, and only the gas flow was optimized.
The elemental analysis of the new adsorbent using energy disperive x-ray spectrometry was carried out with a Philips model XL 30 instrument, placing the samples in stubs and coating them with gold.
Adsorption experiments
The removal of Cu(II) through an adsorption process in aqueous medium is dependent on various factors such as adsorbent quantity, pH, contact time and temperature. The experiments were carried out taking these variables into account.
Pre-weighed samples of the adsorbent and a measured volume of Cu(II) solution were placed in closed 125 mL Erlenmeyer flasks, the system being kept under shaking in a thermostat bath (Shaker Lab-line). The material was filtered and the non-adsorbed metal concentration was determined by atomic adsorption spectrometry (FAAS).
Desorption experiments
The new adsorbent material CTS-XO (0.100 g) was placed in contact with a 50 mL Cu(II) solution in concentration of 10 mg L-1, at optimum adsorption pH (pH 4.0) and kept under shaking for 24 hours. The amount of Cu(II) ions adsorbed per gram of CTS-XO was obtained by the difference between the initial and final concentrations of metal in solution. The adsorbent material complexed with Cu(II) was collected by filtration and washed with an excess of distilled water in order to remove the non-adsorbed metal. The material adsorbent CTS-XO containing Cu(II) was placed in 50 mL of a 0.01 mol L-1 EDTA solution (kept under shaking for 1 hour) and the quantity of desorbed metal was determined by FAAS.
Results and Discussion
Characterization of new adsorbent CTS-XO
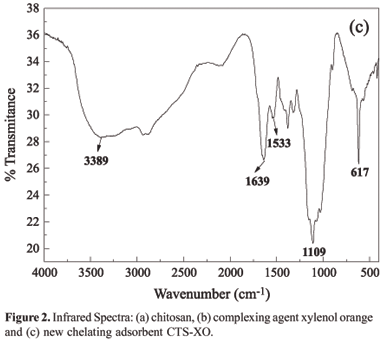
The characterization of the new adsorbent (CTS-XO) was carried out through Fourier-tranformed infrared analysis (FTIR), thermogravimetric analysis (TGA) and elemental analysis using energy disperive X-ray spectrometry (EDX).
The bands obtained in the FTIR spectra of the chitosan (Figure 2a) and of the new adsorbent material (Figure 2c) were very similar, although they showed small differences, which allowed the identification of dye XO in polymeric structure. On comparing the infrared spectra of the chitosan and the new adsorbent material (CTS-XO), it can be observed that a new band appeared at 1533 cm-1, which can be attributed to symmetric bending of the NH3+ group, which is present in the chitosan and is responsible for the interaction of the polymer with the sulfonate groups of the dye[17,18]. The FTIR spectrum of the new adsorbent material showed a shift of the band at 1630 cm-1, which is correlated to the asymmetric stretching of carboxylate ion to 1639 cm-1, due to the probable interaction between protanated amino and sulphonate groups. The broad band of chitosan hides the typical bands of sulphonate groups at 1175 and 1055 cm-1. Another fact, which provides evidence of the impregnation success is the appearance of an elemental sulfur identified by EDX technique, which confirms the presence sulphonate group in the material.
Figure 3 shows the TGA curves for the chitosan, chelating agent and the new chelating adsorbent. The TGA curve for the chitosan shows a thermal degradation at 327ºC, with a polymer mass loss of 65.2%, while the XO showed two decomposition peaks at 226 ºC and 253 ºC with mass loss of 14% and 23%, respectively, being that the first degradation stage relates to the complexing agent xylenol orange and the second corresponding to chitosan8. The decrease in the stability of the CTS-XO in relation to the original polymer can be attributed to ionic interaction between the NH3+ groups of the chitosan and the SO3- groups of the complexing agent. Similar results were found by Maciel and collaborators (2005)[18] and by Lee and collaborators (2003)[19] in the thermal studies involving complexes of chitosan polyelectrolytes. The of temperature peaks related of loss mass observed in the TGA analysis of chitosan, XO and CTS-XO allowed to verify a structural difference between these compounds, confirming the new adsorbent material.
Figure 4 shows the EDX spectrum of the chitosan impregnated with XO. The semi-quantitative analysis revealed a peak centered in 2.2 keV with 2.93% atomic percentage of sulfur in the new material, which also confirms that the impregnation process occurred successfully forming the new chelating adsorbent (CTS-XO).
Effect of pH on the adsorption of Cu(II) by CTS-XO
Figure 5 shows that the adsorption of Cu(II) by the new adsorbent increases with an increase in the solution pH up to a maximum value of 4.0 and then decreases with further increases in the solution pH. At very alkaline pH values, the Cu(II) ion precipitates in the hydroxide form and measurements at these pH values mask any result. At acid pH the decrease in adsorption can be attributed to the protonation of the carboxylate groups of the complexing agent impregnated in the chitosan. Thus, there is competition between the protons and the Cu(II) ions for the adsorption sites at more acid pH values.
At pH higher than 4.0, the decrease in adsorption is attributed to the complexation of Cu(II) íon by citrate buffer; as consequence the ion adsorption is inhibited by the adsorbent[8].
Adsorption Kinetics
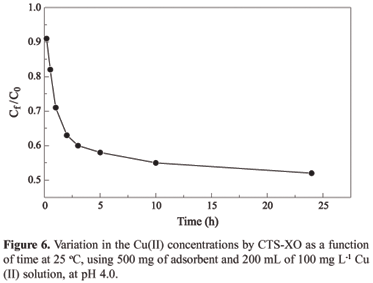
Figure 6 shows the variation in the metal concentrations in the liquid phase (Ct/Co) in relation to time of contact with the adsorbent. Ct and Co corresponds to the metal concentration at time t and the initial concentration, respectively. The adsorption kinetics curve for Cu(II) shows that the adsorption is slow in the initial hours and reaches an adsorption equilibrium in approximately 24 hours.
In order to evaluate the kinetic mechanism which controls the process of Cu(II) metal ion adsorption by CTS-XO, the pseudo first-order, pseudo second-order and intraparticle diffusion models were tested, and the validity of the models could be verified by linear equation analysis log (qe qt) vs. t, (t / qt) vs. t and qtvs. t0.5, respectively. A good correlation of the kinetics data explains the metal ion adsorption mechanism in the solid phase [20-22].
The pseudo first-order equation is represented by Equation 1[17]:
where k1 (min-1) is the pseudo first-order adsorption rate constant; qt is the quantity adsorbed at time t (min), and qe denotes the quantity adsorbed at equilibrium, both in mg g-1. The graph of the log (qe qt) as a function of t, provides the k1 and qe values. The correlation coefficient obtained (R2 = 0.932) did not give a good linearity and a discrepancy is observed, the experimental log qe value is not equal to the intercept of the log (qe qt) vs t graph. When these values were compared with the experimental value there was a deviation of 32%.
It has been observed in several studies that the pseudo first-order equation is inadequate to interpret adsorption kinetics data of dyes and metal ions employing various adsorbents[20].
The pseudo second-order equation has been based on the adsorption capacity at equilibrium may be expressed by Equation 2[20,21]:
where k2 (g mg-1 min-1) is the pseudo second-order adsorption rate constant. From the intercept of the straight line slope of the graph (t/qt) as a function of t, the values of k2 and qe can be obtained.
The graph provided an excellent linearity (R2 = 0.999) with a rate constant (k2) of 1.21x10-3 g mg-1 min-1. A comparison of the experimental value of qe (qe = 17.11) and that obtained from the straight line angular coefficient (qe = 17.62) showed a good agreement with a deviation of 3%.
The adsorption passes through several stages involving the transport of the adsorbate from the aqueous phase to the adsorbent surface and diffusion of the adsorbate in the interior of the adsorbent pores, which is a slow process. The kinetics model of intraparticle diffusion, proposed by Weber and Morris (1963)[22], consists of a simple model in which the intraparticle diffusion rate can be obtained from Equation 3:
When the intraparticle diffusion controls the adsorption kinetics process, the graph of qtvs t0.5 gives a straight line passing through the origin and the angular coefficient gives a rate constant k.
The graph of qtvs t0.5 gave a poor correlation coefficient (R2 = 0.821) and the straight line does not pass through the origin as proposed by the equation, indicating that the intraparticle diffusion is not a determinant factor in the kinetics process.
Based on the analysis of tested kinetic models was determined that the adsorption kinetics of Cu(II) by CTS-XO followed the pseudo second-order model and the adsorption rate was dependent on the Cu(II) ion concentration on the adsorbent surface and on the quantity of Cu(II) ions adsorbed at equilibrium.
Several adsorption kinectic studies using metal íons indicate that the pseudo second-order model also provided the best fit to the experimental data[7,8]
Adsorption isotherms
The equilibrium studies were carried out at optimum adsorption pH with the contact time needed to reach the adsorption equilibrium. For the adsorption data interpretation the Langmuir isotherm model was used due to the homogeneous surface of the adsorbent while the Freundlich isotherm is applied for heterogeneous surfaces[20,21]. The Langmuir isotherm considers the adsorbent surface as homogeneous with identical sites in terms of energy. The Langmuir equation is represented by Equation 4[23,24]:
where, q is the quantity adsorbed (mg g -1), qm is the maximum quantity of adsorption (mg g -1), Kads is the adsorption equilibrium constant, and Ceq is the equilibrium concentration (mg L-1).
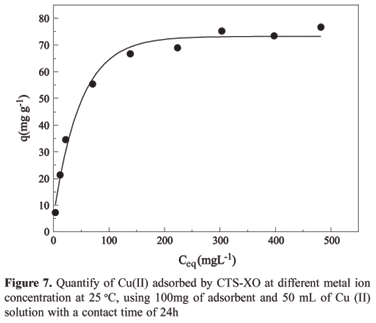
In the adsorption of Cu(II) by CTS-XO represented by Figure 7, the relationship between the quantity of metal ions adsorbed on the adsorbent surface and the concentration remaining in the aqueous phase at equilibrium, can be verified. This relationship showed that the adsorption capacity increases with the equilibrium concentration of Cu(II) ions in solution, reaching progressively the adsorbent saturation.
The adsorption parameters can be determined by transforming the Langmuir equation to a linear form. Equation 5 represents a better linear regression of the isotherm:
The graph of Ceq/q as a function of Ceq allows the calculation of the qm and Kads values. From the adsorption parameters the maximum capacity for the adsorption of the adsorbate by the adsorbent and the Langmuir constant can be evaluated. Figure 8 represents the linearization of the Cu(II) adsorption isotherm according to the Langmuir model.
The straight line equation obtained, Y = 0.391+ 0.012 X, gave a correlation coefficient of 0.999. The value determined for the maximum saturation capacity of the adsorbent monolayer was 81.0 mg of Cu(II) per gram of adsorbent and the Langmuir constant was 3.15 x 10-2 L mg-1.
The adsorption capacity found out our laboratory for Cu(II) (39.7 mg g-1) using chitosan microspheres was approximately twice lower than found in this work[25]. This result was higher than that obtained by Ngah et al (2002)[26] using chitosan crosslinked with glutaraldehyde (59.7 mg g-1) and with epichlorohydrin (62.5 mg g-1). Huang et al (1996)[27] using chitosan not crosslinked on powder form obtained again a value adsorption capacity (45,2 mg g-1) lower than found in this study. However, this result was lower than obtainedby Justi et al (2004) employing chitosan modified with complexation agent BMAMF (109 mg g-1)[8].
In related works, the adsorption of metal íons have been studied by several authors and showed that each chitosan sample has adsorptive properties and, is related to the deacetylation degree, polymerization degree, conformation of polymer chains in aqueous solutions, particle size and porosity. In the adsorption studies, it should be take into account the buffer system and ionic strength of solution.
Desorption studies
Desorption studies using EDTA solution as the eluent were important for the removal of the adsorbed Cu(II) and allowed the possibility of its reuse. The Cu(II) desorption was carried out in duplicate and the average desorption value was 90.2 %.
Conclusions
The techniques of FTIR, TGA and EDX used for the characterization showed that the complexing agent xylenol orange was impregnated in the chitosan surface forming a new adsorbent material. The results indicate that the metal ion adsorption process is dependent on the solution pH, the most probably mechanism for the Cu(II) adsorption being solid phase complexation. The adsorption kinetics followed the pseudo first-order mechanism, which was the model that promoted a better correlation of the experimental data. In the adsorption equilibrium studies the Langmuir equation was used to fit the experimental data obtained giving a maximum capacity of Cu(II) adsorption by CTS-XO of 81.0 mg g-1. The desorption studies carried out revealed that around 90% of the adsorbed metal was removed from the adsorbent material thus enabling its reuse. The results obtained show that the new adsorbent material can be tested in processes of Cu(II) separation, pre-concentration and extraction from aqueous solutions.
Acknowledgements
The authors are grateful to the Universidade Federal de Santa Catarina - UFSC and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq - Brasil, for financial support.
Enviado: 17/10/05
Reenviado: 24/02/06
Aprovado: 06/03/06
- 1. Babel, S.; Kurniawan, T. A. J. Hazard. Mater., B 97, p. 219 (2003).
- 2. Dakiky, M.; Khamis, M.; Manassra, A.; Mereb, M. Adv. Environ. Res., 6, p.533 (2002).
- 3. Tarley, C. R.; Arruda, M. A. Z. Chemosphere, 54, p.987 (2004).
- 4. Pesavento, M.; Baldini, E. Anal. Chim. Acta, 389, p.59 (1999).
- 5. Gurnani, V. Anal. Chim. Acta, 485, p.221 (2003).
- 6. Lee, S-T.; Mi, F-L.; Shen Y-J.; Shyu, S-S. Polymer, 42, p.1879 (2001).
- 7. Spinelli,V. A.; Laranjeira, M. C. M.; Fávere, V. T. React. Funct. Polym., 61, p.347 (2004).
- 8. Justi, C.K.; Laranjeira, M.C.; Neves, A.; Mangrich, A.S.; Favere, V.T. Polymer, 45, p.6285 (2004).
- 9. Kenji Isshiki, K.; Hajime, Y. S.; Nakayam, K. E. Talanta, 224, p.55 (1989).
- 10. Kumar, M. N. V. R. React. Funct. Polym., 46, p.1 (2000).
- 11. Kimura, I.Y.; Furlan, L.; Laranjeira, M. C. M.; Fávere, Int. J. Polym. Mater., 51, p.759 (2002).
- 12. Rodrigues, C.A.; Laranjeira, M. C. M.; Fávere, V.T.; Stadler, E. Polymer, 39, p.5121 (1998).
- 13. Inoue, K.; Yoshizuka, K.; Ohto, K. Anal. Chim. Acta, 388, p.209 (1999).
- 14. Kondo, K.; Nakagawa, S-I.; Matsumoto, M.; Yamashita, T.; Furukawa, I. J. Chem. Eng. Jpn., 30, p.846 (1997).
- 15. Chassary, P.; Vicent, T.; Guibal, E. React. Funct. Polym., 60, p.137 (2004).
- 16. Brajter, K.; Olbrych-Sleszynska, E. Talanta, 30, p.355 (1983).
- 17. Silverstein, R. M.; Bassler. C. G.; Morrill C. T. - Spectrometric Identification of Organic Compounds, 5Ş ed. (1991).
- 18. Maciel, J. S.; Silva, D. A.; Paula, H. C. B.; Paula, R. C. M. Eur. Polym. J., 41, p.2726 (2005).
- 19. Lee, B. S.; Lee, M. Y.; Song, W. K.; Park, H. M. J. Appl. Polym. Sci.., 90, p.925 (2003).
- 20. Ho, Y. S.; McKay, G. Process Biochem. Res., 34, p.451, (1999).
- 21. Ho, Y. S.; McKay, G. Water Res., 33, p.578, (1999).
- 22. Weber, W. J.; Morris, J. C. J. Sanit. Eng. Div. Am. Chem. Soc. Civ. Eng. 89, p.31 (1963).
- 23. Kim, C. Y.; Cho, H. T. J. Appl. Polym. Sci., 63, p.725 (1997).
- 24. Wong, Y. C.; Szeto, Y. S.; Cheung, W. H.; MacKay, G. Process Biochem., 29, p.693 (2004).
- 25. Coelho T. C "Estudos de adsorção dos íons cobre (II) em solução pela microesferas de quitosana reticulada e impregnada com heparina", Dissertação de mestrado, Universidade Federal de Santa Catarina, Brasil (2006).
- 26. Ngah, W. S.; Endud, C. S.; Mayanar, R. React. Funct. Polym., 50, p.181 (2002).
- 27. Huang, C.; Chung, Y.; Liou, M. J. Hazard. Mat., 45, p.265 (1996).
Publication Dates
-
Publication in this collection
02 Oct 2006 -
Date of issue
June 2006
History
-
Accepted
06 Mar 2006 -
Reviewed
24 Feb 2006 -
Received
17 Oct 2005