Abstract
Mechanical recycling of biodegradable plastics has to be encouraged, since the consumption of energy and raw materials can be reduced towards a sustainable development in plastics materials. In this study, the evolution of thermal and mechanical properties, as well as structural changes of poly(hydroxybutyrate) (PHB) up to three extrusion cycles were investigated. Results indicated a significant reduction in mechanical properties already at the second extrusion cycle, with a reduction above 50% in the third cycle. An increase in the crystallinity index was observed due to chemicrystallization process during degradation by chain scission. On the other hand, significant changes in the chemical structure or in thermal stability of PHB cannot be detected by Fourier transform infrared spectroscopy (FTIR) and thermogravimetric analyses (TGA), respectively.
Keywords:
biopolymer; degradation; PHB; recycling; reprocessing
1. Introduction
The rate of municipal solid waste (MSW) generation is rising more than the rate of urbanization around the world[11 Hoornweg, D., & Bhada-Tata, P. (2012). What a waste: a global review of solid waste management. Washington: World Bank.]. By 2025, the volume of MSW generated worldwide is expected to double, reaching an amount of 2.2 billion tonnes per year[11 Hoornweg, D., & Bhada-Tata, P. (2012). What a waste: a global review of solid waste management. Washington: World Bank.]. In general, plastics are of main concern because they represent between 3 and 14.3 wt% of the total MSW, most are from non-renewable sources and have relatively low recycling rates[11 Hoornweg, D., & Bhada-Tata, P. (2012). What a waste: a global review of solid waste management. Washington: World Bank.
2 Thompson, R. C., Moore, C. J., vom Saal, F. S., & Swan, S. H. (2009). Plastics, the environment and human health: current consensus and future trends. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1526), 2153-2166. PMid:19528062. http://dx.doi.org/10.1098/rstb.2009.0053.
http://dx.doi.org/10.1098/rstb.2009.0053...
-33 Davis, G., & Song, J. H. (2006). Biodegradable packaging based on raw materials from crops and their impact on waste management. Industrial Crops and Products, 23(2), 147-161. http://dx.doi.org/10.1016/j.indcrop.2005.05.004.
http://dx.doi.org/10.1016/j.indcrop.2005...
].
A key practice to minimizing the environmental problems associated with solid waste is the practice of the 3R (Reduce, Reuse and Recycle) concept, which contributes to reduce energy and natural resources consumption, extend life cycle of products and landfills, and store carbon for a longer period, all important initiatives within the concept of sustainable development. Another alternative that has gained importance is the replacement of petroleum-based plastics by biopolymers from renewable resources, which are generally compostable and able to close the carbon cycle. According to the European Union Regulation, composting is a considered and accepted way of recovering biodegradable packaging wastes[33 Davis, G., & Song, J. H. (2006). Biodegradable packaging based on raw materials from crops and their impact on waste management. Industrial Crops and Products, 23(2), 147-161. http://dx.doi.org/10.1016/j.indcrop.2005.05.004.
http://dx.doi.org/10.1016/j.indcrop.2005...
]. However, recycling of biopolymers can be an alternative to composting and to reduce carbon dioxide emission, thus meeting the 3R concept. This approach not only saves energy that would be used in polymer synthesis, but also reduces costs associated with bioresources (monomers) used to produce new bioplastics and spares carbon resources. Since biopolymers are commonly used in the production of short-life goods, recycling of these polymers becomes even more important because of the large amount of waste generated in markets, as well as insumes consumed.
Nevertheless, in the case of biodegradable plastics, there are few studies related to their recycling[44 Le Duigou, A., Pillin, I., Bourmaud, A., Davies, P., & Baley, C. (2008). Effect of recycling on mechanical behaviour of biocompostable flax/poly(L-lactide) composites. Composites. Part A, Applied Science and Manufacturing, 39(9), 1471-1478. http://dx.doi.org/10.1016/j.compositesa.2008.05.008.
http://dx.doi.org/10.1016/j.compositesa....
5 Pillin, I., Montrelay, N., Bourmaud, A., & Grohens, Y. (2008). Effect of thermo-mechanical cycles on the physico-chemical properties of poly(lactic acid). Polymer Degradation & Stability, 93(2), 321-328. http://dx.doi.org/10.1016/j.polymdegradstab.2007.12.005.
http://dx.doi.org/10.1016/j.polymdegrads...
6 Nishida, H., Fan, Y., Mori, T., Oyagi, N., Shirai, Y., & Endo, T. (2005). Feedstock recycling of flame-resisting poly(lactic acid) aluminum hydroxide composite. Industrial & Engineering Chemistry Research, 44(5), 1433-1437. http://dx.doi.org/10.1021/ie049208+.
http://dx.doi.org/10.1021/ie049208+...
-77 Sikorska, W., Richert, J., Rydz, J., Musioł, M., Adamus, G., Janeczek, H., & Kowalczuk, M. (2012). Degradability studies of poly(L-lactide) after multi-reprocessing experiments in extruder. Polymer Degradation & Stability, 97(10), 1891-1897. http://dx.doi.org/10.1016/j.polymdegradstab.2012.03.049.
http://dx.doi.org/10.1016/j.polymdegrads...
]. Life cycle analysis[88 Gurieff, N., & Lant, P. (2007). Comparative life cycle assessment and financial analysis of mixed culture polyhydroxyalkanoate production. Bioresource Technology, 98(17), 3393-3403. PMid:17632000. http://dx.doi.org/10.1016/j.biortech.2006.10.046.
http://dx.doi.org/10.1016/j.biortech.200...
] of poly(lactic acid) (PLA), which is one the most studied biopolymers, pointed out that despite of its low environmental impacts, this situation can worsen if recycling is not achieved. According to Shah et al.[99 Shah, A. A., Kato, S., Shintani, N., Kamini, N. R., & Nakajima-Kambe, T. (2014). Microbial degradation of aliphatic and aliphatic-aromatic co-polyesters. Applied Microbiology and Biotechnology, 98(8), 3437-3447. PMid:24522729. http://dx.doi.org/10.1007/s00253-014-5558-1.
http://dx.doi.org/10.1007/s00253-014-555...
], pyrolysis, solvolysis and enzymatic monomer recycling are the most promising feedstock recycling technologies for biodegradable plastics because most biopolymers have heteroatoms in their main chain, which reduce thermal stability and increase susceptibility to hydrolysis. Besides that, studies of biopolymers recyclability by multiple reprocessing are also important due to the fact that the production of biopolymer-based products may involve more than one extrusion step and mechanical recycling is the most energy efficient recycling technology.
Polyhydroxyalcanoates (PHAs) have attracted a great deal of attention due to their types, properties similar to conventional plastics, biodegradability, biocompatibility, renewability, and synthesis by biotechnological processes[1010 Coutinho, B. C., Miranda, G. B., Sampaio, G. R., Souza, L. B. S., Santana, W. J., & Coutinho, H. D. M. (2004). A importância e as vantagens do polihidroxibutirato (plástico biodegradável). Holos, 20(3), 76-81. http://dx.doi.org/10.15628/holos.2004.49.
http://dx.doi.org/10.15628/holos.2004.49...
,1111 Sánchez, R. J., Schripsema, J., Silva, L. F., Taciro, M. K., Pradella, J. G. C., & Gomez, J. G. C. (2003). Medium-chain-length Polyhydroxy-Alkanoic Acids (PHAMCL) produced by Pseudomonas Putida IPT 046 from renewable sources. European Polymer Journal, 39(7), 1385-1394. http://dx.doi.org/10.1016/S0014-3057(03)00019-3.
http://dx.doi.org/10.1016/S0014-3057(03)...
]. Although their discovery occurred early, PHAs were rediscovered in the 1980s, and their use has gained increasing attraction, particularly in the last decades, as a consequence of the reduction and limitation of the fossil fuel resources, allied with the increasing concern about the environmental issues caused by improper manipulation, use and disposal of such non-renewable materials. Among these polymers, biosynthesized polyhydroxybutyrate (PHB), the most common PHA, has a high potential to compete with commodity polymers due to its similarity in crystallinity, melting temperature, tensile strength and Young’s modulus, and competitive costs[1212 Doi, Y. (1990). Microbial polyesters. New York: VCH Publishers Inc.]. Nevertheless, high stiffness, brittleness and poor thermal stability above the melting temperature (a narrow processability window) are some of its drawbacks compared to many conventional polymers[1313 Hablot, E., Bordes, P., Pollet, E., & Averous, L. (2008). Thermal and thermo-mechanical degradation of poly(3-hydroxybutyrate)-based multiphase systems. Polymer Degradation & Stability, 93(2), 413-421. http://dx.doi.org/10.1016/j.polymdegradstab.2007.11.018.
http://dx.doi.org/10.1016/j.polymdegrads...
14 Nguyen, S., Yu, G., & Marchessault, R. (2002). Thermal degradation of poly(3-hydroxyalkanoates): preparation of well-defined oligomers. Biomacromolecules, 3(1), 219-224. PMid:11866577. http://dx.doi.org/10.1021/bm0156274.
http://dx.doi.org/10.1021/bm0156274...
-1515 Sadi, R. K. (2010). Compatibilization and degradation study of Polypropylene/Poly(3-hydroxybutyrate) (PP/PHB) blends (Doctoral thesis). Escola Politécnica, Universidade de São Paulo, São Paulo.].
Feedstock recycling of PHB has already been considered as a viable route to produce end products such as crotonic acid, linear oligomers having crotonate end groups and a cyclic trimer, as well as to produce plasticized PLA by reactive extrusion grafting of PHB degradation products onto PLA chains[1616 Melchiors, M., Keul, H., & Höcker, H. (1996). Depolymerization of poly[(R)-3-hydroxybutyrate] to cyclic oligomers and polymerization of the cyclic trimer: an example of thermodynamic recycling. Macromolecules, 29(20), 6442-6451. http://dx.doi.org/10.1021/ma9604350.
http://dx.doi.org/10.1021/ma9604350...
17 Yang, X., Clénet, J., Xu, H., Odelius, K., & Hakkarainen, M. (2015). Two step extrusion process: from thermal recycling of PHB to plasticized PLA by reactive extrusion grafting of PHB degradation products onto PLA chains. Macromolecules, 48(8), 2509-2518. PMid:27053818. http://dx.doi.org/10.1021/acs.macromol.5b00235.
http://dx.doi.org/10.1021/acs.macromol.5...
18 Spekreijse, J., Le Nôtre, J., Sanders, J. P. M., & Scott, E. L. (2015). Conversion of polyhydroxybutyrate (PHB) to methyl crotonate for the production of biobased monomers. Journal of Applied Polymer Science, 132(35), n/a. http://dx.doi.org/10.1002/app.42462.
http://dx.doi.org/10.1002/app.42462...
-1919 Osanai, Y., Toshima, K., & Matsumura, S. (2003). Enzymatic degradation of poly(R,S-3-hydroxybutanoate) to cyclic oligomers under continuous flow. Green Chemistry, 5(5), 567-570. http://dx.doi.org/10.1039/B304640K.
http://dx.doi.org/10.1039/B304640K...
]. At temperatures a little above the melting point, there is a rapid decrease in molecular weight and a subsequent production of crotonic acid as the main volatile product[2020 Grassie, N., Murray, E. J., & Holmes, P. A. (1984). The thermal degradation of poly(-(d)-b-hydroxybutyric acid): part 1 – Identification and quantitative analysis of products. Polymer Degradation & Stability, 6(1), 47-61. http://dx.doi.org/10.1016/0141-3910(84)90016-8.
http://dx.doi.org/10.1016/0141-3910(84)9...
21 Karlsson, S., Sares, C., Renstad, R., & Albertsson, A. C. (1994). Gas chromatographic, liquid chromatographic and gas chromatographic-mass spectrometric identification of degradation products in accelerated aged microbial polyhydroxyalkanoates. Journal of Chromatography. A, 669(1-2), 97-102. PMid:8075778. http://dx.doi.org/10.1016/0021-9673(94)80341-2.
http://dx.doi.org/10.1016/0021-9673(94)8...
22 Yu, P. H., Chua, H., Huang, A. L., Lo, W., & Chen, G. Q. (1998). Conversion of food industrial wastes into bioplastics. Applied Biochemistry and Biotechnology, 70-72(1), 603-614. PMid:18576025. http://dx.doi.org/10.1007/BF02920172.
http://dx.doi.org/10.1007/BF02920172...
-2323 King, P. P. (1982). Biotechnology. An industrial view. Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 32(1), 2-8. http://dx.doi.org/10.1002/jctb.5030320103.
http://dx.doi.org/10.1002/jctb.503032010...
]. The presence of moisture, fermentation residues, oxygen, metal and alkali catalysts also is known to favor thermal degradation[1414 Nguyen, S., Yu, G., & Marchessault, R. (2002). Thermal degradation of poly(3-hydroxyalkanoates): preparation of well-defined oligomers. Biomacromolecules, 3(1), 219-224. PMid:11866577. http://dx.doi.org/10.1021/bm0156274.
http://dx.doi.org/10.1021/bm0156274...
,2424 Rosa, D. S., & Pântano, R., Fo. (2003). Biodegradação: um ensaio com polímeros. Itatiba: Moara.
25 Foster, L. J. R., & Tighe, B. J. (2005). Centrifugally spun polyhydroxybutyrate fibres: accelerated hydrolytic degradation studies. Polymer Degradation & Stability, 87(1), 1-10. http://dx.doi.org/10.1016/j.polymdegradstab.2003.11.012.
http://dx.doi.org/10.1016/j.polymdegrads...
26 Ariffin, H., Nishida, H., Shirai, Y., & Hassan, M. A. (2010). Highly selective transformation of poly[(R)-3-hydroxybutyric acid] into trans-crotonic acid by catalytic thermal degradation. Polymer Degradation & Stability, 95(8), 1375-1381. http://dx.doi.org/10.1016/j.polymdegradstab.2010.01.018.
http://dx.doi.org/10.1016/j.polymdegrads...
27 Kim, K. J., Doi, Y., & Abe, H. (2008). Effect of metal compounds on thermal degradation behavior of aliphatic poly(hydroxyalkanoic acid)s. Polymer Degradation & Stability, 93(4), 776-785. http://dx.doi.org/10.1016/j.polymdegradstab.2008.01.026.
http://dx.doi.org/10.1016/j.polymdegrads...
-2828 Farid, N. F. S. M., Ariffin, H., Mamat, M. R. Z., Mohd Zahari, M. A. K., & Hassan, M. A. (2015). Non-solvent-based pretreatment of poly(3-hydroxybutyrate) for improved bio-based crotonic acid production. RSC Advances, 5(42), 33546-33553. http://dx.doi.org/10.1039/C5RA03017J.
http://dx.doi.org/10.1039/C5RA03017J...
]. This is an important limitation for processing and consequently, to mechanical recycling. Therefore, there has been a great deal of interest in studying the thermal degradation behavior of PHB and other related poly(hydroxyalkanoate)s[2929 Ariffin, H., Nishida, H., Shirai, Y., & Hassan, M. A. (2008). Determination of multiple thermal degradation mechanisms of poly(3-hydroxybutyrate). Polymer Degradation & Stability, 93(8), 1433-1439. http://dx.doi.org/10.1016/j.polymdegradstab.2008.05.020.
http://dx.doi.org/10.1016/j.polymdegrads...
30 Kopinke, F. D., Remmler, M., & Mackenzie, K. (1996). Thermal decomposition of biodegradable polyesters-I: Poly(β-hydroxybutyric acid). Polymer Degradation & Stability, 52(1), 25-38. http://dx.doi.org/10.1016/0141-3910(95)00221-9.
http://dx.doi.org/10.1016/0141-3910(95)0...
31 Aoyagi, Y., Yamashita, K., & Doi, Y. (2002). Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly [(S)-lactide]. Polymer Degradation & Stability, 76(1), 53-59. http://dx.doi.org/10.1016/S0141-3910(01)00265-8.
http://dx.doi.org/10.1016/S0141-3910(01)...
32 Hong, S. G., Lin, Y. C., & Lin, C. H. (2008). Crystallization and degradation behaviors of treated polyhydroxybutyrates. Reactive & Functional Polymers, 68(11), 1516-1523. http://dx.doi.org/10.1016/j.reactfunctpolym.2008.08.003.
http://dx.doi.org/10.1016/j.reactfunctpo...
33 Kawalec, M., Adamus, G. Y., Kurcok, P., Kowalczuk, M., Foltran, I., Focarete, M. L., & Scandola, M. (2007). Carboxylate-induced degradation of poly(3-hydroxybutyrate)s. Biomacromolecules, 8(4), 1053-1058. PMid:17330956. http://dx.doi.org/10.1021/bm061155n.
http://dx.doi.org/10.1021/bm061155n...
34 Galego, N., & Rozsa, C. (1999). Thermal decomposition of some poly(b-hydroxyalcanoates). Polymer International, 48(12), 1202-1204. http://dx.doi.org/10.1002/(SICI)1097-0126(199912)48:12<1202::AID-PI223>3.0.CO;2-D.
http://dx.doi.org/10.1002/(SICI)1097-012...
-3535 Lehrle, R., Williams, R., French, C., & Hammond, T. (1995). Thermolysis and methanolysis of poly(b-hydroxybutyrate): random scission assessed by statistical analysis of molecular weight distributions. Macromolecules, 28(13), 4408-4414. http://dx.doi.org/10.1021/ma00117a008.
http://dx.doi.org/10.1021/ma00117a008...
]. Also studies seeking to improve the thermal stability of PHB by grafting chemicals in PHB chain[3333 Kawalec, M., Adamus, G. Y., Kurcok, P., Kowalczuk, M., Foltran, I., Focarete, M. L., & Scandola, M. (2007). Carboxylate-induced degradation of poly(3-hydroxybutyrate)s. Biomacromolecules, 8(4), 1053-1058. PMid:17330956. http://dx.doi.org/10.1021/bm061155n.
http://dx.doi.org/10.1021/bm061155n...
,3636 Bahari, K., Mitomo, H., Enjoji, T., Yoshii, F., & Makuuchi, K. (1998). Degradability of poly(3-hydroxybutyrate) and its copolymer grafted with styrene by radiation. Polymer Degradation & Stability, 61(2), 245-252. http://dx.doi.org/10.1016/S0141-3910(97)00147-X.
http://dx.doi.org/10.1016/S0141-3910(97)...
], and adding polymeric additives in PHB matrix[3737 Lee, H. K., Ismail, J., Kammer, H. W., & Bakar, M. A. (2005). Melt reaction in blends of poly(3-hydroxybutyrate) (PHB) and epoxidized natural rubber (ENR-50). Journal of Applied Polymer Science, 95(1), 113-129. http://dx.doi.org/10.1002/app.20808.
http://dx.doi.org/10.1002/app.20808...
,3838 Hong, S. G., Gau, T. K., & Huang, S. C. (2011). Enhancement of the crystallization and thermal stability of polyhydroxybutyrate by polymeric additives. Journal of Thermal Analysis and Calorimetry, 103(3), 967-975. http://dx.doi.org/10.1007/s10973-010-1180-3.
http://dx.doi.org/10.1007/s10973-010-118...
] have been developed.
The thermal degradation behavior of PHB has been discussed in many works[1414 Nguyen, S., Yu, G., & Marchessault, R. (2002). Thermal degradation of poly(3-hydroxyalkanoates): preparation of well-defined oligomers. Biomacromolecules, 3(1), 219-224. PMid:11866577. http://dx.doi.org/10.1021/bm0156274.
http://dx.doi.org/10.1021/bm0156274...
,2020 Grassie, N., Murray, E. J., & Holmes, P. A. (1984). The thermal degradation of poly(-(d)-b-hydroxybutyric acid): part 1 – Identification and quantitative analysis of products. Polymer Degradation & Stability, 6(1), 47-61. http://dx.doi.org/10.1016/0141-3910(84)90016-8.
http://dx.doi.org/10.1016/0141-3910(84)9...
,2121 Karlsson, S., Sares, C., Renstad, R., & Albertsson, A. C. (1994). Gas chromatographic, liquid chromatographic and gas chromatographic-mass spectrometric identification of degradation products in accelerated aged microbial polyhydroxyalkanoates. Journal of Chromatography. A, 669(1-2), 97-102. PMid:8075778. http://dx.doi.org/10.1016/0021-9673(94)80341-2.
http://dx.doi.org/10.1016/0021-9673(94)8...
,3030 Kopinke, F. D., Remmler, M., & Mackenzie, K. (1996). Thermal decomposition of biodegradable polyesters-I: Poly(β-hydroxybutyric acid). Polymer Degradation & Stability, 52(1), 25-38. http://dx.doi.org/10.1016/0141-3910(95)00221-9.
http://dx.doi.org/10.1016/0141-3910(95)0...
,3939 Morikawa, H., & Marchessault, R. H. (1981). Pyrolysis of bacterial polyalkanoates. Canadian Journal of Chemistry, 59(15), 2306-2313. http://dx.doi.org/10.1139/v81-334.
http://dx.doi.org/10.1139/v81-334...
40 Ballistreri, A., Garozzo, D., Giuffrida, M., Impallomeni, G., & Montaudo, G. (1989). Analytical degradation: an approach to the structural analysis of microbial polyesters by different methods. Journal of Analytical and Applied Pyrolysis, 16(3), 239-253. http://dx.doi.org/10.1016/0165-2370(89)80028-2.
http://dx.doi.org/10.1016/0165-2370(89)8...
41 Rosa, D. S., Calil, M. R., Guedes, C. G. F., & Santos, C. E. O. (2001). The Effect of UV-B Irradiation on the biodegradability of poly-β-hydroxybutyrate (PHB) and poly-ε-caprolactone (PCL). Journal of Polymers and the Environment, 9(3), 109-113. http://dx.doi.org/10.1023/A:1020498710586.
http://dx.doi.org/10.1023/A:102049871058...
-4242 Kopinke, F. D., & Mackenzie, K. (1997). Mechanistic aspects of the thermal degradation of poly(lactic acid) and poly(beta-hydroxybutyric acid). Journal of Analytical and Applied Pyrolysis, 40-41, 43-53. http://dx.doi.org/10.1016/S0165-2370(97)00022-3.
http://dx.doi.org/10.1016/S0165-2370(97)...
], in which a random chain scission reaction (β-elimination) involving a six-membered ring transition state (Figure 1) has been considered as the main mechanism based on typical structures of pyrolysis products, i.e., crotonic acid and oligomers with a crotonate end group, i. e., unsaturated end groups. Since the proposed mechanism is a non-radical random chain scission the conventional stabilizers and antioxidant was not efficient to prevent PHB degradation[3838 Hong, S. G., Gau, T. K., & Huang, S. C. (2011). Enhancement of the crystallization and thermal stability of polyhydroxybutyrate by polymeric additives. Journal of Thermal Analysis and Calorimetry, 103(3), 967-975. http://dx.doi.org/10.1007/s10973-010-1180-3.
http://dx.doi.org/10.1007/s10973-010-118...
].
In this work, the effect of multiple reprocessing cycles on the properties of PHB products obtained by more than one reprocessing cycle, as well as in their recyclability was evaluated. Thermal and mechanical properties were measured after each extrusion cycle, and correlated with polymer structural changes.
2. Materials and Methods
2.1 Materials
Poly(3-hydroxybutyrate) (PHB) powder, produced by bacterial fermentation, was kindly supplied by PHB Industrial S/A, Serrana-SP, Brazil, registered under the brand BIOCYCLE® and used as received. This polymer has a melt flow index of 6.5 g/10 min (190 °C, 2.16 kg) and number-average molecular weight (Mn) of 56,650 g.mol-1, weight-average molecular weight (Mw) of 167,223 g.mol-1, and polydispersity index (PDI) of 2.95, as measured by gel permeation chromatography.
2.2 Reprocessing cycles
Extrusions were carried out using an AX Plásticos (São Paulo, Brazil) single screw extruder (ϕ = 16 mm, L/D = 26 and 90 rpm) with Maddock mixing section between the compression zone and the flow control zone up to three times. Temperature profile used was: 165, 170 and 170 °C. The neat PHB powder (virgin polymer) or pellets (extruded polymer) was dried at 60 °C under vacuum for 12 h before each processing cycle. Since it is a first approach on recyclability of PHB, no other materials/additives, such as nucleating agent or plasticizers, were used.
2.3 Compression molding
Compression molded films were prepared in a hydraulic press (capacity of 24kgf) at 170 °C for 1 min at about 10 kgf, followed by quenching in an ice-water bath.
2.4 Mechanical properties
Tensile tests were carried out according to ASTM D 882 at 25 °C and strain rate of 1%.min-1 using a TA Instruments DMA, Q800. The test-specimens with dimensions around (50 × 10 × 0.075) mm were cut from films prepared by compression molding. At least 5 samples were carried out for virgin PHB and after each extrusion cycle. Results were averaged arithmetically.
2.5 Fourier transform infrared (FTIR) spectroscopy
Infrared spectra were obtained by a Nexus 470 Nicolet FTIR spectrophotometer. 32 scans, resolution of 4 cm-1 and interval of 2 cm-1 were used. Analyses were performed in the attenuated total reflectance mode (ATR) by direct analysis of films on SnZn crystal.
2.6 Thermogravimetric analysis (TGA)
Thermogravimetric analyses (TGA) were conducted under 50 mL.min-1 of nitrogen flow, using a Shimadzu TGA 60 apparatus. The samples were heated to 600 °C at 20 °C.min-1. The characteristic degradation temperatures: temperature at the maximum of the DTG curve (Tmax) and the temperature (Tx%) at which the sample loses x% of its initial weight were determined.
2.7 Differential scanning calorimetry (DSC)
Thermograms were obtained using a Shimadzu DSC 60 differential scanning calorimeter (DSC). Calibration was performed with indium and tin in the temperature range from 0 to 350 oC. The sample weight was approximately 6-10 mg. All the samples were heated from 25 °C to 220 °C at 10 °C.min-1 in purge flow of nitrogen at 50 mL/min. Melting enthalpies were determined using constant integration limits. The degree of crystallinity (Xc) was determined using the following equation:
where: ΔHm is the melting enthalpy per unit of weight of PHB samples and ΔH100% denotes enthalpy per unit weight of the 100% crystalline PHB, which is assumed to be 146 J/g[4343 Barham, P. J., Keller, A., Otun, E. L., & Holmes, P. A. (1984). Crystallization and morphology of a bacterial thermoplastic: poly-3-hydroxybutyrate. Journal of Materials Science, 19(9), 2781-2794. http://dx.doi.org/10.1007/BF01026954.
http://dx.doi.org/10.1007/BF01026954...
]. All DSC analysis was taken from the films obtained by compression molding in order to best represent the material undergoing mechanical test. This procedure has been already adopted by Srubar et al.[4444 Srubar, W. V., 3rd, Wright, Z. C., Tsui, A., Michel, A. T., Billington, S. L., & Frank, C. W. (2012). Characterizing the effects of ambient aging on the mechanical and physical properties of two commercially available bacterial thermoplastics. Polymer Degradation & Stability, 97(10), 1922-1929. http://dx.doi.org/10.1016/j.polymdegradstab.2012.04.011.
http://dx.doi.org/10.1016/j.polymdegrads...
].
3. Results and Discussions
3.1 Mechanical properties
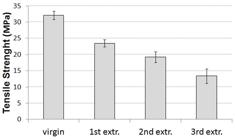
In order to assess the industrial possibility of PHB recycling, its mechanical properties must stay as stable as possible along the processing cycles. A representative tensile curve of virgin PHB was depicted in Figure 2, which shows its brittle nature. Therefore, in this case, the tensile strength is coincident with tensile stress at break. Figure 3 shows the values of tensile strength of PHB, before and after each extrusion cycle. It can be observed that the tensile stress at break decreased from 32.1 MPa to 13.4 MPa after three extrusion cycles. At the 3rd cycle, the tensile stress at break had the same order of magnitude of the tensile strength of LDPE[4545 Coutinho, F. M. B., Mello, I. L., & Santa Maria, L. C. (2003). Polietileno: principais tipos, propriedades e aplicações. Polímeros. Ciência e Tecnologia, 13(1), 1-13. http://dx.doi.org/10.1590/S0104-14282003000100005.
http://dx.doi.org/10.1590/S0104-14282003...
]. However, the elongation at break of these two polymers is very distinct and the tactile sensation of PHB at the 3rd cycle is like a brittle material, which hindered additional extrusion cycles for further characterization of samples.
Representative tensile stress-strain curve of virgin PHB determined at 1 mm.min-1 by using a universal machine Shimadzu AG-X 10kN model, based on the ISO standard 527- 2. The test-specimens, type 5B, were prepared by laser cutting from films prepared by compression molding.
According to Pillin et al.[55 Pillin, I., Montrelay, N., Bourmaud, A., & Grohens, Y. (2008). Effect of thermo-mechanical cycles on the physico-chemical properties of poly(lactic acid). Polymer Degradation & Stability, 93(2), 321-328. http://dx.doi.org/10.1016/j.polymdegradstab.2007.12.005.
http://dx.doi.org/10.1016/j.polymdegrads...
], a similar decrease in tensile strength values was observed only at the 6th injection cycle for PLA. Nevertheless, the properties of PLA stay useful, i.e., above about 10 MPa, until the 7th cycle, the maximum number of cycles evaluated.
The color change of PHB from an off-white powder to opaque brown color was observed after first extrusion, as reported in the literature[4646 El-Hadi, A., Schnabel, R., Straube, E., Müller, G., & Henning, S. (2002). Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polymer Testing, 21(6), 665-674. http://dx.doi.org/10.1016/S0142-9418(01)00142-8.
http://dx.doi.org/10.1016/S0142-9418(01)...
,4747 Sadi, R. K., Fechine, G. J. M., & Demarquette, N. R. (2010). Photodegradation of poly(3-hydroxybutyrate). Polymer Degradation & Stability, 95(12), 2318-2327. http://dx.doi.org/10.1016/j.polymdegradstab.2010.09.003.
http://dx.doi.org/10.1016/j.polymdegrads...
]. Such behavior is accounted for PHB chromophoric carbonyl groups.
The strong decrease in tensile strength is probably ascribed to a reduction in the molecular weight of PHB due to chain scission reactions caused by thermal degradation[1515 Sadi, R. K. (2010). Compatibilization and degradation study of Polypropylene/Poly(3-hydroxybutyrate) (PP/PHB) blends (Doctoral thesis). Escola Politécnica, Universidade de São Paulo, São Paulo.,2020 Grassie, N., Murray, E. J., & Holmes, P. A. (1984). The thermal degradation of poly(-(d)-b-hydroxybutyric acid): part 1 – Identification and quantitative analysis of products. Polymer Degradation & Stability, 6(1), 47-61. http://dx.doi.org/10.1016/0141-3910(84)90016-8.
http://dx.doi.org/10.1016/0141-3910(84)9...
,3131 Aoyagi, Y., Yamashita, K., & Doi, Y. (2002). Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly [(S)-lactide]. Polymer Degradation & Stability, 76(1), 53-59. http://dx.doi.org/10.1016/S0141-3910(01)00265-8.
http://dx.doi.org/10.1016/S0141-3910(01)...
] during extrusion cycles. It is well established that the former properties are strongly affected by chain scission, since the broken chains are confined in the amorphous regions between the lamellae, where tie molecules, which are responsible for the mechanical integrity of semicrystalline polymers, are located[4848 Rabello, M. S., & White, J. R. (1997). Crystallization and melting behavior of photodegraded polypropylene - I. Chemi-crystallization. Polymer, 38(26), 6379-6387. http://dx.doi.org/10.1016/S0032-3861(97)00213-9.
http://dx.doi.org/10.1016/S0032-3861(97)...
].
The hypothesis of mechanical properties decrease with molecular weight was in good agreement with the results achieved by Sadi et al.[4747 Sadi, R. K., Fechine, G. J. M., & Demarquette, N. R. (2010). Photodegradation of poly(3-hydroxybutyrate). Polymer Degradation & Stability, 95(12), 2318-2327. http://dx.doi.org/10.1016/j.polymdegradstab.2010.09.003.
http://dx.doi.org/10.1016/j.polymdegrads...
] and Renstad et al.[4949 Renstad, R., Karlsson, S., & Albertsson, A. C. (1997). Influence of processing parameters on the molecular weight and mechanical properties of poly(3-hydroxybutyrate-3-hydroxyvalerate). Polymer Degradation & Stability, 57(3), 331-338. http://dx.doi.org/10.1016/S0141-3910(97)00028-1.
http://dx.doi.org/10.1016/S0141-3910(97)...
]. In both works, the tensile strength at break for PHB and (poly-3-hydroxy butyrate-co-valerate) (PHBV), respectively, decreased with molecular weight depression. Nevertheless, none of these studies have determined until how many extrusion cycles the mechanical properties of PHB still useful and the rate that decay in the mechanical properties with extrusion cycles occurs. Kendall[5050 Kendall, A. (2012). A life cycle assessment of biopolymer production from material recovery facility residuals. Resources, Conservation and Recycling, 61, 69-74. http://dx.doi.org/10.1016/j.resconrec.2012.01.008.
http://dx.doi.org/10.1016/j.resconrec.20...
], for example, in its life cycle assessment for the production of PHB from material recovery facilities considers that there is no current technology for PHB mechanical recycling, but in this study results show that it is possible to recycle PHB up to three extrusion cycles without any additive to inhibit its degradation or improve its properties. For conventional plastics, it is known that during thermomechanical recycling[5151 Currann, M. A. (1996). Environmental life-cycle assessment. New York: McGraw Hill.
52 Feller, J. F., & Bourmaud, A. (2003). Rheological and calorimetric properties of recycled bisphenol A poly(carbonate). Polymer Degradation & Stability, 82(1), 99-104. http://dx.doi.org/10.1016/S0141-3910(03)00169-1.
http://dx.doi.org/10.1016/S0141-3910(03)...
-5353 Spinace, M. A. S., & De Paoli, M. A. (2004). Nonisothermal crystallization of reprocessed poly(ethylene terephthalate). Journal of Applied Polymer Science, 91(1), 525-531. http://dx.doi.org/10.1002/app.13230.
http://dx.doi.org/10.1002/app.13230...
], mechanical properties decrease with increasing multiple extrusion or injection molding cycles. This trend depends upon the type and chemical nature of the polymer. Nevertheless, blends of recycled polymers with virgin ones, reprocessing with stabilizers and incorporation of reinforcing fillers are alternatives to improve recycled plastics properties[5454 Javierre, C., Claveria, I., Ponz, L., Aisa, J., & Fernandez, A. (2007). Influence of the recycled material percentage on the rheological behaviour of HDPE for injection moulding process. Waste Management (New York, N.Y.), 27(5), 656-663. PMid:16707257. http://dx.doi.org/10.1016/j.wasman.2006.03.005.
http://dx.doi.org/10.1016/j.wasman.2006....
55 Santos, A. S. F., Agnelli, J. A. M., Trevisan, D. W., & Manrich, S. (2002). Degradation and stabilization of polyolefins from municipal plastic waste during multiple extrusions under different reprocessing conditions. Polymer Degradation & Stability, 77(3), 441-447. http://dx.doi.org/10.1016/S0141-3910(02)00101-5.
http://dx.doi.org/10.1016/S0141-3910(02)...
56 Hamzehlou, S. H., & Katbab, A. A. (2007). Bottle-to-bottle recycling of PET via nanostructure formation by melt intercalation in twin screw compounder: improved thermal, barrier, and microbiological properties. Journal of Applied Polymer Science, 106(2), 1375-1382. http://dx.doi.org/10.1002/app.26730.
http://dx.doi.org/10.1002/app.26730...
-5757 Abad, M. J., Ares, A., Barral, L., Cano, J., Díez, F. J., García-Garabal, S., López, J., & Ramírez, C. (2004). Effects of a mixture of stabilizers on the structure and mechanical properties of polyethylene during reprocessing. Journal of Applied Polymer Science, 92(6), 3910-3916. http://dx.doi.org/10.1002/app.20420.
http://dx.doi.org/10.1002/app.20420...
].
Furthermore, changes in mechanical properties could be also attributed to changes in the structure and stability of crystalline state or rearrangement of interlamellar amorphous state[4444 Srubar, W. V., 3rd, Wright, Z. C., Tsui, A., Michel, A. T., Billington, S. L., & Frank, C. W. (2012). Characterizing the effects of ambient aging on the mechanical and physical properties of two commercially available bacterial thermoplastics. Polymer Degradation & Stability, 97(10), 1922-1929. http://dx.doi.org/10.1016/j.polymdegradstab.2012.04.011.
http://dx.doi.org/10.1016/j.polymdegrads...
,5858 Chen, C., Fei, B., Peng, S., Zhuang, Y., Dong, L., & Feng, Z. (2002). The Kinetics of the Thermal Decomposition of Poly(3-hydroxybutyrate) and Maleated Poly(3-hydroxybutyrate). Journal of Applied Polymer Science, 84(9), 1789-1796. http://dx.doi.org/10.1002/app.10463.
http://dx.doi.org/10.1002/app.10463...
59 Koning, G. J. M., Lemstra, P. J., Hill, D. J. T., Carswell, T. G., & O’Donnell, J. H. (1992). Ageing phenomena in bacterial poly[(R)-3-hydroxybutyrate] I. A study on the mobility in poly [(R)-3-hydroxybutyrate] powders by monitoring the radical decay with temperature after radiolysis at 77 K. Polymer, 33(15), 3295-3297. http://dx.doi.org/10.1016/0032-3861(92)90250-Z.
http://dx.doi.org/10.1016/0032-3861(92)9...
-6060 Koning, G. J. M., & Lemstra, P. J. (1993). Crystallization phenomena in bacterial poly[(R)-3- hydroxybutyrate]: 2. Embrittlement and rejuvenation. Polymer, 34(19), 4089-4094. http://dx.doi.org/10.1016/0032-3861(93)90671-V.
http://dx.doi.org/10.1016/0032-3861(93)9...
]. Nevertheless, changes in molecular weight or crystalline and amorphous morphologies are beyond the scope of this study.
3.2 Fourier transform infrared (FTIR) spectroscopy
The original chemical structure of PHB consists of molecules terminated by a hydroxyl and a carboxyl group. The hydroxyl and carboxyl end groups are observed at approximately 3600 cm-1 and 1720 cm-1, respectively. Other characteristics PHB vibrations appear at around 1277cm−1 and 970 cm-1. The peak at 1277 cm-1 denotes the –C-O-C- group and at 970 cm-1 is assigned to bending vibrations of olefinc -C-H[6161 Ong, Y. T., Ahmad, A. L., Zein, S. H. S., Sudesh, K., & Tan, S. H. (2011). Poly(3-hydroxybutyrate)-functionalised multi-walled carbon nanotubes/chitosan green nanocomposite membranes and their application in pervaporation. Separation and Purification Technology, 76(3), 419-427. http://dx.doi.org/10.1016/j.seppur.2010.11.013.
http://dx.doi.org/10.1016/j.seppur.2010....
,6262 Gonzalez, A., Irusta, L., Fernández-Berridi, M. J., Iriarte, M., & Iruin, J. J. (2005). Application of pyrolysis/gas chromatography/Fourier transform infrared spectroscopy and TGA techniques in the study of thermal degradation of poly (3-hydroxybutyrate). Polymer Degradation & Stability, 87(2), 347-354. http://dx.doi.org/10.1016/j.polymdegradstab.2004.09.005.
http://dx.doi.org/10.1016/j.polymdegrads...
].
As the thermal degradation proceeds, it incorporates vinyl (crotonate) ester and carboxyl groups end groups in PHB structure[2020 Grassie, N., Murray, E. J., & Holmes, P. A. (1984). The thermal degradation of poly(-(d)-b-hydroxybutyric acid): part 1 – Identification and quantitative analysis of products. Polymer Degradation & Stability, 6(1), 47-61. http://dx.doi.org/10.1016/0141-3910(84)90016-8.
http://dx.doi.org/10.1016/0141-3910(84)9...
,3030 Kopinke, F. D., Remmler, M., & Mackenzie, K. (1996). Thermal decomposition of biodegradable polyesters-I: Poly(β-hydroxybutyric acid). Polymer Degradation & Stability, 52(1), 25-38. http://dx.doi.org/10.1016/0141-3910(95)00221-9.
http://dx.doi.org/10.1016/0141-3910(95)0...
,3131 Aoyagi, Y., Yamashita, K., & Doi, Y. (2002). Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly [(S)-lactide]. Polymer Degradation & Stability, 76(1), 53-59. http://dx.doi.org/10.1016/S0141-3910(01)00265-8.
http://dx.doi.org/10.1016/S0141-3910(01)...
,4141 Rosa, D. S., Calil, M. R., Guedes, C. G. F., & Santos, C. E. O. (2001). The Effect of UV-B Irradiation on the biodegradability of poly-β-hydroxybutyrate (PHB) and poly-ε-caprolactone (PCL). Journal of Polymers and the Environment, 9(3), 109-113. http://dx.doi.org/10.1023/A:1020498710586.
http://dx.doi.org/10.1023/A:102049871058...
]. Therefore, a gradual increase in crotonate ester groups with extrusions paths can be expected, as well as a decrease in hydroxyl groups present in the original polymer. The absorption band assigned to stretching vibrations of double carbon/carbon bond, -C=C-, is expected to appear at around 1660 cm-1[6363 Vogel, C., Morita, S., Sato, H., Noda, I., Ozaki, Y., & Siesler, H. W. (2007). Thermal Degradation of Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) in Nitrogen and Oxygen Studied by Thermogravimetric–Fourier Transform Infrared Spectroscopy. Applied Spectroscopy, 61(7), 755-764. PMid:17697470. http://dx.doi.org/10.1366/000370207781393370.
http://dx.doi.org/10.1366/00037020778139...
].
FTIR analyses of PHB samples during extrusion cycles are shown in Figure 4, which presents the spectra of virgin PHB and PHB after each extrusion cycle.
The presence of absorption bands associated to the formation of new chemical groups due to degradation mechanisms of polymer was not noticed. According to Yu et al.[6464 Yu, J., Plackett, D., & Chen, L. X. L. (2005). Kinetics and mechanism of the monomeric products from abiotic hydrolysis of poly[(R)-3-hydroxybutyrate] under acidic and alkaline conditions. Polymer Degradation & Stability, 89(2), 289-299. http://dx.doi.org/10.1016/j.polymdegradstab.2004.12.026.
http://dx.doi.org/10.1016/j.polymdegrads...
], the band at 1700-1720 cm-1, assigned to the carbonyl absorption band in the infrared spectra, is shifted to 1654 cm-1 when conjugated with vinyl end groups. Nevertheless, no shift can be observed for this band (Figure 5), corroborating the results of Yu et al.[4444 Srubar, W. V., 3rd, Wright, Z. C., Tsui, A., Michel, A. T., Billington, S. L., & Frank, C. W. (2012). Characterizing the effects of ambient aging on the mechanical and physical properties of two commercially available bacterial thermoplastics. Polymer Degradation & Stability, 97(10), 1922-1929. http://dx.doi.org/10.1016/j.polymdegradstab.2012.04.011.
http://dx.doi.org/10.1016/j.polymdegrads...
] that observed no difference in infrared absorption of PHB film surface even after up to 40 wt% of the PHB film submitted to alkaline hydrolysis had been decomposed into crotonic acid and 3-hydrobutyric acid. In relation to the intensity of absorption band at 1720 cm-1, nothing can be commented, since absorption intensity in ATR/FTIR spectroscopy depends largely on the quality of contact between sample and crystal[6565 Bhargava, R., & Levi, I. W. (Ed.). (2005). Spectrochemical analysis using infrared multichannel detectors. Oxford: Blackwell Publishing. http://dx.doi.org/10.1002/9780470988541.
http://dx.doi.org/10.1002/9780470988541...
].
3.3 Thermogravimetric analysis (TGA)
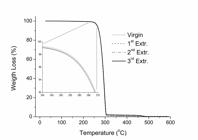
Table 1 shows the values of temperature at the peak of the DTG curve (Tmax) and the temperature at which the sample loss 10% of its initial weight (T10%). A peak on a DTG curve characterizes the temperature at the highest rate of thermal degradation. Figure 6 depict the TGA curves of PHB before and after multiple extrusion cycles.
According to the literature[2020 Grassie, N., Murray, E. J., & Holmes, P. A. (1984). The thermal degradation of poly(-(d)-b-hydroxybutyric acid): part 1 – Identification and quantitative analysis of products. Polymer Degradation & Stability, 6(1), 47-61. http://dx.doi.org/10.1016/0141-3910(84)90016-8.
http://dx.doi.org/10.1016/0141-3910(84)9...
,3030 Kopinke, F. D., Remmler, M., & Mackenzie, K. (1996). Thermal decomposition of biodegradable polyesters-I: Poly(β-hydroxybutyric acid). Polymer Degradation & Stability, 52(1), 25-38. http://dx.doi.org/10.1016/0141-3910(95)00221-9.
http://dx.doi.org/10.1016/0141-3910(95)0...
,3131 Aoyagi, Y., Yamashita, K., & Doi, Y. (2002). Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly [(S)-lactide]. Polymer Degradation & Stability, 76(1), 53-59. http://dx.doi.org/10.1016/S0141-3910(01)00265-8.
http://dx.doi.org/10.1016/S0141-3910(01)...
,4141 Rosa, D. S., Calil, M. R., Guedes, C. G. F., & Santos, C. E. O. (2001). The Effect of UV-B Irradiation on the biodegradability of poly-β-hydroxybutyrate (PHB) and poly-ε-caprolactone (PCL). Journal of Polymers and the Environment, 9(3), 109-113. http://dx.doi.org/10.1023/A:1020498710586.
http://dx.doi.org/10.1023/A:102049871058...
], as PHB degradation proceeds, an ester chain with an unsaturated ester as end group, and an ester chain with a carboxylic acid as end group are formed. Each of these two types of ester may react at a distinct rate. However, COOH and COOR groups have very similar inductive effects[6666 Hoffmann, R. (1970). The norcaradiene cicloheptatriene equilibrium. Tetrahedron Letters, 11(33), 2907-2909. http://dx.doi.org/10.1016/S0040-4039(01)98370-4.
http://dx.doi.org/10.1016/S0040-4039(01)...
], such that any structural changes suffered by PHB during extrusion cycles were not enough to change the thermal behavior of PHB. The TGA curves are all superposed and only a slight shift between them at the beginning of the curve could be verified (Figure 6).
No mass loss was observed until about 225 °C and the temperature at DTG peak (Tmax) was about 300 °C. The thermal decomposition of PHB takes place within a narrow temperature range, i. e., 235 ~ 315 °C. For all samples, the residual weight is lower than 0.5 wt%. Each TGA curve indicates a single step degradation, which means that the thermal degradation of PHB polymer chains occurs by only one mechanism[3939 Morikawa, H., & Marchessault, R. H. (1981). Pyrolysis of bacterial polyalkanoates. Canadian Journal of Chemistry, 59(15), 2306-2313. http://dx.doi.org/10.1139/v81-334.
http://dx.doi.org/10.1139/v81-334...
].
3.4 Differential scanning calorimetry (DSC)
The results of the crystallinity degree (Xc) and melting temperature (Tm) are shown in Table 2.
According to these results, the crystallinity degree increased from 57.7 to 62.2%, this increase was more noticeable from the first to the second extrusion cycle. This evolution of the crystallinity with the number of extrusion cycles is also likely to be ascribed to a gradual decrease of the molecular weight of PHB which enhances the mobility and increases crystallization during the cooling step. This phenomenon is also known as chemicrystallization process[4747 Sadi, R. K., Fechine, G. J. M., & Demarquette, N. R. (2010). Photodegradation of poly(3-hydroxybutyrate). Polymer Degradation & Stability, 95(12), 2318-2327. http://dx.doi.org/10.1016/j.polymdegradstab.2010.09.003.
http://dx.doi.org/10.1016/j.polymdegrads...
]. Corroborating with this hypothesis, there is the tensile strength results that conversely decreased with extrusion cycles, indicating that the increase in crystallinity does not occur with the increase of tie molecules, responsible for transferring stress between two crystalline lamellae. The same behavior was observed for PLA at multiple injections cycle[55 Pillin, I., Montrelay, N., Bourmaud, A., & Grohens, Y. (2008). Effect of thermo-mechanical cycles on the physico-chemical properties of poly(lactic acid). Polymer Degradation & Stability, 93(2), 321-328. http://dx.doi.org/10.1016/j.polymdegradstab.2007.12.005.
http://dx.doi.org/10.1016/j.polymdegrads...
]. Furthermore, contributions of physical processes related to constraints imposed by amorphous chains[6060 Koning, G. J. M., & Lemstra, P. J. (1993). Crystallization phenomena in bacterial poly[(R)-3- hydroxybutyrate]: 2. Embrittlement and rejuvenation. Polymer, 34(19), 4089-4094. http://dx.doi.org/10.1016/0032-3861(93)90671-V.
http://dx.doi.org/10.1016/0032-3861(93)9...
] due to progressive crystallization process of PHB can be disregarded because each measurement was carried out at the same aging time.
The melting temperature decreases slightly at the first extrusion cycle and remains constant for further extrusion cycles. This modification in melting temperature reflects the formation of less perfect crystalline regions, which corroborates with the possibility of molecular weight reduction[2525 Foster, L. J. R., & Tighe, B. J. (2005). Centrifugally spun polyhydroxybutyrate fibres: accelerated hydrolytic degradation studies. Polymer Degradation & Stability, 87(1), 1-10. http://dx.doi.org/10.1016/j.polymdegradstab.2003.11.012.
http://dx.doi.org/10.1016/j.polymdegrads...
].
4. Conclusions
Along multiple extrusion cycles, PHB suffered several changes such as, decrease in mechanical properties and increase in the degree of crystallinity. According to these results, a loss in tensile strength at break of PHB above 50% was observed at the third extrusion cycle. Nevertheless, that decrement in mechanical properties was significant at the secord cycle, i.e., ~ 40%. Also an increase in crystallinity degree was noticed mainly from the first to the second extrusion cycle, which probably could be associated to chemicrystallization process. FTIR results did not show any significant changes in polymeric structures associated to the formation of new chemical groups. Similarly, the thermal stability of PHB along processing cycles exhibited only a trend to decrease the thermal stability with extrusions paths, as evidenced by TGA curves. It is worth to note that the recyclability potential explored in this work refers to property retention up to three extrusion cycles under extreme recycling conditions (100% recycling with no added virgin polymer and no additive or reinforcement). Therefore, improvement in PHB recyclability can be achieved by mixtures with virgin PHB, or incorporation of stabilizers and/or chain extenders to control its degradation during reprocessing.
5. Acknowledgements
The authors gratefully acknowledge to PHB Industrial S.A. for PHB providing the polymer used in this work, to National Foundation for Science and Technology Development (CNPq) for the scholarships and profs. Dr. Daniel D. Melo (UFRN), Dr. Edson N. Ito (UFRN) and Dr. Luiz Fernando Meneses Carvalho (IFPI-Teresina) for their kind help.
6. References
-
1Hoornweg, D., & Bhada-Tata, P. (2012). What a waste: a global review of solid waste management Washington: World Bank.
-
2Thompson, R. C., Moore, C. J., vom Saal, F. S., & Swan, S. H. (2009). Plastics, the environment and human health: current consensus and future trends. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 364(1526), 2153-2166. PMid:19528062. http://dx.doi.org/10.1098/rstb.2009.0053
» http://dx.doi.org/10.1098/rstb.2009.0053 -
3Davis, G., & Song, J. H. (2006). Biodegradable packaging based on raw materials from crops and their impact on waste management. Industrial Crops and Products, 23(2), 147-161. http://dx.doi.org/10.1016/j.indcrop.2005.05.004
» http://dx.doi.org/10.1016/j.indcrop.2005.05.004 -
4Le Duigou, A., Pillin, I., Bourmaud, A., Davies, P., & Baley, C. (2008). Effect of recycling on mechanical behaviour of biocompostable flax/poly(L-lactide) composites. Composites. Part A, Applied Science and Manufacturing, 39(9), 1471-1478. http://dx.doi.org/10.1016/j.compositesa.2008.05.008
» http://dx.doi.org/10.1016/j.compositesa.2008.05.008 -
5Pillin, I., Montrelay, N., Bourmaud, A., & Grohens, Y. (2008). Effect of thermo-mechanical cycles on the physico-chemical properties of poly(lactic acid). Polymer Degradation & Stability, 93(2), 321-328. http://dx.doi.org/10.1016/j.polymdegradstab.2007.12.005
» http://dx.doi.org/10.1016/j.polymdegradstab.2007.12.005 -
6Nishida, H., Fan, Y., Mori, T., Oyagi, N., Shirai, Y., & Endo, T. (2005). Feedstock recycling of flame-resisting poly(lactic acid) aluminum hydroxide composite. Industrial & Engineering Chemistry Research, 44(5), 1433-1437. http://dx.doi.org/10.1021/ie049208+
» http://dx.doi.org/10.1021/ie049208+ -
7Sikorska, W., Richert, J., Rydz, J., Musioł, M., Adamus, G., Janeczek, H., & Kowalczuk, M. (2012). Degradability studies of poly(L-lactide) after multi-reprocessing experiments in extruder. Polymer Degradation & Stability, 97(10), 1891-1897. http://dx.doi.org/10.1016/j.polymdegradstab.2012.03.049
» http://dx.doi.org/10.1016/j.polymdegradstab.2012.03.049 -
8Gurieff, N., & Lant, P. (2007). Comparative life cycle assessment and financial analysis of mixed culture polyhydroxyalkanoate production. Bioresource Technology, 98(17), 3393-3403. PMid:17632000. http://dx.doi.org/10.1016/j.biortech.2006.10.046
» http://dx.doi.org/10.1016/j.biortech.2006.10.046 -
9Shah, A. A., Kato, S., Shintani, N., Kamini, N. R., & Nakajima-Kambe, T. (2014). Microbial degradation of aliphatic and aliphatic-aromatic co-polyesters. Applied Microbiology and Biotechnology, 98(8), 3437-3447. PMid:24522729. http://dx.doi.org/10.1007/s00253-014-5558-1
» http://dx.doi.org/10.1007/s00253-014-5558-1 -
10Coutinho, B. C., Miranda, G. B., Sampaio, G. R., Souza, L. B. S., Santana, W. J., & Coutinho, H. D. M. (2004). A importância e as vantagens do polihidroxibutirato (plástico biodegradável). Holos, 20(3), 76-81. http://dx.doi.org/10.15628/holos.2004.49
» http://dx.doi.org/10.15628/holos.2004.49 -
11Sánchez, R. J., Schripsema, J., Silva, L. F., Taciro, M. K., Pradella, J. G. C., & Gomez, J. G. C. (2003). Medium-chain-length Polyhydroxy-Alkanoic Acids (PHAMCL) produced by Pseudomonas Putida IPT 046 from renewable sources. European Polymer Journal, 39(7), 1385-1394. http://dx.doi.org/10.1016/S0014-3057(03)00019-3
» http://dx.doi.org/10.1016/S0014-3057(03)00019-3 -
12Doi, Y. (1990). Microbial polyesters New York: VCH Publishers Inc.
-
13Hablot, E., Bordes, P., Pollet, E., & Averous, L. (2008). Thermal and thermo-mechanical degradation of poly(3-hydroxybutyrate)-based multiphase systems. Polymer Degradation & Stability, 93(2), 413-421. http://dx.doi.org/10.1016/j.polymdegradstab.2007.11.018
» http://dx.doi.org/10.1016/j.polymdegradstab.2007.11.018 -
14Nguyen, S., Yu, G., & Marchessault, R. (2002). Thermal degradation of poly(3-hydroxyalkanoates): preparation of well-defined oligomers. Biomacromolecules, 3(1), 219-224. PMid:11866577. http://dx.doi.org/10.1021/bm0156274
» http://dx.doi.org/10.1021/bm0156274 -
15Sadi, R. K. (2010). Compatibilization and degradation study of Polypropylene/Poly(3-hydroxybutyrate) (PP/PHB) blends (Doctoral thesis). Escola Politécnica, Universidade de São Paulo, São Paulo.
-
16Melchiors, M., Keul, H., & Höcker, H. (1996). Depolymerization of poly[(R)-3-hydroxybutyrate] to cyclic oligomers and polymerization of the cyclic trimer: an example of thermodynamic recycling. Macromolecules, 29(20), 6442-6451. http://dx.doi.org/10.1021/ma9604350
» http://dx.doi.org/10.1021/ma9604350 -
17Yang, X., Clénet, J., Xu, H., Odelius, K., & Hakkarainen, M. (2015). Two step extrusion process: from thermal recycling of PHB to plasticized PLA by reactive extrusion grafting of PHB degradation products onto PLA chains. Macromolecules, 48(8), 2509-2518. PMid:27053818. http://dx.doi.org/10.1021/acs.macromol.5b00235
» http://dx.doi.org/10.1021/acs.macromol.5b00235 -
18Spekreijse, J., Le Nôtre, J., Sanders, J. P. M., & Scott, E. L. (2015). Conversion of polyhydroxybutyrate (PHB) to methyl crotonate for the production of biobased monomers. Journal of Applied Polymer Science, 132(35), n/a. http://dx.doi.org/10.1002/app.42462
» http://dx.doi.org/10.1002/app.42462 -
19Osanai, Y., Toshima, K., & Matsumura, S. (2003). Enzymatic degradation of poly(R,S-3-hydroxybutanoate) to cyclic oligomers under continuous flow. Green Chemistry, 5(5), 567-570. http://dx.doi.org/10.1039/B304640K
» http://dx.doi.org/10.1039/B304640K -
20Grassie, N., Murray, E. J., & Holmes, P. A. (1984). The thermal degradation of poly(-(d)-b-hydroxybutyric acid): part 1 – Identification and quantitative analysis of products. Polymer Degradation & Stability, 6(1), 47-61. http://dx.doi.org/10.1016/0141-3910(84)90016-8
» http://dx.doi.org/10.1016/0141-3910(84)90016-8 -
21Karlsson, S., Sares, C., Renstad, R., & Albertsson, A. C. (1994). Gas chromatographic, liquid chromatographic and gas chromatographic-mass spectrometric identification of degradation products in accelerated aged microbial polyhydroxyalkanoates. Journal of Chromatography. A, 669(1-2), 97-102. PMid:8075778. http://dx.doi.org/10.1016/0021-9673(94)80341-2
» http://dx.doi.org/10.1016/0021-9673(94)80341-2 -
22Yu, P. H., Chua, H., Huang, A. L., Lo, W., & Chen, G. Q. (1998). Conversion of food industrial wastes into bioplastics. Applied Biochemistry and Biotechnology, 70-72(1), 603-614. PMid:18576025. http://dx.doi.org/10.1007/BF02920172
» http://dx.doi.org/10.1007/BF02920172 -
23King, P. P. (1982). Biotechnology. An industrial view. Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 32(1), 2-8. http://dx.doi.org/10.1002/jctb.5030320103
» http://dx.doi.org/10.1002/jctb.5030320103 -
24Rosa, D. S., & Pântano, R., Fo. (2003). Biodegradação: um ensaio com polímeros Itatiba: Moara.
-
25Foster, L. J. R., & Tighe, B. J. (2005). Centrifugally spun polyhydroxybutyrate fibres: accelerated hydrolytic degradation studies. Polymer Degradation & Stability, 87(1), 1-10. http://dx.doi.org/10.1016/j.polymdegradstab.2003.11.012
» http://dx.doi.org/10.1016/j.polymdegradstab.2003.11.012 -
26Ariffin, H., Nishida, H., Shirai, Y., & Hassan, M. A. (2010). Highly selective transformation of poly[(R)-3-hydroxybutyric acid] into trans-crotonic acid by catalytic thermal degradation. Polymer Degradation & Stability, 95(8), 1375-1381. http://dx.doi.org/10.1016/j.polymdegradstab.2010.01.018
» http://dx.doi.org/10.1016/j.polymdegradstab.2010.01.018 -
27Kim, K. J., Doi, Y., & Abe, H. (2008). Effect of metal compounds on thermal degradation behavior of aliphatic poly(hydroxyalkanoic acid)s. Polymer Degradation & Stability, 93(4), 776-785. http://dx.doi.org/10.1016/j.polymdegradstab.2008.01.026
» http://dx.doi.org/10.1016/j.polymdegradstab.2008.01.026 -
28Farid, N. F. S. M., Ariffin, H., Mamat, M. R. Z., Mohd Zahari, M. A. K., & Hassan, M. A. (2015). Non-solvent-based pretreatment of poly(3-hydroxybutyrate) for improved bio-based crotonic acid production. RSC Advances, 5(42), 33546-33553. http://dx.doi.org/10.1039/C5RA03017J
» http://dx.doi.org/10.1039/C5RA03017J -
29Ariffin, H., Nishida, H., Shirai, Y., & Hassan, M. A. (2008). Determination of multiple thermal degradation mechanisms of poly(3-hydroxybutyrate). Polymer Degradation & Stability, 93(8), 1433-1439. http://dx.doi.org/10.1016/j.polymdegradstab.2008.05.020
» http://dx.doi.org/10.1016/j.polymdegradstab.2008.05.020 -
30Kopinke, F. D., Remmler, M., & Mackenzie, K. (1996). Thermal decomposition of biodegradable polyesters-I: Poly(β-hydroxybutyric acid). Polymer Degradation & Stability, 52(1), 25-38. http://dx.doi.org/10.1016/0141-3910(95)00221-9
» http://dx.doi.org/10.1016/0141-3910(95)00221-9 -
31Aoyagi, Y., Yamashita, K., & Doi, Y. (2002). Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly [(S)-lactide]. Polymer Degradation & Stability, 76(1), 53-59. http://dx.doi.org/10.1016/S0141-3910(01)00265-8
» http://dx.doi.org/10.1016/S0141-3910(01)00265-8 -
32Hong, S. G., Lin, Y. C., & Lin, C. H. (2008). Crystallization and degradation behaviors of treated polyhydroxybutyrates. Reactive & Functional Polymers, 68(11), 1516-1523. http://dx.doi.org/10.1016/j.reactfunctpolym.2008.08.003
» http://dx.doi.org/10.1016/j.reactfunctpolym.2008.08.003 -
33Kawalec, M., Adamus, G. Y., Kurcok, P., Kowalczuk, M., Foltran, I., Focarete, M. L., & Scandola, M. (2007). Carboxylate-induced degradation of poly(3-hydroxybutyrate)s. Biomacromolecules, 8(4), 1053-1058. PMid:17330956. http://dx.doi.org/10.1021/bm061155n
» http://dx.doi.org/10.1021/bm061155n -
34Galego, N., & Rozsa, C. (1999). Thermal decomposition of some poly(b-hydroxyalcanoates). Polymer International, 48(12), 1202-1204. http://dx.doi.org/10.1002/(SICI)1097-0126(199912)48:12<1202::AID-PI223>3.0.CO;2-D
» http://dx.doi.org/10.1002/(SICI)1097-0126(199912)48:12<1202::AID-PI223>3.0.CO;2-D -
35Lehrle, R., Williams, R., French, C., & Hammond, T. (1995). Thermolysis and methanolysis of poly(b-hydroxybutyrate): random scission assessed by statistical analysis of molecular weight distributions. Macromolecules, 28(13), 4408-4414. http://dx.doi.org/10.1021/ma00117a008
» http://dx.doi.org/10.1021/ma00117a008 -
36Bahari, K., Mitomo, H., Enjoji, T., Yoshii, F., & Makuuchi, K. (1998). Degradability of poly(3-hydroxybutyrate) and its copolymer grafted with styrene by radiation. Polymer Degradation & Stability, 61(2), 245-252. http://dx.doi.org/10.1016/S0141-3910(97)00147-X
» http://dx.doi.org/10.1016/S0141-3910(97)00147-X -
37Lee, H. K., Ismail, J., Kammer, H. W., & Bakar, M. A. (2005). Melt reaction in blends of poly(3-hydroxybutyrate) (PHB) and epoxidized natural rubber (ENR-50). Journal of Applied Polymer Science, 95(1), 113-129. http://dx.doi.org/10.1002/app.20808
» http://dx.doi.org/10.1002/app.20808 -
38Hong, S. G., Gau, T. K., & Huang, S. C. (2011). Enhancement of the crystallization and thermal stability of polyhydroxybutyrate by polymeric additives. Journal of Thermal Analysis and Calorimetry, 103(3), 967-975. http://dx.doi.org/10.1007/s10973-010-1180-3
» http://dx.doi.org/10.1007/s10973-010-1180-3 -
39Morikawa, H., & Marchessault, R. H. (1981). Pyrolysis of bacterial polyalkanoates. Canadian Journal of Chemistry, 59(15), 2306-2313. http://dx.doi.org/10.1139/v81-334
» http://dx.doi.org/10.1139/v81-334 -
40Ballistreri, A., Garozzo, D., Giuffrida, M., Impallomeni, G., & Montaudo, G. (1989). Analytical degradation: an approach to the structural analysis of microbial polyesters by different methods. Journal of Analytical and Applied Pyrolysis, 16(3), 239-253. http://dx.doi.org/10.1016/0165-2370(89)80028-2
» http://dx.doi.org/10.1016/0165-2370(89)80028-2 -
41Rosa, D. S., Calil, M. R., Guedes, C. G. F., & Santos, C. E. O. (2001). The Effect of UV-B Irradiation on the biodegradability of poly-β-hydroxybutyrate (PHB) and poly-ε-caprolactone (PCL). Journal of Polymers and the Environment, 9(3), 109-113. http://dx.doi.org/10.1023/A:1020498710586
» http://dx.doi.org/10.1023/A:1020498710586 -
42Kopinke, F. D., & Mackenzie, K. (1997). Mechanistic aspects of the thermal degradation of poly(lactic acid) and poly(beta-hydroxybutyric acid). Journal of Analytical and Applied Pyrolysis, 40-41, 43-53. http://dx.doi.org/10.1016/S0165-2370(97)00022-3
» http://dx.doi.org/10.1016/S0165-2370(97)00022-3 -
43Barham, P. J., Keller, A., Otun, E. L., & Holmes, P. A. (1984). Crystallization and morphology of a bacterial thermoplastic: poly-3-hydroxybutyrate. Journal of Materials Science, 19(9), 2781-2794. http://dx.doi.org/10.1007/BF01026954
» http://dx.doi.org/10.1007/BF01026954 -
44Srubar, W. V., 3rd, Wright, Z. C., Tsui, A., Michel, A. T., Billington, S. L., & Frank, C. W. (2012). Characterizing the effects of ambient aging on the mechanical and physical properties of two commercially available bacterial thermoplastics. Polymer Degradation & Stability, 97(10), 1922-1929. http://dx.doi.org/10.1016/j.polymdegradstab.2012.04.011
» http://dx.doi.org/10.1016/j.polymdegradstab.2012.04.011 -
45Coutinho, F. M. B., Mello, I. L., & Santa Maria, L. C. (2003). Polietileno: principais tipos, propriedades e aplicações. Polímeros. Ciência e Tecnologia, 13(1), 1-13. http://dx.doi.org/10.1590/S0104-14282003000100005
» http://dx.doi.org/10.1590/S0104-14282003000100005 -
46El-Hadi, A., Schnabel, R., Straube, E., Müller, G., & Henning, S. (2002). Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polymer Testing, 21(6), 665-674. http://dx.doi.org/10.1016/S0142-9418(01)00142-8
» http://dx.doi.org/10.1016/S0142-9418(01)00142-8 -
47Sadi, R. K., Fechine, G. J. M., & Demarquette, N. R. (2010). Photodegradation of poly(3-hydroxybutyrate). Polymer Degradation & Stability, 95(12), 2318-2327. http://dx.doi.org/10.1016/j.polymdegradstab.2010.09.003
» http://dx.doi.org/10.1016/j.polymdegradstab.2010.09.003 -
48Rabello, M. S., & White, J. R. (1997). Crystallization and melting behavior of photodegraded polypropylene - I. Chemi-crystallization. Polymer, 38(26), 6379-6387. http://dx.doi.org/10.1016/S0032-3861(97)00213-9
» http://dx.doi.org/10.1016/S0032-3861(97)00213-9 -
49Renstad, R., Karlsson, S., & Albertsson, A. C. (1997). Influence of processing parameters on the molecular weight and mechanical properties of poly(3-hydroxybutyrate-3-hydroxyvalerate). Polymer Degradation & Stability, 57(3), 331-338. http://dx.doi.org/10.1016/S0141-3910(97)00028-1
» http://dx.doi.org/10.1016/S0141-3910(97)00028-1 -
50Kendall, A. (2012). A life cycle assessment of biopolymer production from material recovery facility residuals. Resources, Conservation and Recycling, 61, 69-74. http://dx.doi.org/10.1016/j.resconrec.2012.01.008
» http://dx.doi.org/10.1016/j.resconrec.2012.01.008 -
51Currann, M. A. (1996). Environmental life-cycle assessment New York: McGraw Hill.
-
52Feller, J. F., & Bourmaud, A. (2003). Rheological and calorimetric properties of recycled bisphenol A poly(carbonate). Polymer Degradation & Stability, 82(1), 99-104. http://dx.doi.org/10.1016/S0141-3910(03)00169-1
» http://dx.doi.org/10.1016/S0141-3910(03)00169-1 -
53Spinace, M. A. S., & De Paoli, M. A. (2004). Nonisothermal crystallization of reprocessed poly(ethylene terephthalate). Journal of Applied Polymer Science, 91(1), 525-531. http://dx.doi.org/10.1002/app.13230
» http://dx.doi.org/10.1002/app.13230 -
54Javierre, C., Claveria, I., Ponz, L., Aisa, J., & Fernandez, A. (2007). Influence of the recycled material percentage on the rheological behaviour of HDPE for injection moulding process. Waste Management (New York, N.Y.), 27(5), 656-663. PMid:16707257. http://dx.doi.org/10.1016/j.wasman.2006.03.005
» http://dx.doi.org/10.1016/j.wasman.2006.03.005 -
55Santos, A. S. F., Agnelli, J. A. M., Trevisan, D. W., & Manrich, S. (2002). Degradation and stabilization of polyolefins from municipal plastic waste during multiple extrusions under different reprocessing conditions. Polymer Degradation & Stability, 77(3), 441-447. http://dx.doi.org/10.1016/S0141-3910(02)00101-5
» http://dx.doi.org/10.1016/S0141-3910(02)00101-5 -
56Hamzehlou, S. H., & Katbab, A. A. (2007). Bottle-to-bottle recycling of PET via nanostructure formation by melt intercalation in twin screw compounder: improved thermal, barrier, and microbiological properties. Journal of Applied Polymer Science, 106(2), 1375-1382. http://dx.doi.org/10.1002/app.26730
» http://dx.doi.org/10.1002/app.26730 -
57Abad, M. J., Ares, A., Barral, L., Cano, J., Díez, F. J., García-Garabal, S., López, J., & Ramírez, C. (2004). Effects of a mixture of stabilizers on the structure and mechanical properties of polyethylene during reprocessing. Journal of Applied Polymer Science, 92(6), 3910-3916. http://dx.doi.org/10.1002/app.20420
» http://dx.doi.org/10.1002/app.20420 -
58Chen, C., Fei, B., Peng, S., Zhuang, Y., Dong, L., & Feng, Z. (2002). The Kinetics of the Thermal Decomposition of Poly(3-hydroxybutyrate) and Maleated Poly(3-hydroxybutyrate). Journal of Applied Polymer Science, 84(9), 1789-1796. http://dx.doi.org/10.1002/app.10463
» http://dx.doi.org/10.1002/app.10463 -
59Koning, G. J. M., Lemstra, P. J., Hill, D. J. T., Carswell, T. G., & O’Donnell, J. H. (1992). Ageing phenomena in bacterial poly[(R)-3-hydroxybutyrate] I. A study on the mobility in poly [(R)-3-hydroxybutyrate] powders by monitoring the radical decay with temperature after radiolysis at 77 K. Polymer, 33(15), 3295-3297. http://dx.doi.org/10.1016/0032-3861(92)90250-Z
» http://dx.doi.org/10.1016/0032-3861(92)90250-Z -
60Koning, G. J. M., & Lemstra, P. J. (1993). Crystallization phenomena in bacterial poly[(R)-3- hydroxybutyrate]: 2. Embrittlement and rejuvenation. Polymer, 34(19), 4089-4094. http://dx.doi.org/10.1016/0032-3861(93)90671-V
» http://dx.doi.org/10.1016/0032-3861(93)90671-V -
61Ong, Y. T., Ahmad, A. L., Zein, S. H. S., Sudesh, K., & Tan, S. H. (2011). Poly(3-hydroxybutyrate)-functionalised multi-walled carbon nanotubes/chitosan green nanocomposite membranes and their application in pervaporation. Separation and Purification Technology, 76(3), 419-427. http://dx.doi.org/10.1016/j.seppur.2010.11.013
» http://dx.doi.org/10.1016/j.seppur.2010.11.013 -
62Gonzalez, A., Irusta, L., Fernández-Berridi, M. J., Iriarte, M., & Iruin, J. J. (2005). Application of pyrolysis/gas chromatography/Fourier transform infrared spectroscopy and TGA techniques in the study of thermal degradation of poly (3-hydroxybutyrate). Polymer Degradation & Stability, 87(2), 347-354. http://dx.doi.org/10.1016/j.polymdegradstab.2004.09.005
» http://dx.doi.org/10.1016/j.polymdegradstab.2004.09.005 -
63Vogel, C., Morita, S., Sato, H., Noda, I., Ozaki, Y., & Siesler, H. W. (2007). Thermal Degradation of Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) in Nitrogen and Oxygen Studied by Thermogravimetric–Fourier Transform Infrared Spectroscopy. Applied Spectroscopy, 61(7), 755-764. PMid:17697470. http://dx.doi.org/10.1366/000370207781393370
» http://dx.doi.org/10.1366/000370207781393370 -
64Yu, J., Plackett, D., & Chen, L. X. L. (2005). Kinetics and mechanism of the monomeric products from abiotic hydrolysis of poly[(R)-3-hydroxybutyrate] under acidic and alkaline conditions. Polymer Degradation & Stability, 89(2), 289-299. http://dx.doi.org/10.1016/j.polymdegradstab.2004.12.026
» http://dx.doi.org/10.1016/j.polymdegradstab.2004.12.026 -
65Bhargava, R., & Levi, I. W. (Ed.). (2005). Spectrochemical analysis using infrared multichannel detectors Oxford: Blackwell Publishing. http://dx.doi.org/10.1002/9780470988541
» http://dx.doi.org/10.1002/9780470988541 -
66Hoffmann, R. (1970). The norcaradiene cicloheptatriene equilibrium. Tetrahedron Letters, 11(33), 2907-2909. http://dx.doi.org/10.1016/S0040-4039(01)98370-4
» http://dx.doi.org/10.1016/S0040-4039(01)98370-4
Publication Dates
-
Publication in this collection
29 June 2017 -
Date of issue
Apr-Jun 2017
History
-
Received
11 Dec 2015 -
Reviewed
23 May 2016 -
Accepted
29 June 2016