Abstract
The purpose of this study was to characterize, isolate and chemically modify tiger nut ( Cyperus sculentus L.) starch with octenyl succinic anhydride. The efficiency of the chemical modification was 0.04. Chemical composition, particle morphology (SEM), particle size, X-ray diffraction, infrared analysis, thermogravimetric analysis, differential scanning calorimetry and swelling and solubility power were determined for characterization of the native and modified starch. Both showed similar chemical composition and amylose and amylopectin contents, as well as absorption spectra in the infrared region without modification of the molecular structure and A-type crystalline pattern. The particles of both had an oval and spherical shape. The modified starch was more resistant to temperature and the gelatinization process occurred at 67.52 °C. These results suggest that tiger nut starch has a great industrial potential.
Keywords:
functional properties; octenyl succinic anhydride; tiger nuts starch
1. Introduction
Starch is the main reserve substance in higher plants, supplying 70 to 80% of the calories consumed by man. It is a polysaccharide composed of repeating units of D-glucopyranose, in which D-glucose units are linked by α-1,4 bonds. This natural polymer consists basically of two macromolecules: amylose (15-30%) and amylopectin (85-70%)[11 Lima, B. N. B., Cabral, T. B., Neto, R. P. C., & Tavares, M. I. B. (2012). Estudo do amido de farinhas comerciais comestíveis. Polímeros: Ciência e Tecnologia, 22(5), 486-490. http://dx.doi.org/10.1590/S0104-14282012005000062.
http://dx.doi.org/10.1590/S0104-1428201...
2 Manek, R. V., Builders, P. F., Kolling, W. M., Emeje, M., & Kunle, O. O. (2012). Physicochemical and binder properties of starch obtained from Cyperus esculentus. American Association of Pharmaceutical Scientists PharmSciTech, 13(2), 379-388. http://dx.doi.org/10.1208/s12249-012-9761-z. PMid:22350737.
http://dx.doi.org/10.1208/s12249-012-97...
-33 Builders, P. F., Mbah, C. C., Adama, K. K., & Audu, M. M. (2014). Effect of pH on the physicochemical and binder properties of tiger nut starch. Stärke, 66(3-4), 281-293. http://dx.doi.org/10.1002/star.201300014.
http://dx.doi.org/10.1002/star.20130001...
].
The starch market has been growing and improving in recent years, leading to the search for products with specific characteristics that meet the requirements of these industries[44 Builders, P. F., Anwunobi, P. A., Mbah, C. C., & Adikwu, M. U. (2013). New direct compression excipiente from tiger nut starch: Physicochemical and functional properties. American Association of Pharmaceutical Scientists PharmSciTech, 14(2), 818-827. http://dx.doi.org/10.1208/s12249-013-9968-7. PMid:23649994.
http://dx.doi.org/10.1208/s12249-013-99...
]. The production of modified starches is an alternative that has been developed with the objective of overcoming one or more limitations of native starches, and thus increase the usefulness of this polymer in industrial applications[55 Bemiller, J. N. (1997). Starch modification: challenges and prospects. Stach , 49(4), 127-131. http://dx.doi.org/10.1002/star.19970490402/
http://dx.doi.org/10.1002/star.19970490...
]. The reasons for these modifications involve decreasing the retrogradation and tendency of the pastes to form gels, conferring stability in cooling and thawing processes, improving the texture of the pastes or gels and the formation of films, adding hydrophobic groups and introducing emulsifying power[66 Kweon, D. K., Choi, J. K., Kim, E. K., & Lim, S. T. (2001). Adsorption of divalent metal ions by succinylated and oxidized corn starches. Carbohydrate Polymers, 46(2), 171-177. http://dx.doi.org/10.1016/S0144-8617(00)00300-3.
http://dx.doi.org/10.1016/S0144-8617(00...
]. One of the chemical modification techniques is the treatment of native starches with octenyl succinic anhydride (OSA starches), whose modification provides an amphiphilic character to the starch molecule and, consequently, surface active properties[77 Bhosale, R., & Singhal, R. (2006). Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohydrate Polymers , 66(4), 521-527. http://dx.doi.org/10.1016/j.carbpol.2006.04.007.
http://dx.doi.org/10.1016/j.carbpol.200...
].
Tiger nut (Cyperus esculentus), also called chufa or “junça”, belonging to the Cyperaceae family, is cultivated especially in sandy soils[88 Okoli, C. A. N., Shilling, D. G., Smith, R. L., & Bewick, T. A. (1997). Genetic diversity in purple nutsedge (Cyperus esculentus L.). Biological Control , 8(2), 111-118. http://dx.doi.org/10.1006/bcon.1996.0490.
http://dx.doi.org/10.1006/bcon.1996.049...
]. The plant originates in East Africa and its use is very old. It is a very nutritious tuber, being characterized mainly by the high presence of carbohydrates and oils, which represent around 24.5% of its total composition[99 Yeboah, S. O., Mitei, Y. C., Ngila, J. C., Wessjohann, L., & Schmidt, J. (2011). Compositional and structural studies of the oils from two edible seeds: Tiger nut, Cyperus esculentum , and asiato, Pachira insignis, from Ghana. Food Research International, 47(2), 259-266. http://dx.doi.org/10.1016/j.foodres.2011.06.036.
http://dx.doi.org/10.1016/j.foodres.201...
].
The objective of this study was to characterize native starch isolated from tubers of Cyperus esculentus, and modify it chemically by means of esterification reactions with octenyl succinic anhydride (OSA).
2. Materials and Methods
2.1 Tiger nut starch extraction and chemical modification.
For extracting the tiger nut starch, a sanitization of the tubers was carried out with 10% solution of sodium hypochlorite, followed by milling thereof, by means of a mill type Willye (TE - 680), being previously dried at 50 °C for 48 h. The extraction process was performed according to the procedure described by Guraya et al. (2004)[1010 Kaur, M., Singh, N., Sandhu, K. S., & Guraya, H. S. (2004). Physicochemical, morphological, thermal, and rheological properties of starches separated from kernels of some Indian mango cultivars (Mangifera indica L.). Food Chemistry , 85(1), 131-140. http://dx.doi.org/10.1016/j.foodchem.2003.06.013.
http://dx.doi.org/10.1016/j.foodchem.20...
]. Starch succinylation was carried out in alkaline medium according to the procedure described by Song et al. (2006)[1111 Song, X., He, G., Ruan, H., & Chen, Q. (2006). Preparation and properties of octenyl succinic anhydride modified early indica rice starch. Starch, 58(2), 109-117. http://dx.doi.org/10.1002/star.200500444.
http://dx.doi.org/10.1002/star.20050044...
]. The efficiency of the reaction was performed by determining the degree of substitution according to the procedure of Kweon et al. (2001)[66 Kweon, D. K., Choi, J. K., Kim, E. K., & Lim, S. T. (2001). Adsorption of divalent metal ions by succinylated and oxidized corn starches. Carbohydrate Polymers, 46(2), 171-177. http://dx.doi.org/10.1016/S0144-8617(00)00300-3.
http://dx.doi.org/10.1016/S0144-8617(00...
].
2.2 Granule morphology by Scanning Electron Microscopy. (SEM)
The observation of the starch granule morphology was carried out under scanning electron microscope (Hitachi S5200). The powder sample was placed on an aluminum surface and covered with gold/palladium (40/60) with an acceleration potential of 20.00 kV.
2.3 X-ray Diffraction analysis. (XRD)
The structural characterization of the starch was performed by an X-ray diffraction equipment (Siemens D5000) at room temperature. The samples were packed in aluminum cells and illuminated by 40mA, 45kV CuKα radiation (λ = 1.54056Å). The samples were scanned in the range of 2θ diffraction angles between 5° and 40
2.4 Particle size analysis.
The particle size distribution was obtained using a laser diffraction apparatus (Beckman Coulter LS 13 320) with the sample suspended in water at 20 °C.
2.5 Differential Scanning Calorimetry analysis. (DSC)
The DSC curves were obtained using the NETZSCH STA 409 Pc Luxx equipment, under the following experimental conditions: dynamic nitrogen atmosphere with flow rate of 60 ml/min; heating rate of 10 °C/min, in a range of 40 °C to 250 °C; partially closed aluminum capsule and sample mass around 4.0 mg.
2.6 Analytical methods
The physicochemical analyses of the native and modified starch were performed according to the methods described by AOAC (1997)[1212 Association of official analytical chemists international – AOAC. (1997). Official methods of analysis Chemists. (16. ed.) Gaitherburg: AOAC. ]. The analyzed parameters were: protein, carbohydrate, lipid, moisture, amylose and ash.
3 Results and Discussion
3.1 Tiger nut starch extraction and composition.
The isolation of the tiger nut starch was relatively easy, as there was no interference of other compounds, mainly protein and lipid, on its extraction yield. The yield observed for the tiger nut starch extraction was 33.5%[44 Builders, P. F., Anwunobi, P. A., Mbah, C. C., & Adikwu, M. U. (2013). New direct compression excipiente from tiger nut starch: Physicochemical and functional properties. American Association of Pharmaceutical Scientists PharmSciTech, 14(2), 818-827. http://dx.doi.org/10.1208/s12249-013-9968-7. PMid:23649994.
http://dx.doi.org/10.1208/s12249-013-99...
]. The efficiency of the reaction was performed by determining the degree of substitution (0,04)[1313 Sweedman, M. C., Tizzotti, M. J., Schäfer, C., & Gilbert, R. G. (2013). Structure and physicochemical properties of octenyl succinic anhydride modified starches: a review. Carbohydrate Polymers, 92(1), 905-920. http://dx.doi.org/10.1016/j.carbpol.2012.09.040.
http://dx.doi.org/10.1016/j.carbpol.201...
]. It is observed in Table 1 that native and modified tiger nut starch presented lower values of moisture, ashes, protein and lipid when compared to the values obtained for dry matter. Native tiger nut starch and modified showed higher levels of amylopectin (83.9%; 83,0%) [22 Manek, R. V., Builders, P. F., Kolling, W. M., Emeje, M., & Kunle, O. O. (2012). Physicochemical and binder properties of starch obtained from Cyperus esculentus. American Association of Pharmaceutical Scientists PharmSciTech, 13(2), 379-388. http://dx.doi.org/10.1208/s12249-012-9761-z. PMid:22350737.
http://dx.doi.org/10.1208/s12249-012-97...
,1111 Song, X., He, G., Ruan, H., & Chen, Q. (2006). Preparation and properties of octenyl succinic anhydride modified early indica rice starch. Starch, 58(2), 109-117. http://dx.doi.org/10.1002/star.200500444.
http://dx.doi.org/10.1002/star.20050044...
]
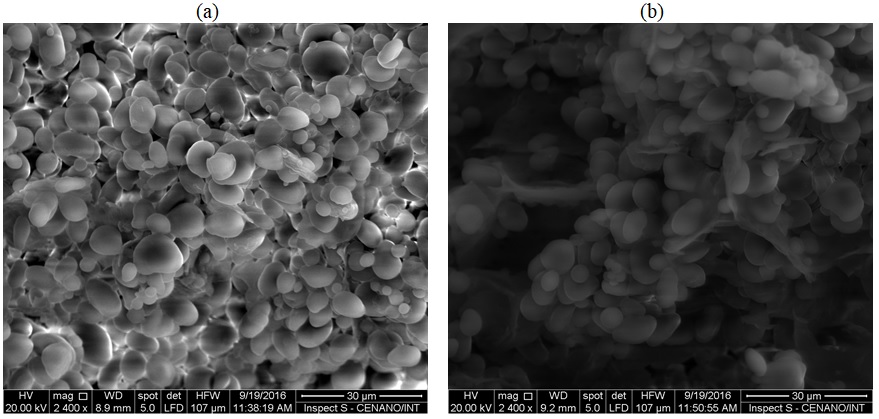
3.2 Morphology and particle size of native and modified starch
According to Figure 1 , it can be seen that the native tiger nut starch and modified granules had a smooth surface with no grooves and, for the most part, oval shapes[1414 Lindeboom, N., Chang, P., & Tyler, R. T. (2004). Analytical, biochemical and physicochemical aspects of starch granules size, with emphasis on small granule starches: a review. Starch, 56(3-4), 89-99. http://dx.doi.org/10.1002/star.200300218.
http://dx.doi.org/10.1002/star.20030021...
,1515 Jing, S., Yan, X., Ouyang, W., Xiang, H., & Ren, Z. (2012). Study on properties of Cyperus esculentus starch grown in Xinjiang, China. Starch , 64(8), 581-589. http://dx.doi.org/10.1002/star.201100129.
http://dx.doi.org/10.1002/star.20110012...
].
Scanning electron micrographs of starch granules obtained from the native and modified tiger nut starch. (a) native starch 2400x; (b) modified starch 2400x.
According to the particle size distribution analysis, the native tiger nut starch presented an average size of 0.182 μm and the modified starch had an average size of 0.156 μm as shown in Figure 2[1515 Jing, S., Yan, X., Ouyang, W., Xiang, H., & Ren, Z. (2012). Study on properties of Cyperus esculentus starch grown in Xinjiang, China. Starch , 64(8), 581-589. http://dx.doi.org/10.1002/star.201100129.
http://dx.doi.org/10.1002/star.20110012...
].
Particle size distribution of tiger nut starch granules. (a) native starch; (b) modifed starch. The line indicates the cumulative distribution of particles.
3.3 X-ray Diffraction (XRD) of native and modified starch
According to Figure 3 , corresponding to the diffractograms of the native and modified tiger nut starch, it was possible to notice that in both samples, the strongest peaks observed occurred approximately at 15°, 17°, 18° and 23° 2θ, a fact that characterizes this starch as A-type crystalline pattern[1616 Zobel, H. F. (1964). X-ray analysis of starch granules. In Whistler, R. L., Smith, R. J., & BeMiller, J. N. (Eds.), Methods in Carbohydrate Chemistry (109–113). New York: Academic Press.
17 Castaño, J., Bouza, R., Rodríguez-Llamazares, S., Carrasco, C., & Vinicius, R. V. B. (2012). Processing and Characterization of starch-based materials from pehuen seeds. Carbohydrate Polymers, 88(1), 299-307. http://dx.doi.org/10.1016/j.carbpol.2011.12.008.
http://dx.doi.org/10.1016/j.carbpol.201...
18 Lima, B. N. B., Cabral, T. B., Neto, R. P. C., & Tavares, M. I. B. (2012). Estudo do amido de farinhas comerciais comestíveis. Polímeros: Ciência e Tecnologia, 22(5), 486-490. http://dx.doi.org/10.1590/S0104-14282012005000062.
http://dx.doi.org/10.1590/S0104-1428201...
-1919 Zavareze, E. R., El Hal Al, S. L. M., Pereira, J. M., Radünz, A. L., Elias, M. C., & Dias, A. R. G. (2009). Caracterização química e rendimento de extração de amido de arroz com diferentes teores de amylose. Brazilian Journal of Food Technology , 1, 24-30. Retrieved in 2017, 08, 15 , from http://bjft.ital.sp.gov.br/artigos/especiais/especial_2009/v11_edesp_06.pdf
http://bjft.ital.sp.gov.br/artigos/espe...
].
3.4 Differential Scanning Calorimetry (DSC) of native and modified tiger nut starch
The curves obtained for the native and modified starch, presented in Figure 4 , were performed with the aim to evaluate, mainly, the gelatinization process thereof.
For the native starch, two endothermic peaks occurred: the first one was in the range of 62.27-75.55 °C (ΔH = 228.86 J.g-1), with maximum at 67.52 °C, related to the gelatinization process of the starch. For the modified starch, only one endothermic peak was observed, occurring in the range of 104.76-117.65 °C (ΔH = 714.28 J.g-1), with maximum at 108.28 °C, related to the melting process of the modified starch.
4. Acknowledgements
This work was financed by CAPES and CNPq. The authors are grateful to the Universidade Federal do Rio de Janeiro, Instituto Federal do for the help given to perform the experimental part.
5. Conclusion
This study explored the physicochemical properties of native and modified starch isolated from tubers of tiger nut (Cyperus esculentus) with respect to their likely potential for the industry as a whole. In addition, the satisfactory modification of tiger nut starch increased the possibilities of use by the industry, as it was shown to be more resistant to temperature as observed by DSC. These facts can raise the importance of the polymer of this tuber as an alternative source of starch, since in Brazil, the tiger nut is constantly eliminated because it is an allelopathic weed.
6. References
-
1Lima, B. N. B., Cabral, T. B., Neto, R. P. C., & Tavares, M. I. B. (2012). Estudo do amido de farinhas comerciais comestíveis. Polímeros: Ciência e Tecnologia, 22(5), 486-490. http://dx.doi.org/10.1590/S0104-14282012005000062.
» http://dx.doi.org/10.1590/S0104-14282012005000062 -
2Manek, R. V., Builders, P. F., Kolling, W. M., Emeje, M., & Kunle, O. O. (2012). Physicochemical and binder properties of starch obtained from Cyperus esculentus. American Association of Pharmaceutical Scientists PharmSciTech, 13(2), 379-388. http://dx.doi.org/10.1208/s12249-012-9761-z. PMid:22350737.
» http://dx.doi.org/10.1208/s12249-012-9761-z -
3Builders, P. F., Mbah, C. C., Adama, K. K., & Audu, M. M. (2014). Effect of pH on the physicochemical and binder properties of tiger nut starch. Stärke, 66(3-4), 281-293. http://dx.doi.org/10.1002/star.201300014.
» http://dx.doi.org/10.1002/star.201300014 -
4Builders, P. F., Anwunobi, P. A., Mbah, C. C., & Adikwu, M. U. (2013). New direct compression excipiente from tiger nut starch: Physicochemical and functional properties. American Association of Pharmaceutical Scientists PharmSciTech, 14(2), 818-827. http://dx.doi.org/10.1208/s12249-013-9968-7. PMid:23649994.
» http://dx.doi.org/10.1208/s12249-013-9968-7 -
5Bemiller, J. N. (1997). Starch modification: challenges and prospects. Stach , 49(4), 127-131. http://dx.doi.org/10.1002/star.19970490402/
» http://dx.doi.org/10.1002/star.19970490402 -
6Kweon, D. K., Choi, J. K., Kim, E. K., & Lim, S. T. (2001). Adsorption of divalent metal ions by succinylated and oxidized corn starches. Carbohydrate Polymers, 46(2), 171-177. http://dx.doi.org/10.1016/S0144-8617(00)00300-3.
» http://dx.doi.org/10.1016/S0144-8617(00)00300-3 -
7Bhosale, R., & Singhal, R. (2006). Process optimization for the synthesis of octenyl succinyl derivative of waxy corn and amaranth starches. Carbohydrate Polymers , 66(4), 521-527. http://dx.doi.org/10.1016/j.carbpol.2006.04.007.
» http://dx.doi.org/10.1016/j.carbpol.2006.04.007 -
8Okoli, C. A. N., Shilling, D. G., Smith, R. L., & Bewick, T. A. (1997). Genetic diversity in purple nutsedge (Cyperus esculentus L.). Biological Control , 8(2), 111-118. http://dx.doi.org/10.1006/bcon.1996.0490.
» http://dx.doi.org/10.1006/bcon.1996.0490 -
9Yeboah, S. O., Mitei, Y. C., Ngila, J. C., Wessjohann, L., & Schmidt, J. (2011). Compositional and structural studies of the oils from two edible seeds: Tiger nut, Cyperus esculentum , and asiato, Pachira insignis, from Ghana. Food Research International, 47(2), 259-266. http://dx.doi.org/10.1016/j.foodres.2011.06.036.
» http://dx.doi.org/10.1016/j.foodres.2011.06.036 -
10Kaur, M., Singh, N., Sandhu, K. S., & Guraya, H. S. (2004). Physicochemical, morphological, thermal, and rheological properties of starches separated from kernels of some Indian mango cultivars (Mangifera indica L.). Food Chemistry , 85(1), 131-140. http://dx.doi.org/10.1016/j.foodchem.2003.06.013.
» http://dx.doi.org/10.1016/j.foodchem.2003.06.013 -
11Song, X., He, G., Ruan, H., & Chen, Q. (2006). Preparation and properties of octenyl succinic anhydride modified early indica rice starch. Starch, 58(2), 109-117. http://dx.doi.org/10.1002/star.200500444.
» http://dx.doi.org/10.1002/star.200500444 -
12Association of official analytical chemists international – AOAC. (1997). Official methods of analysis Chemists (16. ed.) Gaitherburg: AOAC.
-
13Sweedman, M. C., Tizzotti, M. J., Schäfer, C., & Gilbert, R. G. (2013). Structure and physicochemical properties of octenyl succinic anhydride modified starches: a review. Carbohydrate Polymers, 92(1), 905-920. http://dx.doi.org/10.1016/j.carbpol.2012.09.040.
» http://dx.doi.org/10.1016/j.carbpol.2012.09.040 -
14Lindeboom, N., Chang, P., & Tyler, R. T. (2004). Analytical, biochemical and physicochemical aspects of starch granules size, with emphasis on small granule starches: a review. Starch, 56(3-4), 89-99. http://dx.doi.org/10.1002/star.200300218.
» http://dx.doi.org/10.1002/star.200300218 -
15Jing, S., Yan, X., Ouyang, W., Xiang, H., & Ren, Z. (2012). Study on properties of Cyperus esculentus starch grown in Xinjiang, China. Starch , 64(8), 581-589. http://dx.doi.org/10.1002/star.201100129.
» http://dx.doi.org/10.1002/star.201100129 -
16Zobel, H. F. (1964). X-ray analysis of starch granules. In Whistler, R. L., Smith, R. J., & BeMiller, J. N. (Eds.), Methods in Carbohydrate Chemistry (109–113). New York: Academic Press.
-
17Castaño, J., Bouza, R., Rodríguez-Llamazares, S., Carrasco, C., & Vinicius, R. V. B. (2012). Processing and Characterization of starch-based materials from pehuen seeds. Carbohydrate Polymers, 88(1), 299-307. http://dx.doi.org/10.1016/j.carbpol.2011.12.008.
» http://dx.doi.org/10.1016/j.carbpol.2011.12.008 -
18Lima, B. N. B., Cabral, T. B., Neto, R. P. C., & Tavares, M. I. B. (2012). Estudo do amido de farinhas comerciais comestíveis. Polímeros: Ciência e Tecnologia, 22(5), 486-490. http://dx.doi.org/10.1590/S0104-14282012005000062.
» http://dx.doi.org/10.1590/S0104-14282012005000062 -
19Zavareze, E. R., El Hal Al, S. L. M., Pereira, J. M., Radünz, A. L., Elias, M. C., & Dias, A. R. G. (2009). Caracterização química e rendimento de extração de amido de arroz com diferentes teores de amylose. Brazilian Journal of Food Technology , 1, 24-30. Retrieved in 2017, 08, 15 , from http://bjft.ital.sp.gov.br/artigos/especiais/especial_2009/v11_edesp_06.pdf
» http://bjft.ital.sp.gov.br/artigos/especiais/especial_2009/v11_edesp_06.pdf
Publication Dates
-
Publication in this collection
Aug-Sep 2018 -
Date of issue
Sept 2018
History
-
Received
22 Mar 2017 -
Accepted
21 Dec 2017