Abstract
Microcrystalline cellulose synthesized from the waste of Musa balbisiana (BMCC) was characterized to explore the possibility of application in the pharmaceutical industry especially as a drug delivery vehicle. The SEM, XRD and FTIR investigations revealed that the predominantly short, non-aggregated and irregular MCC rods were highly crystalline. The moisture sorption value for BMCC was 5.65%, while total ash was 0.39%. Flow of BMCC was poor, but the product exhibited high hydration (11.7%) and swelling (277.0%) capacities. Preliminary investigation of BMCC tablets containing ascorbic acid carried out in simulated intestinal fluid, showed a concentration dependent retardation of drug release. No cytotoxicity of BMCC was observed in the hemolytic assay. Overall, the study revealed that BMCC can be prepared from an inexpensive and abundant agricultural waste and possesses properties advantageous for application in the pharmaceutical industry and may be explored further in drug delivery research.
Keywords:
drug release; microcrystalline cellulose; Musa balbisiana; physicochemical characteristics
1. Introduction
Microcrystalline cellulose, (MCC), is known as a purified, partially depolymerized cellulose which is prepared by treating alpha cellulose obtained as ‘pulp’ from a fibrous plant with mineral acids. One of the most commonly used direct compression filler-binders is MCC. Due to its excellent binding properties, it is used widely in tablet formulation especially as direct compression excipient. It has been reported[11 Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
] that MCC’s dilution potential is high and performs well in direct compression formulations. It also works well as disintegrants and lubricant as well as diluent or filler in formulation of tablets prepared by wet granulation and as filler in capsules and spheres[22 Höckerfelt, M. H., & Alderborn, G. (2014). The crystallinity of cellulose controls the physical distribution of sorbed water and the capacity to present water for chemical degradation of a solid drug. International Journal of Pharmaceutics, 477(1-2), 326-333. http://dx.doi.org/10.1016/j.ijpharm.2014.10.034. PMid:25455777.
http://dx.doi.org/10.1016/j.ijpharm.2014...
3 Kranz, H., Jürgens, K., Pinier, M., & Siepmann, J. (2009). Drug release from MCC- and carrageenan-based pellets: experiment and theory. European Journal of Pharmaceutics and Biopharmaceutics, 73(2), 302-309. http://dx.doi.org/10.1016/j.ejpb.2009.05.007. PMid:19465119.
http://dx.doi.org/10.1016/j.ejpb.2009.05...
4 Luukkonen, P., Schæfer, T., Podczeck, F., Newton, M., Hellén, L., & Yliruusi, J. (2001). Characterization of microcrystalline cellulose and silicified microcrystalline cellulose wet masses using a powder rheometer. European Journal of Pharmaceutical Sciences, 13(2), 143-149. http://dx.doi.org/10.1016/S0928-0987(00)00197-4. PMid:11297898.
http://dx.doi.org/10.1016/S0928-0987(00)...
5 Mallick, S., Pradhan, S. K., & Mohapatra, R. (2013). Effects of microcrystalline cellulose based comilled powder on the compression and dissolution of ibuprofen. International Journal of Biological Macromolecules, 60(0), 148-155. http://dx.doi.org/10.1016/j.ijbiomac.2013.05.021. PMid:23732329.
http://dx.doi.org/10.1016/j.ijbiomac.201...
-66 Ohwoavworhua, F. O., & Adelakun, T. A. (2010). Non-wood fibre production of microcrystalline cellulose from Sorghum caudatum: characterization and tableting properties. Indian Journal of Pharmaceutical Sciences, 72(3), 295-301. http://dx.doi.org/10.4103/0250-474X.70473. PMid:21188036.
http://dx.doi.org/10.4103/0250-474X.7047...
]. MCC is derived from several sources including gymnosperms (generally conifers) and softwoods, as well as from hardwood dicotyledons. The botanical or biological source has been reported to affect the chemical composition (amounts of lignin, cellulose and hemicellulose) as well as the structural arrangement. These invariably affect the composition of the alpha cellulose and consequently the crystallinity and composition of the final product which is MCC[11 Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
,77 Ohwoavworhua, F. O., & Adelakun, T. A. (2005). Phosphoric acid-mediated depolymerization and decrystallization of α-cellulose obtained from corn cob: preparation of low crystallinity cellulose and some physicochemical properties. Tropical Journal of Pharmaceutical Research, 4(2), 509-516. http://dx.doi.org/10.4314/tjpr.v4i2.14627.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
]. Several researchers[88 Ohwoavworhua, F., Adelakun, T., & Okhamafe, A. (2009). Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: extraction methods and characterization. International Journal of Green Pharmacy, 3(2), 97-104. http://dx.doi.org/10.4103/0973-8258.54895.
http://dx.doi.org/10.4103/0973-8258.5489...
9 Kalita, R. D., Nath, Y., Ochubiojo, M. E., & Buragohain, A. K. (2013). Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids and Surfaces. B, Biointerfaces, 108(0), 85-89. http://dx.doi.org/10.1016/j.colsurfb.2013.02.016. PMid:23524080.
http://dx.doi.org/10.1016/j.colsurfb.201...
-1010 Thoorens, G., Krier, F., Leclercq, B., Carlin, B., & Evrard, B. (2014). Microcrystalline cellulose, a direct compression binder in a quality by design environment: a review. International Journal of Pharmaceutics, 473(1-2), 64-72. http://dx.doi.org/10.1016/j.ijpharm.2014.06.055. PMid:24993785.
http://dx.doi.org/10.1016/j.ijpharm.2014...
] opine that strong hydrogen bonding exists between the cellulose crystals consequently boosting re-aggregation, a precursor to the formation of microcrystalline cellulose.
Musa balbisiana is among the two species (along with M. acuminata) that are wild progenitors of the complex hybrids that make up modern bananas and plantains. The cultivated hybrids are tropical monocot tree-like plants grown in wet tropical areas worldwide, and are the fourth most cultivated food crop in the world, with 2009 global production of 97.4 million tons, harvested from 4.9 million hectares. M. balbisiana is indigenous to Southeast Asia, including China, India, Indonesia (Java), Malaysia, Myanmar, Nepal, New Guinea, Philippines, Sikkim, Sri Lanka, and Thailand, where it majorly grows in ravines in tropical evergreen forests at altitudes of up to 1,100 meters (3,575 feet). In some of these areas, including parts of New Guinea and Thailand, it may have naturalized following cultivation. It is not clear when the first hybrids were made, but archaeological evidence suggests that bananas have been cultivated for at least 7,000 years[1111 Carlos-Amaya, F., Osorio-Diaz, P., Agama-Acevedo, E., Yee-Madeira, H., & Bello-Pérez, L. A. (2011). Physicochemical and digestibility properties of double-modified banana (Musa paradisiaca L.) starches. Journal of Agricultural and Food Chemistry, 59(4), 1376-1382. http://dx.doi.org/10.1021/jf1035004. PMid:21214175.
http://dx.doi.org/10.1021/jf1035004...
,1212 Gogoi, K., Saikia, J. P., & Konwar, B. K. (2013). Immobilizing silver nanoparticles (SNP) on Musa balbisiana cellulose. Colloids and Surfaces. B, Biointerfaces, 102, 136-138. http://dx.doi.org/10.1016/j.colsurfb.2012.07.031. PMid:23010111.
http://dx.doi.org/10.1016/j.colsurfb.201...
].
M. balbisiana is a perennial herbaceous plant with a hard, fibrous “trunk”. It often grows with several pseudo-stems in a cluster. The primary stem bears a single large terminal inflorescence, a spike with pistillate (female) flowers below, and staminate (male) flowers above. This develops into a bunch of bananas, consisting of 8 clusters of 15 or 16 bananas (technically, berries) arranged in two rows. Hybrids have greatly reduced and usually sterile seeds, but in wild types, seeds occupy up to 25% of the fruit. There are Bananas and plantains from hybrids of M. balbisiana, but vary in proportion of sugar to starch[1212 Gogoi, K., Saikia, J. P., & Konwar, B. K. (2013). Immobilizing silver nanoparticles (SNP) on Musa balbisiana cellulose. Colloids and Surfaces. B, Biointerfaces, 102, 136-138. http://dx.doi.org/10.1016/j.colsurfb.2012.07.031. PMid:23010111.
http://dx.doi.org/10.1016/j.colsurfb.201...
].
The novelty of this work lies on the premise that, Banana stem constitutes a waste challenge because they are dumped on the roadsides or into lakes and rivers after the banana fruit has been consumed, thus, constituting environmental hazard. The stem is a very good source of cellulose and its use as a biomass for development of excipients like microcrystalline will not only increase the pool of pharmaceutical excipients available, curbing the environmental menace it constitutes, but equally provide a cheaper alternative to the currently available but expensive microcrystalline cellulose.
There is scanty information on the characterization of the microcrystalline cellulose obtained from Musa balbisiana. Hence this study aims to extract and characterize the microcrystalline cellulose from the waste of Musa balbisiana. Also, the release profile of tablet formulated with this new microcrystalline cellulose was evaluated to determine the suitability of BMCC as an excipient in biopolymer industries.
2. Materials and Methods
2.1 Isolation of α- cellulose
The process outlined by Ohwoavworhoa and Adelakun[11 Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
] was followed; essentially, the banana was peeled and the waste was cut into pieces and dried in a hot air oven. An 80 g quantity of this material was placed in a stainless steel container to which was added 4.0 L of 2% w/v sodium hydroxide and digestion effected for 4 h at 80 °C in a water bath (FGL 1083. Karl Kolb Scientific, West Germany). Following thorough washing and filtration, it was bleached with 2.0 L of a 1:1 aqueous dilution of sodium hypochlorite for 15 min at 80 °C. The material was then washed sufficiently with water and treated with 2.0 L of 17.5% w/v sodium hydroxide at 80 °C for 1 h. The resulting α-cellulose was washed thoroughly with water. The extraction process was then completed by whitening with a 1:2 aqueous dilution of sodium hypochlorite for 15 min at 80 °C and subsequent washing with water until neutral to litmus. The cellulose material was filtered, and the water manually squeezed out using calico cloth to obtain small lumps, which were dried in a fluidized-bed dryer at an inlet air temperature of about 60 °C for 1 h.
2.2 Production of microcrystalline cellulose (MCC)
Again, the process outlined Ohwoavworhoa and Adelakun[11 Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
] was followed; a 50 g quantity of the α- cellulose obtained from the process described above was placed in a glass container and hydrolyzed with 0.8 L of 2.5 N hydrochloric acid at a boiling temperature of 105 °C for 15 min. The hot acid mixture was poured into 2.5 L of cold tap water which was followed by vigorous stirring with a stainless steel spatula and allowed to stand overnight. The microcrystalline cellulose obtained by this process was filtered, washed with water until neutral to litmus, filtered, pressed and dried in a fluidized-bed dryer at an inlet air temperature of about 60 °C for 1 h. Following further milling and sieving, the fraction passing through 650 µm sieve aperture was used for the characterization described below.
2.3 Total ash determination
The total Ash content was estimated by the measurement of the residue left after combustion in a furnace at 550 °C.
2.4 Bulk and tap densities
A 20 g quantity of powder sample was placed in a 50 mL graduated cylinder and the volume, Vo, occupied by the powder without tapping was noted. Using a Stampfvolumeter model STAV 2003 (JEF, Germany), the powder was tapped 100 times, and the volume occupied by the powder after tapping noted as, V100. The bulk, (Db), and tapped densities were then calculated.
2.5 Swelling capacity
The method reported by Ohwoavworhoa, F. O. Adelakun[66 Ohwoavworhua, F. O., & Adelakun, T. A. (2010). Non-wood fibre production of microcrystalline cellulose from Sorghum caudatum: characterization and tableting properties. Indian Journal of Pharmaceutical Sciences, 72(3), 295-301. http://dx.doi.org/10.4103/0250-474X.70473. PMid:21188036.
http://dx.doi.org/10.4103/0250-474X.7047...
] was used and computation was done using the equation below:
V2 is the volume of the hydrated or swollen material and V1 is the tapped volume of the material prior to hydration.
2.6 Hydration capacity
A 1.0 g quantity of each sample was placed in each of four 15 mL plastic centrifuge tubes and 10 mL distilled water was added from a 10 mL measuring cylinder and then stoppered. The contents were mixed on a vortex mixer (Vortex Gennie Scientific Industry, USA) for 2 min. The mixture was allowed to stand for 10 min and immediately centrifuged at 1000 rpm for 10 min on a bench centrifuge (Gallenkamp, England). The supernatant was carefully decanted and the sediment weighed. The hydration capacity was taken as the ratio of the weight of the sediment to the dry sample weight.
2.7 Moisture sorption capacity
A 2 g quantity of the cellulose material was accurately weighed and evenly distributed over the surface of a 70 mm tarred Petri dish. The samples were placed in a large desiccator containing distilled water in its reservoir (RH = 100%) at room temperature and the weight gained by the exposed samples at the end of a five-day period was recorded and the amount of water absorbed was calculated from the weight difference.
2.8 Scanning Electron Microscopy (SEM)
Using a Hitachi S5200 field emission scanning electron microscope (Hitachi High- Technologies Canada, Inc., Ontario, Canada), micrographs of microcrystalline cellulose from Musa balbisiana was obtained. The imaging was done at 1.0 kV accelerating voltage on platinum-coated samples.
2.9 X-Ray Diffraction
Structural characterization of the microcrystalline cellulose molecules was done using a Siemens D5000 X-ray diffractometer (Siemens, Munich, Germany). Powder samples, packed in rectangular aluminum cells were illuminated using CuKa radiation (l = 1.54056 Å) at 45 kV and 40 mA. Samples were scanned between diffraction angles of 5 to 80°. Scan steps of 0.1 were used and the dwell time was 15 s. To reduce the size (kb) contribution to the X-ray signal a nickel filter was used at ambient temperature and three measurements were made.
2.10 Fourier Transform Infrared Spectroscopy (FT-IR)
The IR spectra of BMCC sample were run as KBr pellets on impact 410 Nicolet FTIR spectrometer in the frequency range 4000-500 cm–1.
2.11 Hemolysis test
Hemolysis test was carried out according to the method of Kalita et al.[99 Kalita, R. D., Nath, Y., Ochubiojo, M. E., & Buragohain, A. K. (2013). Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids and Surfaces. B, Biointerfaces, 108(0), 85-89. http://dx.doi.org/10.1016/j.colsurfb.2013.02.016. PMid:23524080.
http://dx.doi.org/10.1016/j.colsurfb.201...
]. Mammalian blood sample from goat was collected in a vial containing 4% trisodium citrate. It was centrifuged at 750 × g. The supernatant was discarded and the precipitate containing the erythrocytes was washed with PBS (pH 7.4) twice at 750 ×g for 10 min. 48.5 mL of PBS was added to 1.5 ml of the erythrocyte suspension and was equally divided in 2 mL tubes. Samples containing three different composition of MCC weighing 10 mg each was added to tubes containing 2 ml of the erythrocyte suspension and incubated for 2 h at 37 °C. After the incubation, the tubes were centrifuged at 750 × g for 10 min. Now, 200 mL of the supernatant was collected in a fresh tube and to it 2.8 mL of PBS was added. The absorbance was taken at 415 nm. PBS was taken as the negative control and TritonX 100 was taken as the positive control.
2.12 Preparation of ascorbic acid tablets
Batches of ascorbic tablets containing 200 mg ascorbic acid per tablet, and BMCC at 25, 50 and 65% were prepared using the direct compression method. Tablet weights; 250, 300 and 330 mg corresponding to BMCC 25, 50 and 65% respectively were produced. The powders were mixed thoroughly with no extra excipients such as diluents, lubricants and glidants prior to compression using a single punch tableting machine (Erweka AR 400, Germany) at uniform compression pressure (8.0 metric tons). Fifty tablets were produced per batch and stored in an air tight container for 24 h preceding release studies.
2.13 Release studies
Release (dissolution) tests were performed on the tablets using an Erweka dissolution test apparatus. The medium used was simulated intestinal fluid, thermostatically maintained at 37 °C at a rotational speed of 50 rpm. Samples of 5 mL were withdrawn at 5 min intervals and replaced with fresh 5 mL of the dissolution medium. The withdrawn samples were analyzed spectrophotometrically at a pre-determined wavelength of 265 nm and the amount of drug released was calculated with reference to the calibration curve (Figure 1).
3. Results and Discussions
3.1 Flow properties
The flow of powdered material is highly important in ascertaining its usage as an excipient in direct compression of tablets. An indirect measure of powder flowability is Hausner index which shows friction between the particles and Carr’s compressibility index which is indicative of the propensity of a volume of a material to decrease on tapping[11 Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
,77 Ohwoavworhua, F. O., & Adelakun, T. A. (2005). Phosphoric acid-mediated depolymerization and decrystallization of α-cellulose obtained from corn cob: preparation of low crystallinity cellulose and some physicochemical properties. Tropical Journal of Pharmaceutical Research, 4(2), 509-516. http://dx.doi.org/10.4314/tjpr.v4i2.14627.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
]. The values are indirectly proportional to the flow character, as the indices increases, the flow character decreases. In general, however, Hausners ratio greater than 1.25, indicates poor flow; Carr’s compressibility index less than 10% indicates excellent flowability and values between 11-15 indicates good flowability while values greater than 38% indicates very poor flow[66 Ohwoavworhua, F. O., & Adelakun, T. A. (2010). Non-wood fibre production of microcrystalline cellulose from Sorghum caudatum: characterization and tableting properties. Indian Journal of Pharmaceutical Sciences, 72(3), 295-301. http://dx.doi.org/10.4103/0250-474X.70473. PMid:21188036.
http://dx.doi.org/10.4103/0250-474X.7047...
,88 Ohwoavworhua, F., Adelakun, T., & Okhamafe, A. (2009). Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: extraction methods and characterization. International Journal of Green Pharmacy, 3(2), 97-104. http://dx.doi.org/10.4103/0973-8258.54895.
http://dx.doi.org/10.4103/0973-8258.5489...
,1313 Ohwoavworhua, F. O., Adelakun, T. A., & Kunle, O. O. (2007). A comparative evaluation of the flow and compaction characteristics of a-cellulose obtained from waste paper. Tropical Journal of Pharmaceutical Research, 6(1), 645-651. http://dx.doi.org/10.4314/tjpr.v6i1.14642.
http://dx.doi.org/10.4314/tjpr.v6i1.1464...
]. In this study, the derived flow parameters (Car’s compressibility index and Hausner’s ratio) show that the powder does not possess excellent flow. Therefore, there is need for inclusion of a glidant or lubricant during tablet compression using this cellulose.
3.2 Total ash content
Total ash value which is an index for purity is found in Table 1, the lower the value, the better the material as it establishes that extreme care was observed during production. In this study, low values were obtained indicating relatively low impurities in the final material.
3.3 Swelling capacity
One of the mechanisms of tablet disintegration is swelling. This swelling can be evaluated by obtaining the hydration and swelling capacities as well as moisture sorption profile of the material. Swelling capacity is indicative of water absorption of the cellulose and this can be seen clearly in Table 2 to be very high. The swelling capacity value shows that the new cellulose increased in volume up to 277% of its original volume. This show that BMCC may be useful in developing a super disintegrant for incorporation in tablet formulation. The mechanism will presumably be by swelling. This high swelling capacity may equally be due to the high porous nature of the extracted cellulose[11 Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
]. High swelling capacity has also been implicated in sustained release formulations[1414 Emeje, M., John-Africa, L., Isimi, Y., Kunle, O., & Ofoefule, S. (2012). Eudraginated polymer blends: a potential oral controlled drug delivery system for theophylline. Acta Pharmaceutica, 62(1), 71-82. http://dx.doi.org/10.2478/v10007-012-0001-6. PMid:22472450.
http://dx.doi.org/10.2478/v10007-012-000...
15 Emeje, M., Nwabunike, P., Isimi, C., Kunle, O., & Ofoefule, S. (2008). Hydro-alcoholic media: am emerging in vitro tool for predicting dose dumping from a controlled release matrices. Journal of Pharmacology and Toxicology, 3(2), 84-92. http://dx.doi.org/10.3923/jpt.2008.84.92.
http://dx.doi.org/10.3923/jpt.2008.84.92...
-1616 Emeje, M. O., Kunle, O. O., & Ofoefule, S. I. (2006). Effect of the molecular size of carboxymethylcellulose and some polymers on the sustained release of theophylline from a hydrophilic matrix. Acta Pharmaceutica, 56(3), 325-335. PMid:19831281.].
3.4 Hydration capacity
The hydration capacity value obtained for the extracted cellulose is shown in the Table 1. The very high hydration capacity shows its capability of absorbing water more than five times its weight. According to Ohwoavworhoa and Adelakun[11 Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
], the hydration capacity of microcrystalline cellulose they obtained from raw cotton of Cochlospermum planchonii was 4.73, the authors concluded that the cellulose was capable of absorbing more water than the standard microcrystalline cellulose, Avicel. In our current report, BMCC hydrates about 11 times more than its original volume. It is therefore predicted that, BMCC may be a good disintegrant in solid dosage preparations, although, it could also be employed as a binder in formulation of controlled delivery systems[1616 Emeje, M. O., Kunle, O. O., & Ofoefule, S. I. (2006). Effect of the molecular size of carboxymethylcellulose and some polymers on the sustained release of theophylline from a hydrophilic matrix. Acta Pharmaceutica, 56(3), 325-335. PMid:19831281.].
3.5 Moisture sorption
Moisture sorption capacity measures how sensitive a material is to moisture, the values for the moisture sorption capacity is found in the Table 1. The crystallite part of the cellulose has been said not to adsorb water invariably water adsorption by cellulose is dependent on the amount of amorphous cellulose present in the material[11 Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
,77 Ohwoavworhua, F. O., & Adelakun, T. A. (2005). Phosphoric acid-mediated depolymerization and decrystallization of α-cellulose obtained from corn cob: preparation of low crystallinity cellulose and some physicochemical properties. Tropical Journal of Pharmaceutical Research, 4(2), 509-516. http://dx.doi.org/10.4314/tjpr.v4i2.14627.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
,88 Ohwoavworhua, F., Adelakun, T., & Okhamafe, A. (2009). Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: extraction methods and characterization. International Journal of Green Pharmacy, 3(2), 97-104. http://dx.doi.org/10.4103/0973-8258.54895.
http://dx.doi.org/10.4103/0973-8258.5489...
]. Thus, the high moisture sorption capacity value recorded indicates the high amount of amorphous cellulose present in BMCC. Moisture sorption capacity also tells us the extent of the stability of any formulation made from the cellulose when stored under humid condition. This property generally gives an insight on the storage condition. Since it is sensitive to moisture, the material should always be stored in air-tight container[11 Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
,77 Ohwoavworhua, F. O., & Adelakun, T. A. (2005). Phosphoric acid-mediated depolymerization and decrystallization of α-cellulose obtained from corn cob: preparation of low crystallinity cellulose and some physicochemical properties. Tropical Journal of Pharmaceutical Research, 4(2), 509-516. http://dx.doi.org/10.4314/tjpr.v4i2.14627.
http://dx.doi.org/10.4314/tjpr.v4i2.1462...
,88 Ohwoavworhua, F., Adelakun, T., & Okhamafe, A. (2009). Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: extraction methods and characterization. International Journal of Green Pharmacy, 3(2), 97-104. http://dx.doi.org/10.4103/0973-8258.54895.
http://dx.doi.org/10.4103/0973-8258.5489...
].
3.6 Scanning Electron Microscopy (SEM)
The scanning electron microscopy of the extracted cellulose is predominantly uniform in shape. Its surface is equally smooth with predominantly rectangular shape (Figure 2). Materials with uniform-sized particles are have found wide use in the pharmaceutical industries[1717 Nwajiobi, C. C., Otaigbe, J. O. E., & Oriji, O. (2019). Isolation and characterization of microcrystalline cellulose from papaya stem. Der Pharma Chemica, 11(3), 19-26. Retrieved in 2018, November 6, from https://www.derpharmachemica.com/pharma-chemica/isolation-and-characterization-of-microcrystalline-cellulose-from-empapayaem-stem-18356.html
https://www.derpharmachemica.com/pharma-...
] we therefore opine that, the potential relevance of BMCC as an excipient in pharmaceutical industry is high.
3.7 Fourier-Transform Infrared Spectroscopy (FTIR)
The FTIR spectra identifies the functional groups present in the material. From Figure 3, the IR peak observed at 3475 is due to cellulose –OH stretching vibrations. The peak at 1436.44 and 1375.87 is attributed to the ‘bending vibrations of –CH2, C-H and C-O of cellulose. The peaks at 1161.78, 1115.30 and 1061.05 is attributed to the ‘deformation of the C-H rocking vibrations[88 Ohwoavworhua, F., Adelakun, T., & Okhamafe, A. (2009). Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: extraction methods and characterization. International Journal of Green Pharmacy, 3(2), 97-104. http://dx.doi.org/10.4103/0973-8258.54895.
http://dx.doi.org/10.4103/0973-8258.5489...
,99 Kalita, R. D., Nath, Y., Ochubiojo, M. E., & Buragohain, A. K. (2013). Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids and Surfaces. B, Biointerfaces, 108(0), 85-89. http://dx.doi.org/10.1016/j.colsurfb.2013.02.016. PMid:23524080.
http://dx.doi.org/10.1016/j.colsurfb.201...
,1818 Pawar, H., & Varkhade, C. (2014). Isolation, characterization and investigation of Plantago ovata husk polysaccharide as superdisintegrant. International Journal of Biological Macromolecules, 69, 52-58. http://dx.doi.org/10.1016/j.ijbiomac.2014.05.019. PMid:24854213.
http://dx.doi.org/10.1016/j.ijbiomac.201...
].
3.8 X-ray diffractometry (XRD)
The X-ray pattern of indicates the crystallinity of a material[99 Kalita, R. D., Nath, Y., Ochubiojo, M. E., & Buragohain, A. K. (2013). Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids and Surfaces. B, Biointerfaces, 108(0), 85-89. http://dx.doi.org/10.1016/j.colsurfb.2013.02.016. PMid:23524080.
http://dx.doi.org/10.1016/j.colsurfb.201...
,1919 Atef, M., Rezaei, M., & Behrooz, R. (2014). Preparation and characterization agar-based nanocomposite film reinforced by nanocrystalline cellulose. International Journal of Biological Macromolecules, 70, 537-544. http://dx.doi.org/10.1016/j.ijbiomac.2014.07.013. PMid:25036597.
http://dx.doi.org/10.1016/j.ijbiomac.201...
,2020 Panda, B., Parihar, A. S., & Mallick, S. (2014). Effect of plasticizer on drug crystallinity of hydroxypropyl methylcellulose matrix film. International Journal of Biological Macromolecules, 67, 295-302. http://dx.doi.org/10.1016/j.ijbiomac.2014.03.033. PMid:24685464.
http://dx.doi.org/10.1016/j.ijbiomac.201...
]. The pronounced peak of BMCC observed at 22.1 (Figure 4) indicates that delignification and depolymerization has been done successfully. The crystalline nature of the material is evident from the sharp intensity observed and the value shows the amount of the crystalline nature; crystallinity is an indication of BMCC’s ordered compact structure.
Whether BMCC had any cell damaging affect was investigated in terms of its hemolytic activity in erythrocytes. It was found that BMCC did not cause any damage to the blood cells (Figures 5 and 6). Its activity even at the highest polymer concentration of 65% was the same as that of the PBS buffer (pH 7.4) after 2 h of incubation which indicated no cytotoxic effect on the cells. This shows the compatibility of BMCC with blood cells and suggests its safety in development of delivery systems to tissues.
3.9 Release profile
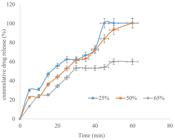
The release of the drug from the formulation was observed to decrease with increase in the concentration of BMCC (Figure 7). The release from tablets containing 25% BMCC was the highest, while tablets containing 65% BMCC had the lowest release. These results corroborate the high swelling capacity of BMCC which is known to decrease drug release as a result of elongated diffusion path length. Therefore, microcrystalline cellulose extracted from Musa balbisiana may be used in formulations meant for sustained or extended drug release..
4. Conclusions
Sequential alkaline and acid treatments of the waste stem of Musa balbisiana led to the production of microcrystalline cellulose (BMCC) with desirable and unique physicochemical properties for potential applications as excipients in pharmaceutical formulations. In general, it was found to be a good direct compression excipient for ascorbic acid and was without cytotoxic effect as evident from the hemolytic assay. The investigation also revealed that BMCC supported a concentration dependent retardation of ascorbic acid release. Thus, the MCC prepared from this agricultural waste may be further explored for application in the pharma industry.
-
How to cite: Emeje, M., Ekpo, M., Olayemi, O., Isimi, C., & Buraghoin, A. (2020). Physicochemical and drug release properties of microcrystalline cellulose derived from Musa balbisiana. Polímeros: Ciência e Tecnologia, 30(1), e2020010. https://doi.org/10.1590/0104-1428.07418
5. References
-
1Ohwoavworhoa, F. O., & Adelakun, T. A. (2005). Some physical characteristics of microcrystalline cellulose obtained from raw cotton of cochlospermum planchonii. Tropical Journal of Pharmaceutical Research, 4(2), 501-507. http://dx.doi.org/10.4314/tjpr.v4i2.14626
» http://dx.doi.org/10.4314/tjpr.v4i2.14626 -
2Höckerfelt, M. H., & Alderborn, G. (2014). The crystallinity of cellulose controls the physical distribution of sorbed water and the capacity to present water for chemical degradation of a solid drug. International Journal of Pharmaceutics, 477(1-2), 326-333. http://dx.doi.org/10.1016/j.ijpharm.2014.10.034 PMid:25455777.
» http://dx.doi.org/10.1016/j.ijpharm.2014.10.034 -
3Kranz, H., Jürgens, K., Pinier, M., & Siepmann, J. (2009). Drug release from MCC- and carrageenan-based pellets: experiment and theory. European Journal of Pharmaceutics and Biopharmaceutics, 73(2), 302-309. http://dx.doi.org/10.1016/j.ejpb.2009.05.007 PMid:19465119.
» http://dx.doi.org/10.1016/j.ejpb.2009.05.007 -
4Luukkonen, P., Schæfer, T., Podczeck, F., Newton, M., Hellén, L., & Yliruusi, J. (2001). Characterization of microcrystalline cellulose and silicified microcrystalline cellulose wet masses using a powder rheometer. European Journal of Pharmaceutical Sciences, 13(2), 143-149. http://dx.doi.org/10.1016/S0928-0987(00)00197-4 PMid:11297898.
» http://dx.doi.org/10.1016/S0928-0987(00)00197-4 -
5Mallick, S., Pradhan, S. K., & Mohapatra, R. (2013). Effects of microcrystalline cellulose based comilled powder on the compression and dissolution of ibuprofen. International Journal of Biological Macromolecules, 60(0), 148-155. http://dx.doi.org/10.1016/j.ijbiomac.2013.05.021 PMid:23732329.
» http://dx.doi.org/10.1016/j.ijbiomac.2013.05.021 -
6Ohwoavworhua, F. O., & Adelakun, T. A. (2010). Non-wood fibre production of microcrystalline cellulose from Sorghum caudatum: characterization and tableting properties. Indian Journal of Pharmaceutical Sciences, 72(3), 295-301. http://dx.doi.org/10.4103/0250-474X.70473 PMid:21188036.
» http://dx.doi.org/10.4103/0250-474X.70473 -
7Ohwoavworhua, F. O., & Adelakun, T. A. (2005). Phosphoric acid-mediated depolymerization and decrystallization of α-cellulose obtained from corn cob: preparation of low crystallinity cellulose and some physicochemical properties. Tropical Journal of Pharmaceutical Research, 4(2), 509-516. http://dx.doi.org/10.4314/tjpr.v4i2.14627
» http://dx.doi.org/10.4314/tjpr.v4i2.14627 -
8Ohwoavworhua, F., Adelakun, T., & Okhamafe, A. (2009). Processing pharmaceutical grade microcrystalline cellulose from groundnut husk: extraction methods and characterization. International Journal of Green Pharmacy, 3(2), 97-104. http://dx.doi.org/10.4103/0973-8258.54895
» http://dx.doi.org/10.4103/0973-8258.54895 -
9Kalita, R. D., Nath, Y., Ochubiojo, M. E., & Buragohain, A. K. (2013). Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids and Surfaces. B, Biointerfaces, 108(0), 85-89. http://dx.doi.org/10.1016/j.colsurfb.2013.02.016 PMid:23524080.
» http://dx.doi.org/10.1016/j.colsurfb.2013.02.016 -
10Thoorens, G., Krier, F., Leclercq, B., Carlin, B., & Evrard, B. (2014). Microcrystalline cellulose, a direct compression binder in a quality by design environment: a review. International Journal of Pharmaceutics, 473(1-2), 64-72. http://dx.doi.org/10.1016/j.ijpharm.2014.06.055 PMid:24993785.
» http://dx.doi.org/10.1016/j.ijpharm.2014.06.055 -
11Carlos-Amaya, F., Osorio-Diaz, P., Agama-Acevedo, E., Yee-Madeira, H., & Bello-Pérez, L. A. (2011). Physicochemical and digestibility properties of double-modified banana (Musa paradisiaca L.) starches. Journal of Agricultural and Food Chemistry, 59(4), 1376-1382. http://dx.doi.org/10.1021/jf1035004 PMid:21214175.
» http://dx.doi.org/10.1021/jf1035004 -
12Gogoi, K., Saikia, J. P., & Konwar, B. K. (2013). Immobilizing silver nanoparticles (SNP) on Musa balbisiana cellulose. Colloids and Surfaces. B, Biointerfaces, 102, 136-138. http://dx.doi.org/10.1016/j.colsurfb.2012.07.031 PMid:23010111.
» http://dx.doi.org/10.1016/j.colsurfb.2012.07.031 -
13Ohwoavworhua, F. O., Adelakun, T. A., & Kunle, O. O. (2007). A comparative evaluation of the flow and compaction characteristics of a-cellulose obtained from waste paper. Tropical Journal of Pharmaceutical Research, 6(1), 645-651. http://dx.doi.org/10.4314/tjpr.v6i1.14642
» http://dx.doi.org/10.4314/tjpr.v6i1.14642 -
14Emeje, M., John-Africa, L., Isimi, Y., Kunle, O., & Ofoefule, S. (2012). Eudraginated polymer blends: a potential oral controlled drug delivery system for theophylline. Acta Pharmaceutica, 62(1), 71-82. http://dx.doi.org/10.2478/v10007-012-0001-6 PMid:22472450.
» http://dx.doi.org/10.2478/v10007-012-0001-6 -
15Emeje, M., Nwabunike, P., Isimi, C., Kunle, O., & Ofoefule, S. (2008). Hydro-alcoholic media: am emerging in vitro tool for predicting dose dumping from a controlled release matrices. Journal of Pharmacology and Toxicology, 3(2), 84-92. http://dx.doi.org/10.3923/jpt.2008.84.92
» http://dx.doi.org/10.3923/jpt.2008.84.92 -
16Emeje, M. O., Kunle, O. O., & Ofoefule, S. I. (2006). Effect of the molecular size of carboxymethylcellulose and some polymers on the sustained release of theophylline from a hydrophilic matrix. Acta Pharmaceutica, 56(3), 325-335. PMid:19831281.
-
17Nwajiobi, C. C., Otaigbe, J. O. E., & Oriji, O. (2019). Isolation and characterization of microcrystalline cellulose from papaya stem. Der Pharma Chemica, 11(3), 19-26. Retrieved in 2018, November 6, from https://www.derpharmachemica.com/pharma-chemica/isolation-and-characterization-of-microcrystalline-cellulose-from-empapayaem-stem-18356.html
» https://www.derpharmachemica.com/pharma-chemica/isolation-and-characterization-of-microcrystalline-cellulose-from-empapayaem-stem-18356.html -
18Pawar, H., & Varkhade, C. (2014). Isolation, characterization and investigation of Plantago ovata husk polysaccharide as superdisintegrant. International Journal of Biological Macromolecules, 69, 52-58. http://dx.doi.org/10.1016/j.ijbiomac.2014.05.019 PMid:24854213.
» http://dx.doi.org/10.1016/j.ijbiomac.2014.05.019 -
19Atef, M., Rezaei, M., & Behrooz, R. (2014). Preparation and characterization agar-based nanocomposite film reinforced by nanocrystalline cellulose. International Journal of Biological Macromolecules, 70, 537-544. http://dx.doi.org/10.1016/j.ijbiomac.2014.07.013 PMid:25036597.
» http://dx.doi.org/10.1016/j.ijbiomac.2014.07.013 -
20Panda, B., Parihar, A. S., & Mallick, S. (2014). Effect of plasticizer on drug crystallinity of hydroxypropyl methylcellulose matrix film. International Journal of Biological Macromolecules, 67, 295-302. http://dx.doi.org/10.1016/j.ijbiomac.2014.03.033 PMid:24685464.
» http://dx.doi.org/10.1016/j.ijbiomac.2014.03.033
Publication Dates
-
Publication in this collection
01 July 2020 -
Date of issue
2020
History
-
Received
06 Nov 2018 -
Reviewed
01 May 2020 -
Accepted
04 May 2020