Abstract
OBJECTIVE: Sepsis is a common condition encountered in hospital environments. There is no effective treatment for sepsis, and it remains an important cause of death at intensive care units. This study aimed to discuss some methods that are available in clinics, and tests that have been recently developed for the diagnosis of sepsis. METHODS: A systematic review was performed through the analysis of the following descriptors: sepsis, diagnostic methods, biological markers, and cytokines. RESULTS: The deleterious effects of sepsis are caused by an imbalance between the invasiveness of the pathogen and the ability of the host to mount an effective immune response. Consequently, the host's immune surveillance fails to eliminate the pathogen, allowing it to spread. Moreover, there is a pro-inflammatory mediator release, inappropriate activation of the coagulation and complement cascades, leading to dysfunction of multiple organs and systems. The difficulty achieve total recovery of the patient is explainable. There is an increased incidence of sepsis worldwide due to factors such as aging population, larger number of surgeries, and number of microorganisms resistant to existing antibiotics. CONCLUSION: The search for new diagnostic markers associated with increased risk of sepsis development and molecules that can be correlated to certain steps of sepsis is becoming necessary. This would allow for earlier diagnosis, facilitate patient prognosis characterization, and prediction of possible evolution of each case. All other markers are regrettably constrained to research units.
Sepsis; methods; cytokines
REVIEW ARTICLE
Diagnostic methods in sepsis: the need of speed
Fernando Rodrigues CoelhoI; Joilson Oliveira MartinsII
ILaboratory Specialist, Instituto de Química, Universidade de São Paulo (USP), São Paulo, SP, Brazil
IIPhD, Assistant Professor, Faculty of Pharmaceutical Sciences, USP, São Paulo, SP, Brazil
Correspondence to Correspondence to: Joilson Oliveira Martins Laboratory of Immunoendocrinology Department of Clinical and Toxicological Analyses Faculty of Pharmaceutical Sciences Universidade de São Paulo Av. Prof. Lineu Prestes, 580 Bloco 17 São Paulo - SP, Brazil CEP: 05508-900 Phone: +55 (11) 3091-3662 Fax: +55 (11) 3813-2197 martinsj@usp.br
SUMMARY
OBJECTIVE: Sepsis is a common condition encountered in hospital environments. There is no effective treatment for sepsis, and it remains an important cause of death at intensive care units. This study aimed to discuss some methods that are available in clinics, and tests that have been recently developed for the diagnosis of sepsis.
METHODS: A systematic review was performed through the analysis of the following descriptors: sepsis, diagnostic methods, biological markers, and cytokines.
RESULTS: The deleterious effects of sepsis are caused by an imbalance between the invasiveness of the pathogen and the ability of the host to mount an effective immune response. Consequently, the host's immune surveillance fails to eliminate the pathogen, allowing it to spread. Moreover, there is a pro-inflammatory mediator release, inappropriate activation of the coagulation and complement cascades, leading to dysfunction of multiple organs and systems. The difficulty achieve total recovery of the patient is explainable. There is an increased incidence of sepsis worldwide due to factors such as aging population, larger number of surgeries, and number of microorganisms resistant to existing antibiotics.
CONCLUSION: The search for new diagnostic markers associated with increased risk of sepsis development and molecules that can be correlated to certain steps of sepsis is becoming necessary. This would allow for earlier diagnosis, facilitate patient prognosis characterization, and prediction of possible evolution of each case. All other markers are regrettably constrained to research units.
Keywords: Sepsis; methods; cytokines.
INTRODUCTION
Sepsis, considered the 10th leading cause of death in the United States (US), is a challenge for medicine today1-5, and its incidence ranges from 76 to 100 cases per 100,000 people. Sepsis and septic shock are among the major causes of death in non-coronary intensive care units (ICU)6-9. In Brazil, the average mortality rate is estimated to be of approximately 29%10. In addition, sepsis is not a reportable disease. As a consequence, it is estimated that these numbers may be higher, since the cause of death might be attributed to other complications. Some studies, for example, have claimed that the mortality rate due to sepsis might vary from 30% to 60%11. In an evaluation in the US, involving more than 6 million hospital records in 7 states, the average number of cases of sepsis was 751,000 per year, with a mortality rate of 28.6%12. Alarming headlines declare that incidence and mortality rates of sepsis are increasing each year. Recently, it was reported that an average of 215,000 people a year died from sepsis between 1979 and 2000 in the US, and an increase of 9% per year of mortality rate6. Aging population, multidrug-resistant microorganisms, popularization of invasive techniques (such as bladder catheters, endotracheal tubes, and intravascular catheters), and increasing number of surgical procedures are among the factors that might explain the higher incidence of sepsis. The peak incidence usually occurs in individuals in the 6th decade. Thus, genetic variation, unconventional lifestyle, and gender are some other factors that may increase the risk of sepsis.
Although much is known about sepsis, the disease has shown a complex clinical and therapeutic profile. Sepsis is a syndrome characterized by a systemic inflammation that can occur in the body as a consequence of a simple infection. Sepsis is actually a counteracting 'misresponse' of the body against infecting microorganisms. This uncontrolled reaction is characterized by a biased system, in favor of pro-inflammatory, pro-coagulant, and over reactive immune inflammatory response13. The magnitude of the response depends on several factors, such as the virulence of the organism, host genetics, and immune status. Moreover, for the progression of the infection to occur, it is necessary that the host cannot contain or destroy the primary infection. Thus, most patients develop either sepsis, or the systemic inflammatory response syndrome (SIRS), which is a clinical pro-inflammatory response, predominantly cytokine-mediated, to a nonspecific insult of either infectious or noninfectious origin. In addition, advances in cellular and molecular biology have demonstrated that bacterial invasion or its by-products (endotoxins, lipopolysaccharide - LPS) need to interact harmfully with the host's immune system for the development of sepsis14. Moreover, among patients who develop sepsis, only a few progress to severe sepsis and are at a higher risk of death15-17.
In the progress of the uncontrolled inflammatory response in sepsis, unpredictable cardiovascular phenomena occur, such as hypovolemia, peripheral vasodilation, myocardial depression, increased endothelial permeability, and hypermetabolism. Patients with sepsis according to a consensus established at the International Conference of Sepsis14 show a diversity of clinical signs. Temperatures can vary from higher than 38ºC to lower than 36ºC, heart rate may be above 90 beats per minute (bpm), tachypnea (FR > 20/min) or hyperventilation (pCO2 < 32 torr) may be present, and white blood cell count may be above 12,000 or below 4,000 cells/µL14,18,19.
Severe sepsis is characterized when there is association of sepsis with organ dysfunction. Septic shock, on the other hand, occurs when resuscitation maneuvers are mandatory due to hypotension or persistent changes in tissue perfusion after conservative attempts of hemodynamic homeostasis maintenance are performed. Nevertheless, the boundaries between severe sepsis, septic shock, and multiple organ dysfunctions are not clearly defined in clinical practice19-21.
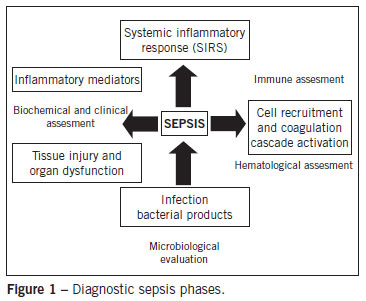
Several inflammatory markers have failed to fulfill the requirements to be eligible for an early and reliable diagnostic predictor of sepsis. Studies17,22 have shown that the best policy is to consider a combination of markers, since the majority of the studies in animal models are not reproducible in humans. This, added to the fact that several markers are commonly found in a wide range of diseases, creates a great difficulty in relating laboratory data to the patients prognosis. Clinical laboratory diagnosis is crucial to avoiding delay in treatment23-25. Thus, one-quarter of patients with sepsis have inadequate treatment and worse prognosis as a consequence of a delayed diagnosis12,17,22. In summary, diagnosis can be considered a two-sided process. Firstly, finding biomarkers capable of monitoring metabolic homeostasis and constantly evaluating patient severity, indicating whether there is systemic or specific organ involvement. Secondly, finding a marker related to pathogen identification, through a quick screening of the patient26. A diagram depicting the diagnostic sepsis phases is presented in Figure 1. In other words, laboratory tests should aim to identify compromised systems or organs, including indicators of inflammatory response in peripheral blood (pro-inflammatory mediators and acute phase indicators) and indicators of organic disorders. Enhanced serum lactate, cytokines, colony stimulating factor, and granulocyte markers of inflammation may be early indicators of more severe conditions, such as SIRS.
METHODS
The data presented in this review originated from a systematic analysis of the PubMed database over the last 10 years by searching for the following terms: sepsis diagnostic, sepsis methods, biological markers, and cytokines. The authors have attempted to summarize and to reference the literature about sepsis and laboratory diagnostics.
HEMATOLOGICAL ASSESSMENT
Blood count analysis and peripheral blood smear tests are usually the first results to be obtained and can provide important information for the clinical management of patients. Frequent leukocytosis is noticeable in patient with sepsis. However, leukopenia or pancytopenia may also be found27. Neutrophilia with a left shift and the presence of immature granulocytes with toxic granulations can be found. The latter, when present in large numbers, is a marker of severity of infection.
Inflammatory mediators cause increased vascular permeability and immune cells chemotaxis to the site28-33. In this process, neutrophils move out of the capillaries, enter the tissues, releasing proteolytic enzymes and reactive oxygen species33-35. Platelets are then attracted to and stick to the damaged endothelium. Platelets and leukocytes occlude the microvasculature leading to a decrease in the blood flow. Following, there is a need to increase oxygen offered to tissues, but this is impossible due to vascular changes.
In addition, there is activation of the coagulation cascade secondary to the complement system, causing a reduction of anticoagulants factors13. In patients with microvascular coagulopathy, it is possible to find different degrees of thrombocytopenia and microangiopathy, with or without the presence of erythroblasts, and schizocytes36. Thrombocytopenia is an independent prognostic marker of mortality in sepsis and should be carefully investigated. The course of sepsis may be the result of drug use, post-transfusion purpura, thrombotic thrombocytopenic purpura, disseminated intravascular coagulation, or heparin- induced thrombocytopenia37. Thus, these candidates for diagnostic markers should be intensively investigated in the near future.
BIOCHEMICAL ASSESSMENT
In sepsis there is a great variation of the patient's electrolytes levels and elevated levels of liver enzymes due to hypoxia. There is also a pronounced hyperglycemia with a hyper- or hypocoagulable state, and metabolic acidosis with respiratory compensation and increased anion gap due to lactic acid production are commonly found38.
The use of biochemical diagnostic markers is vital to determine the prognosis of the patient. The use of gas analysis and other tests such as lactate, albumin, C-reactive protein (PCR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and hemopexin levels are important not only to indicate patient status, but also to give a good picture of the changes in blood homeostasis20,39-41. Gas analysis also provides important information regarding the patient's need for fluid replacement, since the presence of elevated PCR, ALT, and AST is relatively common in sepsis. This may be due to liver injury, post-ischemic condition, and it might also indicate drug toxicity, or be a synonym of direct aggression of the hepatocytes with mitochondrial dysfunction20. Lactate levels, for which tests are available in most hospitals and are relatively low cost, appear to be associated with increased mortality risk in sepsis42.
Albumin is an independent diagnostic marker of severity and mortality in sepsis. During inflammation, there is a leakage of serum albumin to the interstitium and albuminuria in varying degrees. The claimed replacement of exogenous albumin remains controversial in terms of mortality reduction43. The albumin role is better established in patients with liver dysfunction, ascites, and acute lung injury43. Natriuretic peptides (NP) are released by atrial distension and play important role in regulating blood volume, and are considered markers of heart failure. In patients with septic shock, increased levels of NP are associated with higher mortality by myocardial-depression. Recent studies with ICU patients demonstrated that NP levels were significantly higher in survivors than in nonsurvivors, therefore they are a potential indicator of positive prognosis in sepsis44-48.
Blood lactate levels can also be useful in evaluating severe sepsis49-50. Serum lactate is a good indicator of the presence of hypoxic tissue during septic shock, since its production takes place during anaerobic metabolism51. Research conducted by Arnold et al.51 and Nguyen et al.52 provided results suggesting that the clearance lactate rate measure is important in order to identify patients who better respond to treatment and have a more favorable prognosis. In addition, serial measurements of lactate levels in septic patients are more appropriate to assess disease progression rather than a single measurement51. The determination of plasma procalcitonin (PCT) can also be valuable in early diagnosis of patients with severe sepsis53. PCT is the precursor of calcitonin, and higher levels are associated with the development of severe sepsis. Commonly, there is an elevation of PCT levels 4 h after the onset of symptoms, peaking between 8 h and 24 h54. There is evidence that PCT levels are higher in infections caused by Gram-negative than those caused by Gram-positive bacteria55 and it seems to be more specific than PCR18,56.
Ferritin, serum iron binding capacity, and transferrin are tests that must be interpreted with caution in septic patients, since all may be changed due to the presence of high levels of hepcidin57. Ferritin is an acute phase protein that is usually elevated in sepsis and reflects the status of iron stores. The authors of the present study proposed that the level of soluble transferrin receptor should be a good assessment of iron store levels, but even this test has its accuracy reduced in cases of sepsis. The interpretation of these tests should be performed in conjunction with the level of hemoglobin and reticulocytes. Another interesting test reported is insulin sensitivity test58-61. According to Lin et al., glycemic sensitivity reflects the physiological state of the patient together with temperature, heart rate, breathing score, and blood pressure. Careful studies on the changes of these rates would allow physicians to elaborate a mathematical model to predict the onset of sepsis and the need for antibiotics58,62.
MICROBIOLOGICAL EVALUATION
Despite numerous advances in the diagnosis of sepsis, the microbiological evaluation has not yet lost its importance, since it is paramount to identify the causative agent in order to choose an adequate antibiotic therapy. Microbiological evaluation includes direct tests and blood (at least two) or other body fluid culture such as urine, cerebrospinal fluid, feces, secretions, and exudates62,63. Preferably, the sample collection should be performed before the use of antimicrobial therapies. For hospitalized patients, material for culture can also be collected through other methods, including venous or arterial catheters (blood catheters), urinary catheter, tracheotomy (tracheal aspirate), and sutures or scars from recent surgeries62. Although blood culture is the currently adopted method, several patients with sepsis have negative results for this exam. In the ICU, the main causative agents of sepsis are Staphylococcus spp., Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis, Acinetobacter baumannii, and Candida spp.64,65. The use of automated systems for monitoring blood cultures (VITEK, ESP Culture Trek Diagnostic Systems; BacT / Alert and BACTEC the bioMérieux BD) increases the speed and improves their efficiency63,64. Most systems monitor the consumption of carbon dioxide (CO2) by colorimetric methods, generally detecting positivity after 48 hours. An important point to highlight is the way in which the sample collection is performed, in order to avoid false positives and contamination with skin flora.
IMMUNE ASSESSMENT
During an immune response, there is activation of various mechanisms of host defense against a pathogen, such as inflammation, complement and coagulation cascades, polymorfonuclear (PMN) activation, and chemoattraction to the site13,27. These processes are over reactive in SIRS condition. This exacerbated activation of the inflammatory response may lead to greater cell damage, which can culminate with an impairment of the immune response. Despite the fact that only a few tests are commercially available for clinical evaluation purposes, there are several other inflammatory mediators that are screened in the experimental field. Among those markers used as mediators in sepsis there are cytokines such as tumor necrosis factor alpha (TNF)-α, interleukin (IL) -1, IL-6, IL-8, IL-10, and interferon (IFN)-γ66-69, and the presence of bacterial products in the blood or the bacterium itself70-71. IL-1 and TNF-α cytokines are the first cytokines released in an infection and stimulate cellular response. These cytokines release secondary mediators, resulting in chemotaxis and granulocyte activation28,33. These, in turn, lead to another round of cytokine outbreak, which could also give a good picture of the inflammatory process if they could be assessed.
A good example of an acute mediator is IL-6, which is also a predictor of the severity and prognosis of sepsis. Nonetheless, this cytokine lacks specificity, since is likely to be present in high levels in several other inflammatory processes60,72-74. For the same reason, TNF-α cannot be used for the diagnosis of sepsis. IL-10 is an anti-inflammatory cytokine and its level appears to indicate whether the patient is able to respond to an aggression. Bacteria and their by-products, such as LPS and lipoprotein binding protein (LBP), are also good inflammation markers. LBP is an acute phase protein involved in immune response mediated by endotoxin that allows the endotoxin to bind to CD14 receptor, which subsequently activates the expression of toll-like receptors (TLR)-2 and TLR-4 and triggers gene transcription75.
Unfortunately, all these markers are common to many different inflammation types and are also found in simple infections, and thus, are not good predictors of inflammation severity. Also, it is necessary to process the sample immediately, because these proteins are labile and can easily change or be degraded. Once again, as previously mentioned, it is imperative to find a "pathognomonic" marker for sepsis that can routinely be used for clinical diagnosis.
MOLECULAR DIAGNOSIS
The use of molecular biology techniques to diagnose new cases of sepsis is necessary. This has been strongly encouraged by the requirement of smaller samples, capable of providing reliable results and earlier diagnosis. These techniques can detect the presence of LPS in the blood, expression of High-Mobility Group Box (HMGB) -1 or even identify bacterial DNA76. These tests, however, are not 100% accurate, but they do strongly indicate the presence of sepsis. Detection of bacterial DNA fragments by real-time polymerase chain reaction (RT-PCR) in blood samples, or 16S rRNA fragments of Gram-positive and Gram-negative bacteria and Candida in the 18S rRNA might be very promising to help early detection of sepsis, since they have shown a high degree of specificity and sensitivity. The main disadvantages of these techniques are the high costs, the lack of standardization, and the need for skilled personnel to perform them.
The ideal test should be precise, affordable, reproducible, fast, and show high specificity and sensibility, being able to accurately evaluate the patient during different stages of the condition. Until now, none of the tests fulfilled these conditions.
SCORING PREDISPOSITION TO SEPSIS
The criteria for diagnosis of sepsis was established in 1991, and revised only at the International Sepsis Definitions Conference in 200137. The risk and individual symptoms during sepsis were defined as PIRO: predisposition to infection and response to organ dysfunction37,77. This score is important to establish a correct and personalized treatment implementation in sepsis. The PIRO score uses several indicators such as prior co-morbidities, gender, age, culture and characterization of the sensitivity of the microorganism, SIRS, manifestations of sepsis, shock, PCR, and failure rate of organ dysfunction37. Another widely used diagnostic criterion is the age acute physiology and chronic health examination (APACHE)78-79, but it is more restricted and sometimes fails to differentiate sepsis from SIRS. In addition, the information obtained from the monitoring of indicators of severity (sepsis-related organ failure assessment - SOFA) may be more appropriate in many cases80.
CONCLUSION
Rapid diagnosis is essential in the case of sepsis. Laboratory findings are important and represent a two-sided process. The first side is responsible for monitoring changes in metabolic homeostasis and patient evaluation; indicating severity of the disease and whether there is involvement of specific organs or entire systems. The second refers to pathogen identification through a microbiological screening of the patient.
Several indicators might be used for this purpose: pro-inflammatory mediators, acute phase indicators, and pathogen metabolites. Lactate levels, serum cytokines, presence of colony stimulating factors, and plasma nitric oxide levels may be early indicators of SIRS, but remain restricted to research units.
An ideal test should allow for a fast and precise diagnosis, be reproducible, affordable, and have high sensitivity and specificity. Despite several candidates such as blood culture, serum lactate, and PCT levels, a combination of tests is still compulsory for the diagnosis of sepsis. In a field where speed and accuracy are needed, a gold standard test for sepsis is still searched for. Because health is so precious, knowledge must rise to meet current needs.
ACKNOWLEDGEMENTS
The authors apologize to the many researchers whose work they have not been able to discuss in this limited review. Joilson Oliveira Martins is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Projeto Universal), and Pró-reitoria de Pesquisa da Universidade de São Paulo (Projeto I and Novos Docentes), Brazil.
Submitted on:10/26/2011
Approved on: 05/12/2012
Conflict of interest: None.
Study conducted at Department of Clinical and Toxicological Analyses, Faculty of Pharmaceutical Sciences, Universidade de São Paulo, São Paulo, SP, Brazil
- 1. Suffredini AF, Munford RS. Novel therapies for septic shock over the past 4 decades. JAMA. 2011;306:194-9.
- 2. Mangia CM, Kissoon N, Branchini OA, Andrade MC, Kopelman BI, Carcillo J. Bacterial sepsis in Brazilian children: a trend analysis from 1992 to 2006. PLoS One. 2011;6:e14817.
- 3. Booker E. Sepsis, severe sepsis, and septic shock: current evidence for emergency department management. Emerg Med Pract. 2011;13:1-22.
- 4. Hecksher CA, Lacerda HR, Maciel MA. Characteristics and outcomes of patients treated with drotrecogin alpha and other interventions of the "surviving sepsis" campaign in clinical practice. Rev Bras Ter Intensive. 2008;20:135-43.
- 5. Pizzolatti ML, Moritz RD, Andrade J. Analysis of knowledge among emergency medicine physicians about systemic inflammatory responses syndrome (SIRS), sepsis, severe sepsis and septic shock definitions criteria. Rev Bras Ter Intensive. 2004;16:210-4.
- 6. Schefold JC, Hasper D, Jörres A. Organ crosstalk in critically ill patients: hemofiltration and immunomodulation in sepsis. Blood Purif. 2009;28:116-23.
- 7. Sales-Júnior JAL, David CM, Hatum R, Souza PCSP, Japiassú A, Pinheiro CTS, et al. An epidemiological study of sepsis in intensive care units. Sepsis Brazil Study. Rev Bras Ter Intensive. 2006;18:9-17.
- 8. Rosolem MM, Rabello LS, Lisboa T, Caruso P, Costa RT, Leal JV, et al. Critically ill patients with cancer and sepsis: clinical course and prognostic factors. J Crit Care. 2012;27(3):301-7.
- 9. Salluh JI, Rabello LS, Rosolem MM, Soares M, Bozza FA, Verdeal JC, et al. The impact of coagulation parameters on the outcomes of patiet alents with severe community-acquired pneumonia requiring intensive care unit admission. J Crit Care. 2011;26:496-501.
- 10. Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, et al. Brazilian Sepsis Epidemiological Study. Brazilian Sepsis Epidemiological Study (BASES study). Crit Care. 2004; 8:R251-R26060.
- 11. Lin J, Lee DS, Chase JG, Hann CE, Lotz T, Wong XW. Stochastic modelling of insulin sensitivity variability in critical care. Biomedical Signal Processing & Control. 2006;1:229-42.
- 12. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-10.
- 13. Villar J, Flores C, Perez-Mendez L. Genetic determinants of survival in sepsis and acute lung injury. Minerva Anestesiol. 2008;74:341-5.
- 14. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Conference: definition for sepsis and organ failure and guidelines for the use for innovative therapies in sepsis. Chest. 1992;101:1644-1655.
- 15. Kellum JA, Angus DC. Genetic variation and risk of sepsis. Minerva Anestesiol. 2003;69:245-53.
- 16. Elias AC, Matsuo T, Grion CM, Cardoso LT, Verri PH. Incidence and risk factors for sepsis in surgical patients: a cohort study. J Crit Care. 2012;27(2):159-66.
- 17. Shiramizo SC, Marra AR, Durão MS, Paes ÂT, Edmond MB, Pavão dos Santos OF. Decreasing mortality in severe sepsis and septic shock patients by implementing a sepsis bundle in a hospital setting. PLoS One. 2011;6:e26790.
- 18. Dahaba AA, Metzler H. Procalcitonins role in the sepsis cascade. Is procalcitonin a sepsis marker or mediator? Minerva Anestesiol. 2009;75:447-52.
- 19. Katz DV, Troster EJ, Vaz FA. Dopamine and kidney in sepsis: a systematic review. Rev Assoc Med Bras. 2003;49:317-25.
- 20. Carvalho PRA, Trotta EA. Avanços no diagnóstico e tratamento da sepse. J Pediatr. 2003;79:S195-S204.
- 21. Salles MJ, Sprovieri SR, Bedrikow R, Pereira AC, Cardenuto SL, Azevedo PR, et al. Systemic inflammatory response syndrome/sepsis - review and terminology and physiopathology study. Rev Assoc Med Bras. 1999;45:86-92.
- 22. Ventetuolo CE, Levy MM. Biomarkers: diagnosis and risk assessment in sepsis. Clin Chest Med. 2008;29:591-603.
- 23. Xing K, Murthy S, Liles WC, Singh JM. Clinical utility of biomarkers of endothelial activation in sepsis - a systematic review. Crit Care. 2012;16:R7.
- 24. Chew MS, Ihrman L, During J, Bergenzaun L, Ersson A, Unden J, et al. Extravascular lung water index improves the diagnostic accuracy of lung injury in patients with shock. Crit Care. 2012;16:R1.
- 25. Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306:2614-5.
- 26. Becker JU, Theodosis C, Jacob ST, Wira CR, Groce NE. Surviving sepsis in lowincome and middle-income countries: new directions for care and research. Lancet Infect Dis. 2009;9:577-82.
- 27. Jacobi J. Pathophysiology of sepsis. Am J Health Syst Pharm. 2002;15:S3-8.
- 28. Oliveira C, Xavier RA, Anjos-Vallota E, Martins JO, Silveira VL, Gonçalves LR, et al. Effect of plant neutrophil elastase inhibitor on cell migration, adhesion and cytokine release on inflammatory conditions. Br J Pharmacol. 2010;161:899-910.
- 29. Oliveira WR, Cavassani SS, Maganhin CC, Carbonel AA, Simões MJ, Simões RS, et al. Histomorphologic and respiratory aspects of acute lung injury in rats induced by experimental sepsis and under pentoxifylline treatment. Rev Assoc Med Bras. 2009;55:127-31.
- 30. Costantini TW, Deree J, Martins JO, Loomis W, Bansal V, Coimbra R. A novel fluid resuscitation strategy modulates pulmonary transcription factor activation after hemorrhagic shock. Clinics. 2010;65:621-8.
- 31. Sunahara KK, Martins JO. Alveolar macrophages in diabetes: friends or foes? J Leukoc Biol. 2012;91(6):871-6.
- 32. Legrand M, Max A, Peigne V, Mariotte E, Canet E, Debrumetz A, et al. Survival in neutropenic patients with severe sepsis or septic shock. Crit Care Med. 2012;40:43-9.
- 33. Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. 2007;40:1037-44.
- 34. Sunahara KKS, Sannomiya P, Martins JO. Briefs on insulin and innate immune response. Cell Physiol Biochem. 2012;29(1-2):1-8.
- 35. Souza YM, Fontes B, Martins JO, Sannomiya P, Brito GS, Younes RN, et al. Evaluation of the effects of ozone therapy in the treatment of intra-abdominal infection in rats. Clinics. 2010;65:195-202.
- 36. De Backer D, Donadello K, Taccone FS, Ospina-Tascon G, Salgado D, Vincent JL. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 2011;1:27.
- 37. Wang Z, Yu Z, Su J, Cao L, Zhao X, Ruan C. Sepsis-induced disseminated intravascular coagulation with features of thrombotic thrombocytopenic purpura: a fatal fulminant syndrome. Clin Appl Thromb Hemost. 2011;17:251-3.
- 38. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. For the International Sepsis Definitions Conference. 2001 SCCM/ESICM/ ACCP/ATS/ SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250-6
- 39. Claessens YE, Schmidt J, Batard E, Grabar S, Jegou D, Hausfater P, et al. Can C-reactive protein, procalcitonin and mid-regional pro-atrial natriuretic peptide measurements guide choice of in-patient or out-patient care in acute pyelonephritis? Biomarkers In Sepsis (BIS) multicentre study. Clin Microbiol Infect. 2010;16:753-60.
- 40. Ceccon ME, Vaz FA, Diniz EM, Okay TS. Interleukins 6 and C-reactive protein for the diagnosis of late onset sepsis in the newborn infant. Rev Assoc Med Bras. 2006;52:79-85.
- 41. Hatzistilianou M. Diagnostic and prognostic role of procalcitonin in infections. ScientificWorldJournal. 2010;10:1941-6.
- 42. Karon B, Scott R, Burritt M, Santrach P. Comparison of lactate values between point-of-care and central laboratory analyzers. Am J Clin Pathol. 2007;128: 168-71.
- 43. Han J, Martin GS. Does albumin fluid resuscitation in sepsis save lives? Crit Care Med. 2011;39:418-9.
- 44. Rudiger A, Gasser S, Fischler M, Hornemann T, Von Eckardstein A, Maggiorini M. Comparable increase of B-type natriuretic peptide and aminoterminal pro-B-type natriuretic peptide levels in patients with severe sepsis, septic shock, and acute heart failure. Crit Care Med. 2006;34:2140-4.
- 45. Guinard-Barbier S, Grabar S, Chenevier-Gobeaux C, Quinquis L, Schmidt J, Kierzek G, et al. Is mid-regional pro-atrial natriuretic peptide (MRproANP) an accurate marker of bacteremia in pyelonephritis? Biomarkers. 2011;16:355-63.
- 46. Iapichino G, Marzorati S, Umbrello M, Baccalini R, Barassi A, Cainarca M, et al. Daily monitoring of biomarkers of sepsis in complicated long-term ICU-patients: can it support treatment decisions? Minerva Anestesiol. 2010;76:814-23.
- 47. Claessens YE, Mathevon T, Kierzek G, Grabar S, Jegou D, Batard E, et al. Accuracy of C-reactive protein, procalcitonin, and mid-regional pro-atrial natriuretic peptide to guide site of care of community-acquired pneumonia. Intensive Care Med. 2010;36:799-809.
- 48. Nguyen HB, Van Ginkel C, Batech M, Banta J, Corbett SW. Comparison of predisposition, insult/infection, response, and organ dysfunction, acute physiology and chronic health evaluation ii, and mortality in emergency department sepsis in patients meeting criteria for early goal-directed therapy and the severe sepsis resuscitation bundle. J Crit Care. 2011;Oct25. [Epub ahead of print]
- 49. Friedman G, Berlot G, Kahn RJ, Vincent JL. Combined measurements of blood lactate concentrations and gastric intramucosal pH in patients with severe sepsis. Crit Care Med. 1995;23:1184-93.
- 50. Hajjar LA, Nakamura RE, de Almeida JP, Fukushima JT, Hoff PM, Vincent JL, et al. Lactate and base deficit are predictors of mortality in critically ill patients with cancer. Clinics. 2011;66:2037-42.
- 51. Arnold RC, Shapiro NI, Jones AE, Schorr C, Pope J, Casner E, et al. Emergency Medicine Shock Research Network Investigators. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock. 2009;32:35-9.
- 52. Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin AM, Ressler J, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637-42.
- 53. Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med. 2008;9:407-13.
- 54. Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother. 2011;66:1133-40.
- 55. Charles PE, Ladoire S, Aho S. et al. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram-negative or Gram-positive bacteria. BMC Infect Dis. 2008;8:38.
- 56. Ferreira AM, Sakr Y. Organ dysfunction: general approach, epidemiology, and organ failure scores. Semin Respir Crit Care Med. 2011;32:543-51.
- 57. Bullen J, Griffiths E, Rogers H, Ward G. Sepsis: the critical role of iron. Microbes Infect. 2000;2:409-15.
- 58. Lin J, Parente JD, Chase JG, Shaw GM, Blakemore AJ, Lecompte AJ, et al. Development of a model-based clinical sepsis biomarker for critically ill patients. Comput Methods Programs Biomed. 2011;102:149-55.
- 59. De Oliveira Martins J, Meyer-Pflug AR, Alba-Loureiro TC, Melbostad H, Costa da Cruz JW, Coimbra R, et al. Modulation of lipopolysaccharide-induced acute lung inflammation: Role of insulin. Shock. 2006;25:260-6.
- 60. Martins JO, Ferracini M, Anger DB, Martins DO, Ribeiro-Jr LF, Sannomiya P, et al. Signaling pathways and mediators in LPS-induced lung inflammation in diabetic rats: role of insulin. Shock. 2009;31:404-9.
- 61. Martins JO, Ferracini M, Anger DB, Martins DO, Ribeiro-Jr LF, Sannomiya P, et al. Signaling pathways and mediators in LPS-induced lung inflammation in diabetic rats: role of insulin. Shock. 2010;33:76-82.
- 62. Mackenzie I, Lever A. Management of sepsis. BMJ. 2007;335:929-32.
- 63. Riedel S, Carroll KC. Blood cultures: key elements for best practices and future directions. J Infect Chemother. 2010;16:301-16.
- 64. Molina JM, Cordoba J, Ramirez P, Gobernado M. Deteccion automatica de bacterias y hongos en sangre. Enferm Infecc Microbiol Clin. 2008;26:75-80.
- 65. Ferreira LE, Dalposso K, Hackbarth BB, Gonçalves AR, Westphal GA, França PHC, et al. Molecular panel for detection of sepsis-related microorganisms. Rev Bras Ter Intensiva. 2011;23:36-40.
- 66. Deree J, Martins JO, Melbostad H, Putnam JG, de Campos T, Hoyt DB, et al. Insights into the regulation of TNF-α production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition. Clinics. 2008;63:321-8.
- 67. Anjos-Valotta EA, Martins JO, Oliveira MA, Casolari DA, Britto LR, Tostes RC, et al. Inhibition of tumor necrosis factor-alpha-induced intercellular adhesion molecule-1 expression in diabetic rats: role of insulin. Inflamm Res. 2006;55:16-22.
- 68. Martins JO, Ferracini M, Ravanelli N, Landgraf RG, Jancar S. Insulin inhibits LPS-induced signaling pathways in alveolar macrophages. Cell Physiol Biochem. 2008;21:297-304.
- 69. Martins JO, Ferracini M, Ravanelli N, Landgraf RG, Jancar S. Insulin suppresses LPS-induced iNOS and COX-2 expression and NF-kappaB activation in alveolar macrophages. Cell Physiol Biochem. 2008;22:279-86.
- 70. Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699-713.
- 71. Schuerholz T, Marx G. Management of sepsis. Minerva Anestesiol. 2008;74:181-95.
- 72. Martins JO, Wittlin BM, Anger DBC, Martins DO, Sannomiya P, Jancar S. Early phase of allergic airway inflammation in diabetic rats: role of insulin on the signaling pathways and mediators. Cell Physiol Biochem. 2010;26:739-48.
- 73. Rosário AL, Park M, Brunialti MK, Mendes M, Rapozo M, Fernandes D, et al. SvO(2)-guided resuscitation for experimental septic shock: effects of fluid infusion and dobutamine on hemodynamics, inflammatory response, and cardiovascular oxidative stress. Shock. 2011;36:604-12.
- 74. Skrupky LP, Kerby PW, Hotchkiss RS. Advances in the management of sepsis and the understanding of key immunologic defects. Anesthesiology. 2011;115:1349-62.
- 75. Zweigner J, Gramm HJ, Singer OC, Wegscheider K, Schumann RR. High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood. 2001;98:3800-8.
- 76. Rubulotta F, Marshall JC, Ramsay G, Nelson D, Levy M, Williams M. Predisposition, insult/infection, response, and organ dysfunction: a new model for staging severe sepsis. Crit Care Med. 2009;37:1329-35.
- 77. Westh H, Lisby G, Breysse F, Böddinghaus B, Chomarat M, Gant V, et al. Multiplex real-time PCR and blood culture for identification of bloodstream pathogens in patients with suspected sepsis. Clin Microbiol Infect. 2005;15:544-51.
- 78. Couto DO, Peixoto-Júnior AA, Farias JLM, Sales DB, Lima JPA, Rodrigues RS, et al. Gender and mortality in sepsis: do sex hormones impact the outcome? Rev Bras Ter Intensiva. 2011;23:297-303.
- 79. Siqueira-Batista R, Gomes AP, Calixto-Lima L, Vitorino RR, Perez MCA, Mendonça EG, et al. Sepsis: an update. Rev Bras Ter Intensiva. 2011;23:207-16.
- 80. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-10.
Correspondence to:
Publication Dates
-
Publication in this collection
24 Aug 2012 -
Date of issue
Aug 2012
History
-
Received
26 Oct 2011 -
Accepted
12 May 2012