Abstract
Elicitors activate the defense mechanism in plants to resist pathogens. Ulvans and glucuronans can act as elicitors, and their activity seems to be related to the sulfate groups, rhamnose and uronic acid monosaccharides. Chichá gum (CHG), which also contains rhamnose and uronic acid, was sulfated with chlorosulfonic acid/N,N-dimethylformamide and deacetylated with sodium hydroxide solution. The changes were confirmed by infrared spectroscopy. Carbon-13 NMR revealed that sulfation occurred in galactose and rhamnose units. The apples were sprayed with water (negative control), deacetylated chichá gum (DCHG), and sulfated chichá gum (SCHG). The activity of enzymes guaiacol peroxidase and polyphenol oxidases and the lignin content were compared with those under the action of a commercial elicitor, benzothiadiazole. DCHG, and especially SCHG, increased the activity of the two enzymes. Only fruits treated with SCHG showed a significant (p<0.05) increase in lignin content. The plant exudate can be one abundant, renewable and safe source of elicitors.
Keywords:
benzothiadiazole (BTH); Sterculia striata; elicitor; polysaccharide; sulfation
1. Introduction
Elicitors are molecules capable of induce defense responses. Because these molecules can protect plants against pathogens, they are promising for reducing the use of agrochemicals[11 Iriti, M., & Vitalini, S. (2021). Plant immunity and crop yield: a sustainable approach in agri-food systems. Vaccines, 9(2), 1-3. http://dx.doi.org/10.3390/vaccines9020121. PMid:33546315.
https://doi.org/10.3390/vaccines9020121...
]. Among the various compounds that may act as elicitors, there are the polysaccharides from seaweed[22 Abouraïcha, E., El Alaoui-Talibi, Z., El Boutachfaiti, R., Petit, E., Courtois, B., Courtois, J., & El Modafar, C. (2015). Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Scientia Horticulturae, 181, 121-128. http://dx.doi.org/10.1016/j.scienta.2014.11.002.
http://dx.doi.org/10.1016/j.scienta.2014...
,33 Abouraïcha, E. F., El Alaoui-Talibi, Z., Tadlaoui-Ouafi, A., El Boutachfaiti, R., Petit, E., Douira, A., Courtois, B., Courtois, J., & El Modafar, C. (2017). Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. Journal of Applied Phycology, 29(1), 471-480. http://dx.doi.org/10.1007/s10811-016-0926-0.
http://dx.doi.org/10.1007/s10811-016-092...
]. The most promising algae-derived polysaccharides for defense induction in plant tissue are ulvans and glucuronans, which are widely available due to the abundance of green algae[33 Abouraïcha, E. F., El Alaoui-Talibi, Z., Tadlaoui-Ouafi, A., El Boutachfaiti, R., Petit, E., Douira, A., Courtois, B., Courtois, J., & El Modafar, C. (2017). Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. Journal of Applied Phycology, 29(1), 471-480. http://dx.doi.org/10.1007/s10811-016-0926-0.
http://dx.doi.org/10.1007/s10811-016-092...
,44 Stadnik, M. J., & Freitas, M. B. (2014). Algal polysaccharides as source of plant resistance inducers. Tropical Plant Pathology, 39(2), 111-118. http://dx.doi.org/10.1590/S1982-56762014000200001.
http://dx.doi.org/10.1590/S1982-56762014...
].
Ulvans protected tomato plants against Alternaria solani and Xanthomonas vesicatoria[55 Ramkissoon, A., Ramsubhag, A., & Jayaraman, J. (2017). Phytoelicitor activity of three Caribbean seaweed species on suppression of pathogenic infections in tomato plants. Journal of Applied Phycology, 29(6), 3235-3244. http://dx.doi.org/10.1007/s10811-017-1160-0.
http://dx.doi.org/10.1007/s10811-017-116...
] and apple fruits against Penicillium expansum and Botrytis cinerea[22 Abouraïcha, E., El Alaoui-Talibi, Z., El Boutachfaiti, R., Petit, E., Courtois, B., Courtois, J., & El Modafar, C. (2015). Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Scientia Horticulturae, 181, 121-128. http://dx.doi.org/10.1016/j.scienta.2014.11.002.
http://dx.doi.org/10.1016/j.scienta.2014...
]. Ulvans are basically composed of rhamnose (16.8‒45.0%), sulfate groups (16.0‒23.2%), uronic acids (6.5‒19%), xylose (2.1‒12.0%), iduronic acid (1.1‒9.1%), and glucose (0.5‒6.4%)[44 Stadnik, M. J., & Freitas, M. B. (2014). Algal polysaccharides as source of plant resistance inducers. Tropical Plant Pathology, 39(2), 111-118. http://dx.doi.org/10.1590/S1982-56762014000200001.
http://dx.doi.org/10.1590/S1982-56762014...
]. According El Modafar et al. (2012)[66 El Modafar, C., Elgadda, M., El Boutachfaiti, R., Abouraicha, E., Zehhara, N., & Petit, E. (2012). Induction of natural defence accompanied by salicylic acid dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Scientia Horticulturae, 138, 55-63. http://dx.doi.org/10.1016/j.scienta.2012.02.011.
http://dx.doi.org/10.1016/j.scienta.2012...
] the ability of ulvans to induce plant defense may be related to the presence of rhamnose and sulfate groups. Glucuronans, to a lesser extent, and oligoglucuronans, to a greater extent, protected tomato seedlings against Fusarium oxysporum f. sp. Lycopersici[66 El Modafar, C., Elgadda, M., El Boutachfaiti, R., Abouraicha, E., Zehhara, N., & Petit, E. (2012). Induction of natural defence accompanied by salicylic acid dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Scientia Horticulturae, 138, 55-63. http://dx.doi.org/10.1016/j.scienta.2012.02.011.
http://dx.doi.org/10.1016/j.scienta.2012...
] and apples against Penicillium expansum and Botrytis cinerea[33 Abouraïcha, E. F., El Alaoui-Talibi, Z., Tadlaoui-Ouafi, A., El Boutachfaiti, R., Petit, E., Douira, A., Courtois, B., Courtois, J., & El Modafar, C. (2017). Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. Journal of Applied Phycology, 29(1), 471-480. http://dx.doi.org/10.1007/s10811-016-0926-0.
http://dx.doi.org/10.1007/s10811-016-092...
]. As glucuronans are composed primarily of residues of glucuronic acids, these residues may be related to the defense-inducing activity.
Three polysaccharide subunits are reportedly related to the elicitation of apples defenses: rhamnose, uronic acid, and sulfate groups. A polysaccharide extracted from the exudate of Sterculia striata, commonly known as chichá gum (CHG), has - in addition to xylose (5.6‒7.7%), galactose (19.3‒23.4%), and acetyl groups (9.6‒10.7%) - rhamnose (23.1‒28.8%), and uronic acids (42.2‒49.2%)[77 Brito, A. C. F., Sierakowski, M. R., Reicher, F., Feitosa, J. P. A., & Paula, R. C. M. (2005). Dynamic rheological study of Sterculia striata and karaya polysaccharides in aqueous solution. Food Hydrocolloids, 19(5), 861-867. http://dx.doi.org/10.1016/j.foodhyd.2004.10.035.
http://dx.doi.org/10.1016/j.foodhyd.2004...
,88 Brito, A. C. F., Silva, D. A., Paula, R. C. M., & Feitosa, J. P. A. (2004). Sterculia striata exudate polysaccharide: characterization, rheological properties and comparison with Sterculia urens (karaya) polysaccharide. Polymer International, 53(8), 1025-1032. http://dx.doi.org/10.1002/pi.1468.
http://dx.doi.org/10.1002/pi.1468...
]. Chemical modifications that remove acetyl groups (deacetylation) and add sulfate groups (sulfation), could make the composition of CHG more similar to that of polysaccharides from green algae, making CHG more interesting for apple elicitation tests. In addition, the synthesis and physiological role of sulfated polysaccharides has receiving great attention so far and is another novelty in this work.
Apples are one of the most pesticide-treated crops[99 Tzatzarakis, M., Kokkinakis, M., Renieri, E., Goumenou, M., Kavvalakis, M., Vakonaki, E., Chatzinikolaou, A., Stivaktakis, P., Tsakiris, I., Rizos, A., & Tsatsakis, A. (2020). Multiresidue analysis of insecticides and fungicides in apples from the Greek market. Applying an alternative approach for risk assessment. Food and Chemical Toxicology, 140, 111262. http://dx.doi.org/10.1016/j.fct.2020.111262. PMid:32198030.
http://dx.doi.org/10.1016/j.fct.2020.111...
]. The ‘Pink Lady’ cultivar was selected as it is susceptible to diseases and thus requires high use of agrochemicals[1010 Marolleau, B., Gaucher, M., Heintz, C., Degrave, A., Warneys, R., Orain, G., Lemarquand, A., & Brisset, M. N. (2017). When a plant resistance inducer leaves the lab for the field: integrating ASM into routine apple protection practices. Frontiers of Plant Science, 8, 1938. http://dx.doi.org/10.3389/fpls.2017.01938. PMid:29255473.
http://dx.doi.org/10.3389/fpls.2017.0193...
]. The objective of this work is to deacetylate and sulfate (for the first time) CHG, characterize the derivatives, and evaluate the ability of these polysaccharides to enhance the protection of “Pink Lady” apples against pathogens. The results are compared with those of a widely used synthetic elicitor, benzothiadiazole (BTH). The carcinogenicity of BTH is classified as Category 1A[1111 SYNGENTA. (2015). Material safety data sheet (MSDS) Byon®, Syngenta, 4/16/2015. Retrieved in 2020, October 5, from https://assets.greenbook.net/M114161.pdf
https://assets.greenbook.net/M114161.pdf...
], which means: “known to have carcinogenic potential for humans[1212 McGregor, D., Boobis, A., Binaglia, M., Botham, P., Hoffstadt, L., Hubbard, S., Petry, T., Riley, A., Schwartz, D., & Hennes, C. (2010). Guidance for the classification of carcinogens under the Globally Harmonised System of Classification and Labelling of Chemicals (GHS). Critical Reviews in Toxicology, 40(3), 245-285. http://dx.doi.org/10.3109/10408440903384717. PMid:20014893.
http://dx.doi.org/10.3109/10408440903384...
].
2. Materials and Methods
2.1 Materials
Sterculia striata exudate was obtained from a tree located in Fortaleza-Ceará (Brazil). BTH sodium hypochlorite, phosphate buffered saline (pH 7.4), citric acid, bovine serum albumin, monolignol (guaiacol) peroxidase, guaiacol, hydrogen peroxide, pyrocathecol, acetone, thioglycolic acid, and 2-hydroxypropyl ether were supplied by Sigma-Aldrich, São Paulo, Brazil. Sodium hydroxide, N,N-dimethylformamide (DMF), and chlorosulfonic acid (CSA) were obtained from Vetec, Brazil. Sodium chloride, hydrochloric acid, and ethanol were purchased from Synth, São Paulo, Brazil.
2.2 Isolation of CHG
The gum was isolated by the methodology of Brito et al.[88 Brito, A. C. F., Silva, D. A., Paula, R. C. M., & Feitosa, J. P. A. (2004). Sterculia striata exudate polysaccharide: characterization, rheological properties and comparison with Sterculia urens (karaya) polysaccharide. Polymer International, 53(8), 1025-1032. http://dx.doi.org/10.1002/pi.1468.
http://dx.doi.org/10.1002/pi.1468...
], with adaptations. An exudate aqueous solution (1% w/v) was filtered through a sintered plate funnel (G1) and the pH was adjusted to 7.0 by adding of 1.0 mol.L‒1 NaOH. Sodium chloride (half of the exudate mass) was added. After 3 h, the gum was precipitated in 2 volumes of ethanol and filtered through a sintered plate funnel (G4). The precipitate was washed three times with ethanol and dried in a desiccator under vacuum. The obtained sample is denoted as CHG.
2.3 Sulfation of chichá gum
The sulfation of CHG was based on the method reported by Pires et al. (2013)[1313 Pires, N. R., Cunha, P. L. R., Maciel, J. S., Angelim, A. L., Melo, V. M. M., Paula, R. C. M., & Feitosa, J. P. A. (2013). Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydrate Polymers, 91(1), 92-99. http://dx.doi.org/10.1016/j.carbpol.2012.08.011. PMid:23044109.
http://dx.doi.org/10.1016/j.carbpol.2012...
], with several modifications. 1 g sample of the polysaccharide and 50 mL of DMF were placed in a round-bottom flask (250 mL). After 15 h under stirring, another 50 mL aliquot of DMF was added. In an ice bath, 6 mL of CSA was slowly added to the solution, and the reaction was allowed to run for 3 h at room temperature (around 28 °C). Addition of 200 mL of ethanol stopped the reaction. To improve the precipitation of the sulfated derivative, 0.5 g of NaCl was added. The precipitate was retained in a sintered plate funnel (G3), washed twice with ethanol, and dissolved in 200 mL of distilled water. The pH was adjusted to 7.0 with 1.0 mol.L‒1 NaOH, and the solution was dialyzed against distilled water. The sample was recovered by lyophilization, and the sulfated gum (SCHG) was obtained.
2.4 Deacetylation of CHG
The procedure reported by Brito et al. (2005)[77 Brito, A. C. F., Sierakowski, M. R., Reicher, F., Feitosa, J. P. A., & Paula, R. C. M. (2005). Dynamic rheological study of Sterculia striata and karaya polysaccharides in aqueous solution. Food Hydrocolloids, 19(5), 861-867. http://dx.doi.org/10.1016/j.foodhyd.2004.10.035.
http://dx.doi.org/10.1016/j.foodhyd.2004...
] was used for deacetylation of chichá gum. CHG aqueous solution (200 mL, 1% w/v) was mixed with 200 mL of 1.0 mol.L‒1 NaOH solution under stirring. After 20 min, the mixture was neutralized using 6.0 mol.L‒1 HCl, and dialyzed for 4 days, while monitoring the conductivity. The solid obtained by lyophilization is denoted as deacetylayed chichá gum (DCHG).
2.5 Characterization of chichá gum and its derivatives
2.5.1 Determination of the degree of sulfation of SCHG
The percentage of carbon (%C) and sulfur (%S) in the sulfated derivative was measured using Perkin Elmer 2400 Series II CHNS/O microanalyzer (Massachusetts, US). Equation 1 was used to calculate the degree of sulfation (DS):
where Ms and Mc are the atomic mass of sulfur and carbon, respectively.
2.5.2 Fourier-transform infrared spectroscopy (FTIR)
The absorption spectra of CHG, SCHG, and DCHG in the infrared region were obtained for the samples in KBr pellets by using a Shimadzu IRT Race100 FTIR spectrophotometer, in the range of 400‒4000 cm‒1.
2.5.3 Thermal analysis (TGA, DSC)
Samples of the gums (10 mg) were subjected to thermogravimetric analysis on TGAQ50 equipment (TA Instruments) under synthetic air atmosphere at a heating rate of 10 °C min‒1. The dehydration and total decomposition rates were identified to determine the moisture and ash content, respectively. The differential scanning calorimetry curves were obtained on Shimadzu DSC50 equipment under nitrogen atmosphere using a flow of 50 mL min‒1 and 4.0 mg of sample, in the temperature range of 27‒450 °C, at a heating rate of 10 °C min‒1.
2.5.4 Gel permeation chromatography (GPC)
Solutions of the gums (0.1% w/v) in 0.1 mol L‒1 sodium nitrate were prepared by magnetic stirring in a water-bath (70 °C) for 12 h, and filtered through a 0.45 μm Milipore membrane. Shimadzu equipment (Kyoto, Japan) consisting of a pump (LC10AD) coupled to a refractive index detector (RID6A) was employed. A Phenomenex precolumn PolySepGFCP (35 mm × 7.80 mm) and PolySepGFCP linear column (300 mm × 7.80 mm) were used for the separation process. A 0.1 mol L‒1 NaNO3 solution was used as the eluent at a flow rate of 1.0 mL min‒1 and sample volume was 50 μL. The standard curve for the molar mass determination was constructed using polystyrene sulfonate standards (logM = 13.92 – 0.99Ve) according to Dupont[1414 Dupont, A.-L. (2002). Study of the degradation of gelatin in paper upon aging using aqueous size-exclusion chromatography. Journal of Chromatography. A, 950(1-2), 113-124. http://dx.doi.org/10.1016/S0021-9673(02)00010-9. PMid:11990984.
http://dx.doi.org/10.1016/S0021-9673(02)...
].
2.5.5 Carbon-13 nuclear magnetic resonance (13C-NMR)
The gums were dissolved in D2O with 1% sodium 2,2-dimethylsilapentane-5-sulfonate (DSS) for zero calibration of the chemical shift. CHG was subjected to partial acid hydrolysis to improve the spectral resolution. The spectra were obtained at 70 °C on a Bruker Avance RX500 model spectrometer (Massachusetts, US).
2.6 Biochemical tests
2.6.1 Material preparation and fruit treatments
The polysaccharides and BTH were dissolved in distilled water at a concentration of 5 mg mL‒1 for DCHG and SCHG[22 Abouraïcha, E., El Alaoui-Talibi, Z., El Boutachfaiti, R., Petit, E., Courtois, B., Courtois, J., & El Modafar, C. (2015). Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Scientia Horticulturae, 181, 121-128. http://dx.doi.org/10.1016/j.scienta.2014.11.002.
http://dx.doi.org/10.1016/j.scienta.2014...
,33 Abouraïcha, E. F., El Alaoui-Talibi, Z., Tadlaoui-Ouafi, A., El Boutachfaiti, R., Petit, E., Douira, A., Courtois, B., Courtois, J., & El Modafar, C. (2017). Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. Journal of Applied Phycology, 29(1), 471-480. http://dx.doi.org/10.1007/s10811-016-0926-0.
http://dx.doi.org/10.1007/s10811-016-092...
] and 0.4 mg mL‒1 for BTH[1010 Marolleau, B., Gaucher, M., Heintz, C., Degrave, A., Warneys, R., Orain, G., Lemarquand, A., & Brisset, M. N. (2017). When a plant resistance inducer leaves the lab for the field: integrating ASM into routine apple protection practices. Frontiers of Plant Science, 8, 1938. http://dx.doi.org/10.3389/fpls.2017.01938. PMid:29255473.
http://dx.doi.org/10.3389/fpls.2017.0193...
] (positive control, Ctrl+). ‘Pink Lady’ apples were harvested in Vacaria, Rio Grande do Sul state (Brazil) and the fruits were further sanitized in 2% (v/v) sodium hypochlorite solution. Once dried, the apples were sprayed with distilled water (negative control, Ctrl‒), the gums, and BTH. In a completely randomized experiment, three apple samples (per treatment) were taken at times of 12, 24, 48, and 72 h after spraying (HAS) for pulp sampling. The soluble protein, extracted lignin, and enzyme activity were evaluated. For the chosen time interval, the apples were subjected to B. D. O (Biochemical Oxygen Demand) chamber conditions for 24 h in the dark at 25 ºC.
2.6.2 Enzyme and lignin determination
Pulp samples were taken from apple fruits with a spudger and homogenized for 2 min in a mortar in 1:3 (w/v) dilution with 100 mmol L‒1 PBS pH 7.4 buffer containing 25 mmol L‒1 citric acid (cooled). After gauze filtration, the fresh material was centrifuged at 12,400 ×g for 10 min at 4 °C. The crude extract obtained from the supernatant was used for enzymatic determinations[1515 Cavalcanti, F. R., Resende, M. L. V., Carvalho, C. P. S., Silveira, J. A. G., & Oliveira, J. T. A. (2007). An aqueous suspension of Crinipellis perniciosa mycelium activates tomato defence responses against Xanthomonas vesicatoria. Crop Protection (Guildford, Surrey), 27(5), 729-738. http://dx.doi.org/10.1016/j.cropro.2006.06.012.
http://dx.doi.org/10.1016/j.cropro.2006....
]. The soluble protein (mgP mL‒1) from the crude extracts was pre-determined using 0.3 mol L‒1 bovine serum albumin (BSA)[1616 Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3. PMid:942051.
http://dx.doi.org/10.1016/0003-2697(76)9...
].
To determine the enzyme activity, 100 μL of crude extract (supernatant) was used as the substrate in the enzyme mixtures comprising 2.0 mL of a solution containing 50 mmol L-1 sodium acetate buffer (pH 6.8), 20 mmol L-1 guaiacol and 30 mmol L-1 hydrogen peroxide for guaiacol peroxidase (GPX, EC 1.11.1.7). The procedure was the same for polyphenol oxidases (PPO, EC 1.10.3.1), however without hydrogen peroxide and with 30 mmol L‒1 pyrocathecol instead of guaiacol. The activity of the two enzymes was recorded over the course of 10 min using a spectrophotometer. The GPX activity and PPO were read at 480 and 410 nm, respectively[1515 Cavalcanti, F. R., Resende, M. L. V., Carvalho, C. P. S., Silveira, J. A. G., & Oliveira, J. T. A. (2007). An aqueous suspension of Crinipellis perniciosa mycelium activates tomato defence responses against Xanthomonas vesicatoria. Crop Protection (Guildford, Surrey), 27(5), 729-738. http://dx.doi.org/10.1016/j.cropro.2006.06.012.
http://dx.doi.org/10.1016/j.cropro.2006....
]. The relative activity unit (UA) of both oxidases was defined as the change in the enzyme absorbance at the respective wavelengths by one milligram of soluble protein per min (UA mg.P‒1min‒1).
For the lignin determination, 0.2 g fresh samples of ‘Pink Lady’ apple pulps were powdered in a mortar with liquid nitrogen for 3 min. The samples were incubated in 85% acetone for 48 h. After centrifugation at 8,000 ×g for 15 min at 7 °C, the dried precipitate was resuspended in 5 mL thioglycolic acid diluted in 2 mol L‒1 hydrochloric acid (1:10, v/v). The suspensions were incubated for 4 h at 25 °C and centrifuged at 8,000 × g. The supernatant was then transferred to a 20 mL tube; 200 µL of 10 mol L‒1 hydrochloric acid was added and the mixture was incubated in an ice-bath for 4 h. After centrifugation at 8.000 × g, the pellet was homogenized in 5 mL of 0.5 mol L‒1 NaOH and the absorbance was measured at 280 nm. Thioglycolic acid derivatives (lignin) were measured by comparison with a 10–100 µg mL‒1 standard curve for 2-hydroxypropyl ether[1717 Monties, B. (1989). Lignins. In P. M. Dey, & J. B. Harborne (Eds.), Methods in plant biochemistry (Vol. 1, pp. 113-158). New York: Academic Press. https://doi.org/10.1016/B978-0-12-461011-8.50010-X
https://doi.org/10.1016/B978-0-12-461011...
].
2.6.4 Statistical analysis
Regarding enzyme responses, descriptive statistics and standard deviations between gum-, Bion- treatments and water-pre-treated (Ctrl-) apples were compared with vertical bars beside averages in each hour of time interval. For the lignin determinations, normality (Shapiro-Wilk), homoscedasticity (Bartlett), ANOVA and Tukey tests were run at 5% significance with a specific R script (R version 3.5.0 - The R Foundation, 2018).
3. Results and Discussions
3.1 Characterization of chichá gum and its sulfated and deacetylated derivatives
The isolation, sulfation, and deacetylation yields were 52, 105, and 76%, respectively. A yield exceeding 100% is justified because hydrogen atoms (1.0 g mol-1) in the molecular structure are replaced by SO3Na groups (103.1 g mol‒1) during the sulfation process. Other authors have observed sulfation yields above 100%[1818 Xing, R., Liu, S., Yu, H., Guo, Z., Li, Z., & Li, P. (2005). Preparation of high molecular weight and high sulfate content chitosans and their potential antioxidant activity in vitro. Carbohydrate Polymers, 61(2), 148-154. http://dx.doi.org/10.1016/j.carbpol.2005.04.007.
http://dx.doi.org/10.1016/j.carbpol.2005...
,1919 Vikhoreva, G., Bannikova, G., Stolbushkina, P., Panov, A., Drozd, N., Makarov, V., Varlamov, V., & Galbraikh, L. (2005). Preparation and anticoagulant activity of a low-molecular-weight sulfated chitosan. Carbohydrate Polymers, 62(4), 327-332. http://dx.doi.org/10.1016/j.carbpol.2005.05.022.
http://dx.doi.org/10.1016/j.carbpol.2005...
]. The percentage of sulfur and degree of sulfation achieved were 8.7% and 0.82, respectively.
3.1.1 Infrared absorption spectroscopy (FTIR)
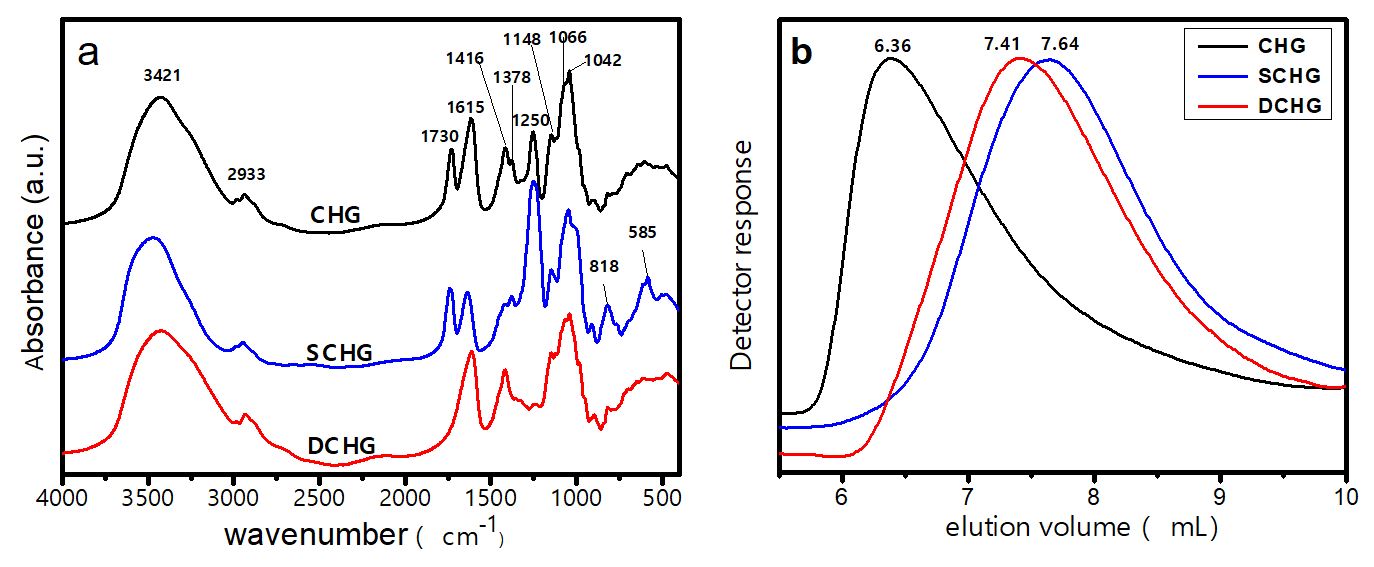
The spectra of chichá gum and its derivatives are shown in Figure 1a. The band at 3421 cm‒1 is attributed to the stretching of the O-H bonds[2020 Gangapuram, B. R., Bandi, R., Dadigala, R., Kotu, G. M., & Guttena, V. (2017). Facile green synthesis of gold nanoparticles with carboxymethyl gum karaya, selective and sensitive colorimetric detection of copper (II) ions. Journal of Cluster Science, 28(5), 2873-2890. http://dx.doi.org/10.1007/s10876-017-1264-3.
http://dx.doi.org/10.1007/s10876-017-126...
]. This band was narrower for the SCHG, plausibly because for each sulfate group inserted, one O-H group is lost. For the DCHG, this band was slightly wider. The bandwidth is related to intermolecular interactions. Stronger interaction leads to a wider bandwidth[2121 Patra, N., Vojtová, L., & Martinová, L. (2015). Deacetylation induced changes in thermal properties of Sterculia urens gum. Journal of Thermal Analysis and Calorimetry, 122(1), 235-240. http://dx.doi.org/10.1007/s10973-015-4680-3.
http://dx.doi.org/10.1007/s10973-015-468...
]. The bands at 2933 and 1378 cm‒1 were attributed to C-H (CH3) stretching and deformation, respectively[2121 Patra, N., Vojtová, L., & Martinová, L. (2015). Deacetylation induced changes in thermal properties of Sterculia urens gum. Journal of Thermal Analysis and Calorimetry, 122(1), 235-240. http://dx.doi.org/10.1007/s10973-015-4680-3.
http://dx.doi.org/10.1007/s10973-015-468...
,2222 Padil, V. V. T., Senan, C., & Černík, M. (2015). Dodecenylsuccinic anhydride derivatives of gum karaya (Sterculia urens): Preparation, characterization, and their antibacterial properties. Journal of Agricultural and Food Chemistry, 63(14), 3757-3765. http://dx.doi.org/10.1021/jf505783e. PMid:25797306.
http://dx.doi.org/10.1021/jf505783e...
]. These two bands were similar for CHG and SCHG because the sulfation process does not change the number of C-H bonds in the macromolecule. For DCHG, the band at 2933 cm‒1 became narrower and that at 1378 cm‒1 practically disappeared due to loss of the acetyl groups.
The bands at 1148, 1066, and 1042 cm‒1 (fingerprint region) correspond to the stretching vibrations of various C-O-C bonds present in carbohydrates in general[2222 Padil, V. V. T., Senan, C., & Černík, M. (2015). Dodecenylsuccinic anhydride derivatives of gum karaya (Sterculia urens): Preparation, characterization, and their antibacterial properties. Journal of Agricultural and Food Chemistry, 63(14), 3757-3765. http://dx.doi.org/10.1021/jf505783e. PMid:25797306.
http://dx.doi.org/10.1021/jf505783e...
]. The band at 1730 cm‒1, which was absent in the FTIR profile of DCHG, and present in that of CHG and SCHG, is assigned to C=O from the acetyl groups. Intensification of the band at 1250 cm‒1 was observed for SCHG. This absorbance is assigned to the S=O bonds of sulfate groups, and also to the acetyl group[2121 Patra, N., Vojtová, L., & Martinová, L. (2015). Deacetylation induced changes in thermal properties of Sterculia urens gum. Journal of Thermal Analysis and Calorimetry, 122(1), 235-240. http://dx.doi.org/10.1007/s10973-015-4680-3.
http://dx.doi.org/10.1007/s10973-015-468...
,2323 Salehi, P., Dashti, Y., Tajabadi, F. M., Safidkon, F., & Rabei, R (2011). Structural and compositional characteristics of a sulfated galactan from the red alga Gracilaria psispérsica. Carbohydrate Polymers, 83(4), 1570-1574. http://dx.doi.org/10.1016/j.carbpol.2010.10.017.
http://dx.doi.org/10.1016/j.carbpol.2010...
]. For this reason, this band is not present in the spectrum of DCHG. The bands appearing at 818 and 584 cm‒1 are due to the asymmetric and symmetric stretching of the O=S=O bonds of the sulfate group, respectively[2424 Cakić, M., Nikolić, G., Ilić, L., & Stanković, S. (2005). Synthesis and FTIR characterization of same dextran sulphates. Chemical Industry & Chemical Engineering Quarterly, 1(2), 74-78. http://dx.doi.org/10.2298/CICEQ0502074C.
http://dx.doi.org/10.2298/CICEQ0502074C...
].
3.1.2 Gel permeation chromatography (GPC)
Figure 1b shows that the modified polysaccharides required a larger volume of eluent for detection. This suggests that sulfation and deacetylation degrade the polysaccharide. The average molar masses (Mw and Mn) of CHG are 15.1 × 106 and 0.42 × 106 g mol‒1, respectively. Average molar masses of the same order of magnitude were reported for a similar polysaccharide (karaya gum), i.e., Mw = 16.1 × 106 and Mn = 1.1 × 106 g mol‒1 [2525 Postulkova, H., Chamradova, I., Pavlinak, D., Humpa, O., Jancar, J., & Vojtova, L. (2017). Study of effects and conditions on the solubility of natural polysaccharide gum karaya. Food Hydrocolloids, 67, 148-156. http://dx.doi.org/10.1016/j.foodhyd.2017.01.011.
http://dx.doi.org/10.1016/j.foodhyd.2017...
]. After deacetylation of CHG, the Mw decreased to 4.8 × 106 g mol‒1. A similar value was observed for deacetylated karaya gum, i.e., 1.8 × 106 g mol‒1 [2222 Padil, V. V. T., Senan, C., & Černík, M. (2015). Dodecenylsuccinic anhydride derivatives of gum karaya (Sterculia urens): Preparation, characterization, and their antibacterial properties. Journal of Agricultural and Food Chemistry, 63(14), 3757-3765. http://dx.doi.org/10.1021/jf505783e. PMid:25797306.
http://dx.doi.org/10.1021/jf505783e...
]. Opposite results, i.e., an increase in the Mw after deacetylation of karaya gum (2‒5 × 106 to 12‒16 × 106 g.mol‒1) was reported by Le Cerf, Irinei, & Muller (1990)[2626 Le Cerf, D. L., Irinei, F., & Muller, G. (1990). Solution properties of gum exudates from Sterculia urens (karaya Gum). Carbohydrate Polymers, 13(4), 375-386. http://dx.doi.org/10.1016/0144-8617(90)90037-S.
http://dx.doi.org/10.1016/0144-8617(90)9...
]. However, the authors explained that deacetylation enables water solubilization of high-Mw polymeric chains at high pH, while solubilization of the original gum without adjusting the pH was only partial, where only fractions of lower Mw were soluble. The molar mass of SCHG declined to 3.8 × 106 g mol‒1 (around four times), but no sulfation studies were found for karaya or CHG to compare.
3.1.3 Thermal analysis (TGA, DSC)
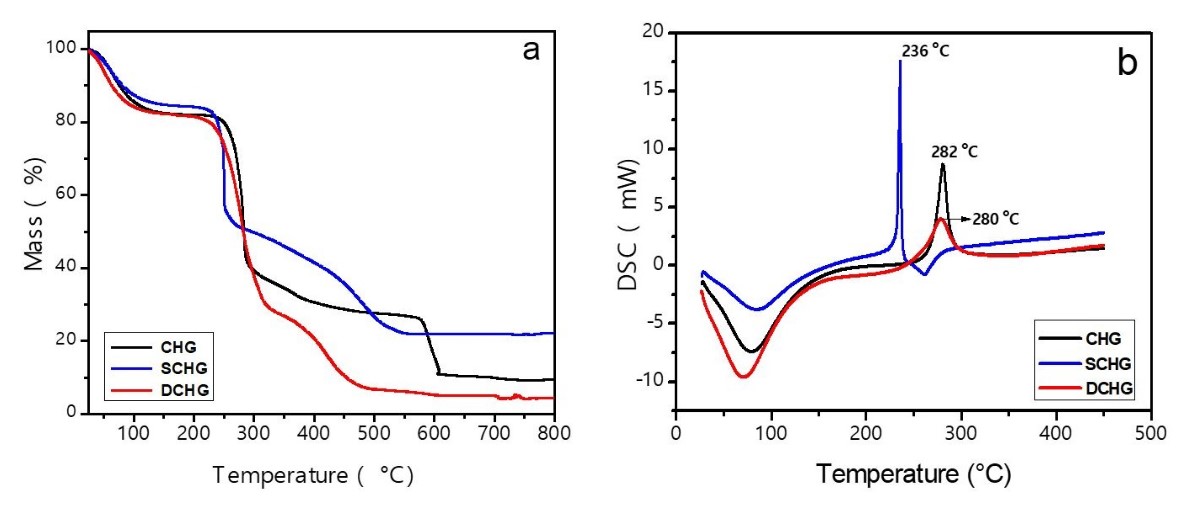
SCHG and DCHG were less thermally stable than the CHG (Figura 2a). Degradation began at a lower temperature for SCHG and DCHG (217.5±0.5 °C) than for CHG (236 °C). The moisture content of CHG is close to that of DCHG (18.1±0.1%) and is greater than that of SCHG (15.7%). These values are in the moisture content range of karaya gum: 13%[2727 Singh, B., Sharma, V., & Pal, L. (2011). Formation of Sterculia polysaccharide networks by gamma rays induced graft copolymerization for biomedical applications. Carbohydrate Polymers, 86(3), 1371-1380. http://dx.doi.org/10.1016/j.carbpol.2011.06.041.
http://dx.doi.org/10.1016/j.carbpol.2011...
] and 20%[2222 Padil, V. V. T., Senan, C., & Černík, M. (2015). Dodecenylsuccinic anhydride derivatives of gum karaya (Sterculia urens): Preparation, characterization, and their antibacterial properties. Journal of Agricultural and Food Chemistry, 63(14), 3757-3765. http://dx.doi.org/10.1021/jf505783e. PMid:25797306.
http://dx.doi.org/10.1021/jf505783e...
]. The moisture content of DCHG was higher than the values reported for deacetylated karaya gum, i.e., 13‒15%[2222 Padil, V. V. T., Senan, C., & Černík, M. (2015). Dodecenylsuccinic anhydride derivatives of gum karaya (Sterculia urens): Preparation, characterization, and their antibacterial properties. Journal of Agricultural and Food Chemistry, 63(14), 3757-3765. http://dx.doi.org/10.1021/jf505783e. PMid:25797306.
http://dx.doi.org/10.1021/jf505783e...
]. More residue was generated at 800 °C for SCHG (21.8%) than for CHG (9.2%) and DCHG (4.6%). The increase in the amount of residue after sulfation is due to the presence of the sodium (Na+) counter ion of the sulfate groups[1313 Pires, N. R., Cunha, P. L. R., Maciel, J. S., Angelim, A. L., Melo, V. M. M., Paula, R. C. M., & Feitosa, J. P. A. (2013). Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydrate Polymers, 91(1), 92-99. http://dx.doi.org/10.1016/j.carbpol.2012.08.011. PMid:23044109.
http://dx.doi.org/10.1016/j.carbpol.2012...
]. The lower value for DCHG compared with that of CHG may be due to unintended purification of the gum during deacetylation.
Figure 2b shows the DSC curves of CHG and its derivatives. Two events were observed for all polysaccharides. The first (endothermic) is related to water loss, and the second (exothermic) to thermal decomposition. The enthalpy of water loss followed the order: DCHG (677 J g‒1) > CHG (575 J g‒1) > SCHG (345 J g‒1). The order was maintained even when the difference in the moisture content of the samples was taken into account. The maximum degradation occurred at 280±2 °C for the CHG and DCHG, and at 236 °C for the sulfated polysaccharide. The enthalpy of degradation was lowest for SCHG (117 J g‒1), whereas the values for DCHG and CHG were close, i.e., 156 and 163 J g‒1, respectively. Sulfation apparently weakened the chemical bonds of the polysaccharides.
3.1.4 Carbon-13 nuclear magnetic resonance (13C-NMR)
Figure 3 shows the 13C-NMR spectrum of SCHG. The signal/noise ratio is somewhat poor; nevertheless, important information can be derived. The spectra of CHG and DCHG presented in Supplementary Material Figure S1 are similar to those reported by Brito et al.[88 Brito, A. C. F., Silva, D. A., Paula, R. C. M., & Feitosa, J. P. A. (2004). Sterculia striata exudate polysaccharide: characterization, rheological properties and comparison with Sterculia urens (karaya) polysaccharide. Polymer International, 53(8), 1025-1032. http://dx.doi.org/10.1002/pi.1468.
http://dx.doi.org/10.1002/pi.1468...
]. The absence of the band at 23.31 ppm ppm in the DCHG spectrum reaffirms the success of deacetylation and corroborates the FTIR results. The spectra of CHG and DCHG show signals at 60.46, 61.70, 61.21, and 61.63 ppm. These peaks are absent in the profile of SCHG, and can be attributed to carbon 6 of the galactose residues[2828 Singh, B., & Singh, B. (2017). Influence of graphene-oxide nanosheets impregnation on properties of Sterculia gum-polyacrylamide hydrogel formed by radiation induced polymerization. International Journal of Biological Macromolecules, 99, 699-712. http://dx.doi.org/10.1016/j.ijbiomac.2017.03.037. PMid:28284934.
http://dx.doi.org/10.1016/j.ijbiomac.201...
,2929 Wang, J., Guo, H., Zhang, J., Wang, X., Zhao, B., Yao, J., & Wang, Y. (2010). Sulfated modification, characterization and structure–antioxidant relationships of Artemisia sphaerocephala polysaccharides. Carbohydrate Polymers, 81(4), 897-905. http://dx.doi.org/10.1016/j.carbpol.2010.04.002.
http://dx.doi.org/10.1016/j.carbpol.2010...
]. Another possibility is that the peaks may be attributed to the 4-O-methyl glucuronic or 4-O-methyl- galacturonic group, but this polysaccharide does not possess these residues[88 Brito, A. C. F., Silva, D. A., Paula, R. C. M., & Feitosa, J. P. A. (2004). Sterculia striata exudate polysaccharide: characterization, rheological properties and comparison with Sterculia urens (karaya) polysaccharide. Polymer International, 53(8), 1025-1032. http://dx.doi.org/10.1002/pi.1468.
http://dx.doi.org/10.1002/pi.1468...
]. The two new peaks that emerged in the 13C-NMR spectrum of SCHG at 68.86 and 70.07 ppm, attributed to the sulfated carbon 6 of the galactose residues, shifted by ~6 ppm to lower-field. The peak at 23.31 ppm in the 13C-NMR spectrum of SCHG is due not only to methyl acetyl groups[88 Brito, A. C. F., Silva, D. A., Paula, R. C. M., & Feitosa, J. P. A. (2004). Sterculia striata exudate polysaccharide: characterization, rheological properties and comparison with Sterculia urens (karaya) polysaccharide. Polymer International, 53(8), 1025-1032. http://dx.doi.org/10.1002/pi.1468.
http://dx.doi.org/10.1002/pi.1468...
,2525 Postulkova, H., Chamradova, I., Pavlinak, D., Humpa, O., Jancar, J., & Vojtova, L. (2017). Study of effects and conditions on the solubility of natural polysaccharide gum karaya. Food Hydrocolloids, 67, 148-156. http://dx.doi.org/10.1016/j.foodhyd.2017.01.011.
http://dx.doi.org/10.1016/j.foodhyd.2017...
], but also to the methyl groups of sulfated rhamnose, and was shifted by ~4 ppm. The 13C-NMR spectra of sulfated materials are known to be more complicated because the sulfate groups withdraw electrons from the carbons to which they bond, shifting the signals of these carbons to lower-field, and donate electrons to neighboring carbons, shifting these carbon signals to higher-field[2929 Wang, J., Guo, H., Zhang, J., Wang, X., Zhao, B., Yao, J., & Wang, Y. (2010). Sulfated modification, characterization and structure–antioxidant relationships of Artemisia sphaerocephala polysaccharides. Carbohydrate Polymers, 81(4), 897-905. http://dx.doi.org/10.1016/j.carbpol.2010.04.002.
http://dx.doi.org/10.1016/j.carbpol.2010...
,3030 Yang, X. B., Gao, X. D., Han, F., & Tan, R. X. (2005). Sulfation of a polysaccharide produced by a marine filamentous fungus Phoma herbarum YS4108 alters its antioxidant properties in vitro. Biochimica et Biophysica Acta, 1725(1), 120-127. http://dx.doi.org/10.1016/j.bbagen.2005.06.013. PMid:16054758.
http://dx.doi.org/10.1016/j.bbagen.2005....
].
3.2 Enzymes and lignins related to plant defense in ‘Pink Lady’ apples exposed to elicitors
The activity of the guaiacol peroxidase (GPX) and polyphenoloxidase (PPO) enzymes was recorded for gum-treated and water-treated (Ctrl‒) ‘Pink Lady’ apple pulps. The BTH was adopted as a positive control to test the elicitor properties of the gums. The GPX activity increased significantly (p<0.05) with DCHG and SCHG at 12‒48 h (Figure 4a and b). BTH (0.4 mg mL‒1) induced increase in the GPX activity at 12 h, while there was no significant increase (p>0.05) at the other times. GPX plays an essential role in lignin biosynthesis in plant tissues as these enzymes catalyze the cross-linking of phenylpropanoid route monomers (coniferyl, sinapyl, and p-coumaryl alcohols) and their coupling in the subunits of heterogeneous polyphenols. Lignin is associated with the induction of plant defense as it strengthens the cell walls, making it difficult for pathogens to enter[3131 Lee, M.-H., Jeon, H. S., Kim, S. H., Chung, J. H., Roppolo, D., Lee, H.-J., Cho, H. J., Tobimatsu, Y., Ralph, J., & Park, O. K. (2019). Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. The EMBO Journal, 38(23), e101948. http://dx.doi.org/10.15252/embj.2019101948. PMid:31559647.
http://dx.doi.org/10.15252/embj.20191019...
,3232 Passardi, F., Cosio, C., Penel, C., & Dunand, C. (2005). Peroxidases have more functions than a Swiss army knife. Plant Cell Reports, 24(5), 255-265. http://dx.doi.org/10.1007/s00299-005-0972-6. PMid:15856234.
http://dx.doi.org/10.1007/s00299-005-097...
].
GPX activity in Pink Lady apples after spraying with distilled water (control, Ctrl), and DCHG (a), SCHG (b), and BTH (c).
The activity of PPO in apple pulp increased (p<0.05) in the interval 12‒48 h exposure to DCHG (Figure 5b), as reported for GPX. However, in apples exposed to SCHG and Bion, a more transient PPO response was triggered. PPO activity peaked for 24 h for both, but much less intense for apples treated with Bion. At 48 and 72 h, PPO activity was less than to control for fruits treated with SCHG. There are few specific studies to reveal details of the post-harvest enzyme responses in apples. What can be factually inferred is that SCHG gum apparently could promote greater GPX responses at the expense of PPO, and this seems to indicate that GPX may have compensated PPO in the enhanced polymerization of the lignin monomers observed in SCHG treated apples. PPOs are also considered enzymes that are markers of the resistance of plants against pathogens. These enzymes oxidize phenolic compounds to toxic quinones that can act against invading pathogens[33 Abouraïcha, E. F., El Alaoui-Talibi, Z., Tadlaoui-Ouafi, A., El Boutachfaiti, R., Petit, E., Douira, A., Courtois, B., Courtois, J., & El Modafar, C. (2017). Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. Journal of Applied Phycology, 29(1), 471-480. http://dx.doi.org/10.1007/s10811-016-0926-0.
http://dx.doi.org/10.1007/s10811-016-092...
] and are key enzymes for the synthesis of lignin[3333 Lu, Y.-C., Lu, Y., & Fan, X. (2020). Structure and Characteristics of Lignin. In S. Sharma & A. Kumar. Lignin biosynthesis and transformation for industrial applications (pp. 31-32). Switzerland: Springer. http://dx.doi.org/10.1007/978-3-030-40663-9_2
http://dx.doi.org/10.1007/978-3-030-4066...
].
PPO activity in Pink Lady apples after spraying with distilled water (control, Ctrl), and DCHG (a), SCHG (b), and BTH (c).
Because the two enzymes analyzed are involved in the synthesis of lignin, the content of lignin in the fruits was quantified 72 h after treatment (Figure 6). As expected, due to the increase in the activity of GPX and PPO, the fruits treated with DCHG, SCHG, and BTH presented lignin levels numerically higher than that of the control. However, only the fruits treated with SCHG showed a significant (p<0.05) increase in the lignin content. The lignin content of the fruits treated with DCHG and BTH was statistically similar to that of the control (p>0.05). The weak induction of defense compounds (GPX, PPO and lignin) of BTH in “Pink Lady” apples can be explained by the reported low performance of BTH in this cultivar. Marolleau et al.[1010 Marolleau, B., Gaucher, M., Heintz, C., Degrave, A., Warneys, R., Orain, G., Lemarquand, A., & Brisset, M. N. (2017). When a plant resistance inducer leaves the lab for the field: integrating ASM into routine apple protection practices. Frontiers of Plant Science, 8, 1938. http://dx.doi.org/10.3389/fpls.2017.01938. PMid:29255473.
http://dx.doi.org/10.3389/fpls.2017.0193...
] treated Elstar, Fuji, Gala, Golden, and “Pink Lady” apple cultivars with BTH, and reported that the “Pink Lady” cultivar had the lowest level of defense induction. The results suggest 'de novo' lignin synthesis triggered by the gums, mainly SCHG, in “Pink Lady” apples. The gum that induced the strongest defense response is the one that simultaneously possesses the three residues suggested by the literature as responsible for the induction of defense compounds: rhamnose, uronic acid, and sulfate groups. This work reaffirms the importance of these residues and suggests that plant exudate polysaccharides should be further explored, as they may be a promising source of defense-inducing compounds in plants.
Lignin content (TGA derivatives, mg g‒1 FW) extracted from apple (cv. Pink Lady) pulps 72 h after spraying with elicitors. The control (Ctrl‒) apples were sprayed with distilled water. Same letters indicate that the values did not differ significantly according to Tukey’s test (p>0.05).
4. Conclusions
The polysaccharide from Sterculia striata (chichá gum,CHG) was deacetylated (DCHG) and, for the first time, sulfated (SCHG). The derivatives were less thermally stable and the average molar mass was lower than that of the original gum. Sulfation occurred at carbon 6 of the galactose and rhamnose residues. Both derivatives of CHG have the ability to induce increased defense-related enzyme activity in “Pink Lady” apples. The amount of lignin extracted from the apples was greater than the control only for fruits sprayed with SCHG. The best defense induction was achieved with the polysaccharide (SCHG) that contained the three subunits associated with plant defense: rhamnose, uronic acid, and sulfate groups. This study demonstrates that plant exudate can be an abundant, renewable and safety source of elicitors.
Supplementary Material
Supplementary material accompanies this paper.
Table S1: Molar mass of crude (GCH), sulfated (SCHG) and deacetylated (DCHG) chichá gum.
Figure S1: Carbon-13 nuclear magnetic resonance spectra of crude (CHG) and deacetylated (DCHG) chichá gum.
This material is available as part of the online article from http://www.scielo.br/POLIMEROS
https://minio.scielo.br/documentstore/1678-5169/cgGR3syLKsQZb68KhMYRwjq/a571cc5d4d119d7b59f8d493fa9b3627bbef4b08.pdf5. Acknowledgements
This work was supported by the Coordination Foundation for the Improvement of Higher Education Personnel (CAPES-Brazil), CNPq, the Cearense Foundation for Research Support (FUNCAP-CE-Brazil), INOMAT, and the Brazilian Agricultural Research Corporation (Embrapa-Brazil).
-
How to cite: Lima, C. P. C., Oster, A. H., Cavalcanti, F. R., Paula, R. C. M., & Feitosa, J. P. A. (2021). Induction of defense in apples by sulfated and deacetylated chichá gum. Polímeros: Ciência e Tecnologia, 31(1), e2021010. https://doi.org/10.1590/0104-1428.08820
6. References
-
1Iriti, M., & Vitalini, S. (2021). Plant immunity and crop yield: a sustainable approach in agri-food systems. Vaccines, 9(2), 1-3. http://dx.doi.org/10.3390/vaccines9020121. PMid:33546315.
» https://doi.org/10.3390/vaccines9020121 -
2Abouraïcha, E., El Alaoui-Talibi, Z., El Boutachfaiti, R., Petit, E., Courtois, B., Courtois, J., & El Modafar, C. (2015). Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Scientia Horticulturae, 181, 121-128. http://dx.doi.org/10.1016/j.scienta.2014.11.002
» http://dx.doi.org/10.1016/j.scienta.2014.11.002 -
3Abouraïcha, E. F., El Alaoui-Talibi, Z., Tadlaoui-Ouafi, A., El Boutachfaiti, R., Petit, E., Douira, A., Courtois, B., Courtois, J., & El Modafar, C. (2017). Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. Journal of Applied Phycology, 29(1), 471-480. http://dx.doi.org/10.1007/s10811-016-0926-0
» http://dx.doi.org/10.1007/s10811-016-0926-0 -
4Stadnik, M. J., & Freitas, M. B. (2014). Algal polysaccharides as source of plant resistance inducers. Tropical Plant Pathology, 39(2), 111-118. http://dx.doi.org/10.1590/S1982-56762014000200001
» http://dx.doi.org/10.1590/S1982-56762014000200001 -
5Ramkissoon, A., Ramsubhag, A., & Jayaraman, J. (2017). Phytoelicitor activity of three Caribbean seaweed species on suppression of pathogenic infections in tomato plants. Journal of Applied Phycology, 29(6), 3235-3244. http://dx.doi.org/10.1007/s10811-017-1160-0
» http://dx.doi.org/10.1007/s10811-017-1160-0 -
6El Modafar, C., Elgadda, M., El Boutachfaiti, R., Abouraicha, E., Zehhara, N., & Petit, E. (2012). Induction of natural defence accompanied by salicylic acid dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Scientia Horticulturae, 138, 55-63. http://dx.doi.org/10.1016/j.scienta.2012.02.011
» http://dx.doi.org/10.1016/j.scienta.2012.02.011 -
7Brito, A. C. F., Sierakowski, M. R., Reicher, F., Feitosa, J. P. A., & Paula, R. C. M. (2005). Dynamic rheological study of Sterculia striata and karaya polysaccharides in aqueous solution. Food Hydrocolloids, 19(5), 861-867. http://dx.doi.org/10.1016/j.foodhyd.2004.10.035
» http://dx.doi.org/10.1016/j.foodhyd.2004.10.035 -
8Brito, A. C. F., Silva, D. A., Paula, R. C. M., & Feitosa, J. P. A. (2004). Sterculia striata exudate polysaccharide: characterization, rheological properties and comparison with Sterculia urens (karaya) polysaccharide. Polymer International, 53(8), 1025-1032. http://dx.doi.org/10.1002/pi.1468
» http://dx.doi.org/10.1002/pi.1468 -
9Tzatzarakis, M., Kokkinakis, M., Renieri, E., Goumenou, M., Kavvalakis, M., Vakonaki, E., Chatzinikolaou, A., Stivaktakis, P., Tsakiris, I., Rizos, A., & Tsatsakis, A. (2020). Multiresidue analysis of insecticides and fungicides in apples from the Greek market. Applying an alternative approach for risk assessment. Food and Chemical Toxicology, 140, 111262. http://dx.doi.org/10.1016/j.fct.2020.111262 PMid:32198030.
» http://dx.doi.org/10.1016/j.fct.2020.111262 -
10Marolleau, B., Gaucher, M., Heintz, C., Degrave, A., Warneys, R., Orain, G., Lemarquand, A., & Brisset, M. N. (2017). When a plant resistance inducer leaves the lab for the field: integrating ASM into routine apple protection practices. Frontiers of Plant Science, 8, 1938. http://dx.doi.org/10.3389/fpls.2017.01938 PMid:29255473.
» http://dx.doi.org/10.3389/fpls.2017.01938 -
11SYNGENTA. (2015). Material safety data sheet (MSDS) Byon®, Syngenta, 4/16/2015. Retrieved in 2020, October 5, from https://assets.greenbook.net/M114161.pdf
» https://assets.greenbook.net/M114161.pdf -
12McGregor, D., Boobis, A., Binaglia, M., Botham, P., Hoffstadt, L., Hubbard, S., Petry, T., Riley, A., Schwartz, D., & Hennes, C. (2010). Guidance for the classification of carcinogens under the Globally Harmonised System of Classification and Labelling of Chemicals (GHS). Critical Reviews in Toxicology, 40(3), 245-285. http://dx.doi.org/10.3109/10408440903384717 PMid:20014893.
» http://dx.doi.org/10.3109/10408440903384717 -
13Pires, N. R., Cunha, P. L. R., Maciel, J. S., Angelim, A. L., Melo, V. M. M., Paula, R. C. M., & Feitosa, J. P. A. (2013). Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydrate Polymers, 91(1), 92-99. http://dx.doi.org/10.1016/j.carbpol.2012.08.011 PMid:23044109.
» http://dx.doi.org/10.1016/j.carbpol.2012.08.011 -
14Dupont, A.-L. (2002). Study of the degradation of gelatin in paper upon aging using aqueous size-exclusion chromatography. Journal of Chromatography. A, 950(1-2), 113-124. http://dx.doi.org/10.1016/S0021-9673(02)00010-9 PMid:11990984.
» http://dx.doi.org/10.1016/S0021-9673(02)00010-9 -
15Cavalcanti, F. R., Resende, M. L. V., Carvalho, C. P. S., Silveira, J. A. G., & Oliveira, J. T. A. (2007). An aqueous suspension of Crinipellis perniciosa mycelium activates tomato defence responses against Xanthomonas vesicatoria. Crop Protection (Guildford, Surrey), 27(5), 729-738. http://dx.doi.org/10.1016/j.cropro.2006.06.012
» http://dx.doi.org/10.1016/j.cropro.2006.06.012 -
16Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3 PMid:942051.
» http://dx.doi.org/10.1016/0003-2697(76)90527-3 -
17Monties, B. (1989). Lignins. In P. M. Dey, & J. B. Harborne (Eds.), Methods in plant biochemistry (Vol. 1, pp. 113-158). New York: Academic Press. https://doi.org/10.1016/B978-0-12-461011-8.50010-X
» https://doi.org/10.1016/B978-0-12-461011-8.50010-X -
18Xing, R., Liu, S., Yu, H., Guo, Z., Li, Z., & Li, P. (2005). Preparation of high molecular weight and high sulfate content chitosans and their potential antioxidant activity in vitro. Carbohydrate Polymers, 61(2), 148-154. http://dx.doi.org/10.1016/j.carbpol.2005.04.007
» http://dx.doi.org/10.1016/j.carbpol.2005.04.007 -
19Vikhoreva, G., Bannikova, G., Stolbushkina, P., Panov, A., Drozd, N., Makarov, V., Varlamov, V., & Galbraikh, L. (2005). Preparation and anticoagulant activity of a low-molecular-weight sulfated chitosan. Carbohydrate Polymers, 62(4), 327-332. http://dx.doi.org/10.1016/j.carbpol.2005.05.022
» http://dx.doi.org/10.1016/j.carbpol.2005.05.022 -
20Gangapuram, B. R., Bandi, R., Dadigala, R., Kotu, G. M., & Guttena, V. (2017). Facile green synthesis of gold nanoparticles with carboxymethyl gum karaya, selective and sensitive colorimetric detection of copper (II) ions. Journal of Cluster Science, 28(5), 2873-2890. http://dx.doi.org/10.1007/s10876-017-1264-3
» http://dx.doi.org/10.1007/s10876-017-1264-3 -
21Patra, N., Vojtová, L., & Martinová, L. (2015). Deacetylation induced changes in thermal properties of Sterculia urens gum. Journal of Thermal Analysis and Calorimetry, 122(1), 235-240. http://dx.doi.org/10.1007/s10973-015-4680-3
» http://dx.doi.org/10.1007/s10973-015-4680-3 -
22Padil, V. V. T., Senan, C., & Černík, M. (2015). Dodecenylsuccinic anhydride derivatives of gum karaya (Sterculia urens): Preparation, characterization, and their antibacterial properties. Journal of Agricultural and Food Chemistry, 63(14), 3757-3765. http://dx.doi.org/10.1021/jf505783e PMid:25797306.
» http://dx.doi.org/10.1021/jf505783e -
23Salehi, P., Dashti, Y., Tajabadi, F. M., Safidkon, F., & Rabei, R (2011). Structural and compositional characteristics of a sulfated galactan from the red alga Gracilaria psispérsica. Carbohydrate Polymers, 83(4), 1570-1574. http://dx.doi.org/10.1016/j.carbpol.2010.10.017
» http://dx.doi.org/10.1016/j.carbpol.2010.10.017 -
24Cakić, M., Nikolić, G., Ilić, L., & Stanković, S. (2005). Synthesis and FTIR characterization of same dextran sulphates. Chemical Industry & Chemical Engineering Quarterly, 1(2), 74-78. http://dx.doi.org/10.2298/CICEQ0502074C
» http://dx.doi.org/10.2298/CICEQ0502074C -
25Postulkova, H., Chamradova, I., Pavlinak, D., Humpa, O., Jancar, J., & Vojtova, L. (2017). Study of effects and conditions on the solubility of natural polysaccharide gum karaya. Food Hydrocolloids, 67, 148-156. http://dx.doi.org/10.1016/j.foodhyd.2017.01.011
» http://dx.doi.org/10.1016/j.foodhyd.2017.01.011 -
26Le Cerf, D. L., Irinei, F., & Muller, G. (1990). Solution properties of gum exudates from Sterculia urens (karaya Gum). Carbohydrate Polymers, 13(4), 375-386. http://dx.doi.org/10.1016/0144-8617(90)90037-S
» http://dx.doi.org/10.1016/0144-8617(90)90037-S -
27Singh, B., Sharma, V., & Pal, L. (2011). Formation of Sterculia polysaccharide networks by gamma rays induced graft copolymerization for biomedical applications. Carbohydrate Polymers, 86(3), 1371-1380. http://dx.doi.org/10.1016/j.carbpol.2011.06.041
» http://dx.doi.org/10.1016/j.carbpol.2011.06.041 -
28Singh, B., & Singh, B. (2017). Influence of graphene-oxide nanosheets impregnation on properties of Sterculia gum-polyacrylamide hydrogel formed by radiation induced polymerization. International Journal of Biological Macromolecules, 99, 699-712. http://dx.doi.org/10.1016/j.ijbiomac.2017.03.037 PMid:28284934.
» http://dx.doi.org/10.1016/j.ijbiomac.2017.03.037 -
29Wang, J., Guo, H., Zhang, J., Wang, X., Zhao, B., Yao, J., & Wang, Y. (2010). Sulfated modification, characterization and structure–antioxidant relationships of Artemisia sphaerocephala polysaccharides. Carbohydrate Polymers, 81(4), 897-905. http://dx.doi.org/10.1016/j.carbpol.2010.04.002
» http://dx.doi.org/10.1016/j.carbpol.2010.04.002 -
30Yang, X. B., Gao, X. D., Han, F., & Tan, R. X. (2005). Sulfation of a polysaccharide produced by a marine filamentous fungus Phoma herbarum YS4108 alters its antioxidant properties in vitro. Biochimica et Biophysica Acta, 1725(1), 120-127. http://dx.doi.org/10.1016/j.bbagen.2005.06.013 PMid:16054758.
» http://dx.doi.org/10.1016/j.bbagen.2005.06.013 -
31Lee, M.-H., Jeon, H. S., Kim, S. H., Chung, J. H., Roppolo, D., Lee, H.-J., Cho, H. J., Tobimatsu, Y., Ralph, J., & Park, O. K. (2019). Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. The EMBO Journal, 38(23), e101948. http://dx.doi.org/10.15252/embj.2019101948 PMid:31559647.
» http://dx.doi.org/10.15252/embj.2019101948 -
32Passardi, F., Cosio, C., Penel, C., & Dunand, C. (2005). Peroxidases have more functions than a Swiss army knife. Plant Cell Reports, 24(5), 255-265. http://dx.doi.org/10.1007/s00299-005-0972-6 PMid:15856234.
» http://dx.doi.org/10.1007/s00299-005-0972-6 -
33Lu, Y.-C., Lu, Y., & Fan, X. (2020). Structure and Characteristics of Lignin. In S. Sharma & A. Kumar. Lignin biosynthesis and transformation for industrial applications (pp. 31-32). Switzerland: Springer. http://dx.doi.org/10.1007/978-3-030-40663-9_2
» http://dx.doi.org/10.1007/978-3-030-40663-9_2
Publication Dates
-
Publication in this collection
09 July 2021 -
Date of issue
2021
History
-
Received
01 Oct 2020 -
Reviewed
28 Feb 2021 -
Accepted
24 Mar 2021