IMAGEM EM MEDICINA
Renal replacement lipomatosis and xanthogranulomatous pyelonephritis: differential diagnosis
Frederico R. RomeroI; Roberto PilatiII; Maria Fernanda Sales Ferreira CabocloIII; Antônio de Pádua Gomes SilvaIV; Marco Aurélio CravoV; Thadeu Brenny FilhoII
IPhD in General Surgery at Universidade Federal do Paraná; Urologist at Hospital São Vicente de Curitiba, Curitiba, PR
IIMD, Urologist, Hospital São Vicente de Curitiba, Curitiba, PR
IIIMD, Radiologist, Hospital São Vicente de Curitiba, Curitiba, PR
IVMD, Pathologist, Citopar - Centro de Citopatologia Paraná Ltda., Curitiba, PR
VMD, Pathologist, Consulpat - Laboratório de Patologia e Citologia, Curitiba, PR
Correspondence to Correspondence to: Frederico R. Romero Rua Emiliano Perneta, 653 - apto. 41 Curitiba - PR - CEP: 80420-080 frederico.romero@gmail.com
Renal replacement lipomatosis (RRL) is a relatively uncommon entity, although misdiagnosis - mainly with xanthogranulomatous pyelonephritis (XGP) - due to lack of awareness by urologists, radiologists, and pathologists may be responsible for underreporting1,2.
We illustrate a case of RRL that was initially misdiagnosed as XGP, and compare it with a classic case of XGP, underscoring the similarities and the differences between them.
Patient 1
A 63 year-old morbid obese (BMI = 52 kg/m2) female was admitted to the hospital with right flank pain. She had a medical history of open cholecystectomy and inferior median laparotomy for gynaecological surgery. Investigation revealed urinary tract infection with E. coli and Klebsiella sp. Creatinine clearance was 80 mL/min and hemogram was unremarkable.
Plain radiography demonstrated a right renal staghorn calculus. Computed tomography (CT) scan showed an enlarged right kidney with hydronephrosis, parenchymal atrophy, and calculi located in the right renal pelvis, with marked fatty proliferation within the right renal sinus (Figure 1A). The case was initially misdiagnosed as XGP, and transperitoneal laparoscopic nephrectomy was offered to the patient.
During surgery, large amount of perirenal and hilar fat was identified. Renal artery and vein were dissected from surrounding fat tissue, and sequentially clipped with Hem-o-lok clips. There were no perirenal adhesions or infiltration, commonly observed when approaching XGP. The kidney was dissected free within Gerota's fascia and removed through the previous median laparotomy incision. Operative time was 180 minutes and estimated blood loss was 300 mL. The specimen was 10 x 8 x 7 cm in size, and was pathologically diagnosed as renal replacement lypomatosis (Figure 2). Postoperative course was uneventful and the patient was discharged home at day number 3.
Patient 2
A 51 year-old obese (BMI = 32 kg/m2) female was admitted to the hospital with right flank pain. She had a history of weight loss of 20 kg in the last 12 months, and open surgical drainage for a right flank abscess five months earlier that persisted with small amount of purulent discharge until one month previously. Investigation revealed urinary tract infection with E. coli.
Plain radiography demonstrated a right renal staghorn calculus. A CT scan showed an enlarged right kidney with hydronephrosis, calculi located in the right renal pelvis, multiple low attenuation areas with peripheral parenchyma enhancement, thickening of Gerota's fascia, and densification of perirenal and periureteral fat, all of which suggested the diagnosis of XGP. There were no residual perinephric collections (Figure 1B).
During right-sided transperitoneal laparoscopic nephrectomy, there were intense perirenal adhesions, with firm attachments between Gerota's fascia and the anterior aspect of inferior vena cava (IVC). While trying to identify the plane between the IVC and the ureter at the lower pole of the kidney, we inadvertently entered the interaortocaval space and, without realizing it, lifted the IVC anteriorly. Dissection over this area resulted in accidental tearing of a lumbar vein, which was managed laparoscopically.
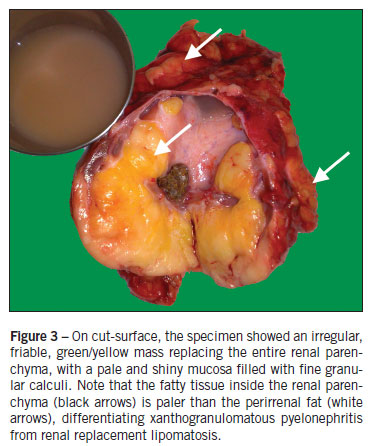
Renal artery and vein were not distinctly identified. All vascular structures were controlled with Hem-o-lok clips and harmonic scalpel. The kidney was dissected free inside Gerota's fascia, with small purulent discharge during dissection of the upper pole. The specimen was removed through the posterior lumbar incision used for abscess drainage. Operative time was 150 minutes. Estimated blood loss was 500 mL, and the patient received two units of blood intraoperatively. The specimen was 11 x 9 x 8 cm in size, with a fibrous, lobulated, yellow-tan capsule. Pathology confirmed the diagnosis of xanthogranulomatous pyelonephritis (Figure 3). Postoperatively, she received ceftriaxone and metronidazole for 7 days, with no complications except for one episode of unexplained fever, and was discharged home on post-operative day number 8.
Discussion
Renal replacement lypomatosis and xanthogranulomatous pyelonephritis have similar etiopathogenic, clinical and radiological features. Both are characterized by atrophy and destruction of renal parenchyma, often associated with unilateral chronic renal infection, hydronephrosis or pyonephrosis, and calculous disease. The main difference between them is that in RRL, first reported by Brown in 18613, the atrophic renal parenchyma is replaced by fatty tissue proliferation3-6. In XGP, initially described as staphylomycosis in 1916 by Schlogenhaufer, xanthoma cells (lipid-laden macrophages) infiltrate and substitute necrotic renal tissue resulting in a lipomatous degeneration4,7;.
RRL, renal sinus lipomatosis, and fibrolipomatosis of the kidney represent a spectrum of changes in which normal renal sinus and perirenal fat increase in amount and replace the renal parenchyma. Renal sinus lipomatosis, the mildest form, is associated with obesity, renal atrophy of varying causes (e.g. aging and atherosclerosis), Cushing's syndrome or the use of exogenous steroids4-6. This mild form infrequently produces symptoms because of the absence of caliceal obstruction5, and is a common finding at autopsy4. Invasion of adipose cells from the peripelvic fat into the kidney occurs along the blood vessels in the renal sinus3. At the other end of the spectrum is RRL, where the entire renal parenchyma is replaced with adipose tissue, usually secondary to calculous disease and longstanding inflammatory/infectious disease (e.g. renal tuberculosis)4,6. In this group there is no invasion from without. Proliferation of fat cells in the interstitium of the kidney is mediated by connective tissue cells3.
Pathogenesis of XGP is not fully understood. It begins within the pelvis and calyces and subsequently extends into and destroys renal parenchymal and adjacent tissues. All theories agree that the primary factors involved in the development of XGP are bacterial infection, obstruction and calculous disease. Other possible interrelated factors include venous occlusion and hemorrhage, abnormal lipid metabolism, lymphatic blockage, failure of antimicrobial therapy, altered immunologic competence, and renal ischemia7.
RRL and XGP usually occur between the fifth and seventh decade of life. The patient may be asymptomatic or present a varied clinical picture related to the primary disease, the most frequent manifestations being urinary tract infections, flank pain, weight loss, hematuria, fever, and palpable mass6-8.
CT scan is the most valuable method for differentiating RRL and XGP8. In XGP, it demonstrates a large reniform mass with a central staghorn calculus, and peripheral enhancement that may correspond to compressed residual parenchyma or a capsule of inflammatory tissue8-10. Renal parenchyma is replaced by multiple low attenuation (-15 to 29 HU) areas with radial distribution, which represent dilated calyces and abscess cavities filled with pus and debris, frequently described as a "bunch of grapes" or a "bear claw"8,10. True fat density is usually not seen8. Air inside the urinary tract and perinephric extension to adjacent organs are also indicative of XGP9,10. In RRL, the characteristic distribution of adipose mass within the renal sinus and perirenal space, with areas of negative attenuation values similar to those of adipose tissue (confirmed by a value of -20 HU or lower) help in the diagnosis5,6. Even though the CT scan illustrated in Figure 1A is characteristic of RRL, we missed the diagnosis because of our lack of knowledge about this clinical entity.
During surgery, although the fat in renal lipomatosis is tougher and more fibrous than normal fat3, severe adhesions and infiltration observed in XGP are usually not present in RRL. Further, the renal hilum may not be distinguished within the dense perihilar fibrosis present in XGP, while it is relatively easily dissected in renal lipomatosis. This is especially important when approaching the kidney by laparoscopy. XGP is considered a relative contraindication to laparoscopy because of increased difficulty, higher morbimortality, and greater conversion rates. However, patients diagnosed with XGP and described as having no adhesions, infiltrations or fibrosis during laparoscopic nephrectomy should be critically investigated as having RRL misdiagnosed as XGP, as in the first patient illustrated herein.
Pathologically, the kidney is usually enlarged and presents with a gross fibrofatty appearance. When the specimen is opened, only a thin rim or shell of atrophied renal parenchyma if found, with bright-yellow fat tissue in the renal sinus that is similar to the perirenal fat in RRL, and a pale-yellow fatty tissue in XGP. Histologically, there is increase of lipid-laden macrophages (xanthoma cells) inside the renal parenchyma in XGP, whereas RRL contains large fat cells outside the renal parenchyma2,4,5, with sharp demarcation between the adipose tissue and the renal parenchyma, showing that there is no real invasion of the kidney by fat but merely replacement of fat as it atrophies1,3,4.
Additionally though very rare, XGP and RLL may coexist. Other focal fatty lesions such as lipomas, angiomyolipomas, and liposarcomas must be considered. These lesions, however, are usually not associated with parenchymal atrophy or staghorn calculi, and frequently produce a mass effect on the intrarenal collecting system2,5.
Renal replacement lipomatosis should always be kept in mind by clinicians, urologists, and radiologists when evaluating a patient with suspicion of xanthogranulomatous pyelonephritis. Specific imaging, operative, and pathological differences may provide clues for the differential diagnosis.
Study conducted at Hospital São Vicente de Curitiba; Citopar - Centro de Citopatologia Paraná Ltda; Consulpat - Laboratório de Patologia e Citologia, Curitiba, PR
- 1. Shah VB, Rupani AB, Deokar MS, Pathak HR. Idiopathic renal replacement lipomatosis: a case report and review of literature. Indian J Pathol Microbiol. 2009;52:552-3.
- 2. Xu Y, Liu RL, Zhang ZH, Zhao WM, Yang QC. Renal replacement lipomatosis. Eur Surg Res. 2006;38:385-7.
- 3. Dukes CE. The pathology of renal lipomatosis. Proc Royal Soc Med. 1938;31:1361-4.
- 4. Ambos MA, Bosniak MA, Gordon R, Madayag MA. Replacement lipomatosis of the kidney. AJR Am J Roentgenol. 1978;130:1087-91.
- 5. Kocaoglu M, Bozlar U, Sanal HT, Guvenc I. Replacement lipomatosis: CT and MRI findings of a rare renal mass. Br J Radiol. 2007;80:e287-9.
- 6. Yagci C, Kosucu P, Yorubulut M, Akyar S. Renal replacement lipomatosis: ultrasonography and computed tomography findings. Eur Radiol. 1999;9:1599-601.
- 7. Sharma S, Jhobta A, Goyal D, Surya M, Sumala, Negi A. Ureteral involvement in xanthogranulomatous pyelonephritis - rare manifestation. Ind J Radiol Imag. 2006;16:243-5.
- 8. D'Ippolito G, Tokechi D, Shigueoka DC, Ajzen S. Tomographic aspects of xanthogranulomatous pyelonephritis and related complications. São Paulo Med J. 1996;114:1091-6.
- 9. Calisir C, Can C, Kebapci M. Renal replacement lipomatosis: multidetector-row computed tomography findings in one case. Acta Radiol. 2007;48:242-5.
- 10. Korkes F, Favoretto RL, Bróglio M, Silva CA, Castro MG, Perez MD. Xanthogranulomatous pyelonephritis: clinical experience with 41 cases. Urology 2008;71:178-80.
Correspondence to:
Publication Dates
-
Publication in this collection
10 June 2011 -
Date of issue
June 2011