Summary
Aromatase inhibitors have emerged as an alternative endocrine therapy for the treatment of hormone sensitive breast cancer in postmenopausal women. The use of third-generation inhibitors represented by exemestane, letrozol and anastrozole is currently indicated. Anastrozole is a nonsteroidal compound and a potent selective inhibitor of the aromatase enzyme. Although a few studies have shown that its pharmacodynamic and pharmacokinetic properties may be affected by interindividual variability, this drug has been recently used in all configurations of breast cancer treatment. In metastatic disease, it is currently considered the first-line treatment for postmenopausal women with estrogen receptor-positive breast tumors. Anastrozole has shown promising results in the adjuvant treatment of early-stage breast cancer in postmenopausal women. It has also achieved interesting results in the chemoprevention of the disease. Therefore, due to the importance of anastrozole both for endocrine treatment and chemoprevention of hormone-sensitive breast cancer in postmenopausal women, we proposed the current literature review in the SciELO and PubMed database of articles published in the last 10 years.
Keywords:
aromatase inhibitors; chemoprevention; breast neoplasms; pharmacokinetics
Resumo
Os inibidores de aromatase têm emergido como uma endocrinoterapia alternativa para o tratamento de câncer de mama sensível a hormônios em mulheres pós-menopáusicas. A utilização de inibidores de terceira geração, representados por exemestano, letrozol e anastrozol, é atualmente indicada. Anastrozol é um composto não esteroide e um inibidor potente e seletivo da enzima aromatase. Embora alguns estudos tenham demonstrado que as suas propriedades farmacodinâmicas e farmacocinéticas podem ser afetadas pela variabilidade interindividual, esse fármaco tem sido recentemente utilizado em todas as configurações de tratamento do câncer de mama. Na doença metastática, é atualmente considerado o tratamento de primeira linha em mulheres pós-menopáusicas com tumores de mama e receptor de estrogênio positivo. O anastrozol tem mostrado resultados promissores no tratamento adjuvante do câncer de mama em estágio inicial em mulheres na pós-menopausa. Ele também conseguiu resultados interessantes na quimioprevenção da doença. Portanto, em virtude da importância do anastrozol tanto no tratamento endócrino quanto na quimioprevenção do câncer de mama hormoniossensível em mulheres na pós-menopausa, propusemos a atual revisão da literatura na base de dados SciELO e PubMed de artigos publicados nos últimos 10 anos.
Palavras-chave:
inibidores da aromatase; quimioprevenção; neoplasias da mama; farmacocinética
Introduction
Breast cancer is one of the most commonly diagnosed types of cancer in women, presenting high incidence rates in developed regions of the world compared with developing ones. Incidence rates of the disease range from 27 cases per 100,000 women in Eastern Africa to 96 cases per 100,000 women in Western Europe.11 DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014; 64(1):52-62.,22 Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136(5):E359-86. Breast cancer is characterized as a multifactorial disease and its development has been reported as the result of complex interactions between an individual's genoma and the environment.33 Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004; 6(5):213-8. Prolonged exposure to estrogene plus progesterone plays a significant role in the etiology of breast carcinoma and biosynthesis pathway of estrogens is thus an important therapeutic target.44 Key T, Appleby P, Barnes I, Reeves G; Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002; 94(8):606-16.
The main enzyme involved in estrogen biosynthesis is CYP19A1 or aromatase that belongs to the cytochrome P450 family and is predominantly located in the liver, adrenal glands and fatty tissue.55 Lake DE, Hudis C. Aromatase inhibitors in breast cancer: an update. Cancer Control. 2002; 9(6):490-8. However, the source of estrogen varies widely between premenopausal and postmenopausal women (Figure 1).66 Freedman OC, Verma S, Clemons MJ. Pre-menopausal breast cancer and aromatase inhibitors: treating a new generation of women. Breast Cancer Res Treat. 2006; 99(3):241-7. In premenopausal women, the main source of estrogen is the ovary, while in postmenopausal women, estrogen is derived from the conversion of androgens into estrogens (through the aromatase enzyme). In particular, testosterone is converted into estradiol, androstenedione into estrone and 16-alpha-hydroxytestosterone into estriol (Figure 2), originating from the peripheral tissues, including the skin, fatty tissue and breast. Therefore, the aromatase enzyme directly affects estrogen biosynthesis in the breast and it is believed that this enzyme plays an important role in the progression of breast cancer.77 Geisler J, Lønning PE. Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res. 2005; 11(8):2809-21.,88 Di Nardo G, Gilardi G. Human aromatase: perspectives in biochemistry and biotechnology. Biotechnol Appl Biochem. 2013; 60(1):92-101.
Molecular structure of the androgen substrates of aromatase and the corresponding estrogen products.
Aromatase inhibitors (AIs) have recently been approved as a first-line endocrine therapy for postmenopausal women with hormone-sensitive and metastatic breast cancer.99 Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract. 2007; 61(12):2051-63.,1010 Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ; Panel members. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009; 20(8):1319-29. There are three generations of AIs and the last, represented by exemestane, letrozol and anastrozole, is the one with the most widely recommended drugs, due to their high specificity for the aromatase enzyme and less adverse effects compared with the previous generations of AI drugs (Figure 3). Exemestane is a steroidal compound that forms covalent bonds with aromatase, and this type of inhibition is irreversible and can only be overcome by the synthesis of a new enzyme. Letrozol and anastrozole are nonsteroidal compounds with reversible action and are competitive AIs.1111 Lombardi P. Exemestane, a new steroidal aromatase inhibitor of clinical relevance. Biochim Biophys Acta. 2002; 1587(2-3):326-37.,1212 Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003; 348(24):2431-42.
Chemical structures of currently used antiaromatase compounds. A. Steroidal aromatase inactivator. B. Nonsteroidal aromatase inhibitors.
Anastrozole and all third-generation compounds have become endocrine drugs of choice for postmenopausal breast cancer patients, as they are associated with a stronger activity and better general tolerability compared with tamoxifen (TAM), a first-generation selective estrogen receptor modulator (SERM)1313 Gobbi S, Rampa A, Belluti F, Bisi A. Nonsteroidal aromatase inhibitors for the treatment of breast cancer: an update. Anticancer Agents Med Chem. 2014; 14(1):54-65. that has been associated with potentially fatal adverse effects such as an increased incidence of endometrial cancer, thromboembolism and cerebrovascular event.1414 Pan K, Chlebowski RT. Adjuvant endocrine therapy of perimenopausal and recently postmenopausal women with hormone receptor-positive breast cancer. Clin Breast Cancer. 2014; 14(3):147-53. However, there are few studies particularly on the pharmacodynamic and pharmacokinetic properties of anastrozole and its use in the chemoprevention and treatment of breast cancer.
In this review, we will discuss the pharmacodynamic and pharmacokinetic properties of anastrozole, as well as its use in the chemoprevention and treatment of breast cancer.
Pharmacodynamic properties of anastrozole
Anastrozole is a derivative of benzotriazole marketed as ARIMIDEX® by AstraZeneca Pharmaceuticals LP. Similarly to other AIs, it has an inhibitory action on aromatase, thus blocking the conversion of testosterone into estradiol and androstenedione into estrone (Figure 4).1515 Haynes BP, Dowsett M, Miller WR, Dixon JM, Bhatnagar AS. The pharmacology of letrozole. J Steroid Biochem Mol Biol. 2003; 87(1):35-45.
16 Kelly CM, Buzdar AU. Anastrozole. Expert Opin Drug Saf. 2010; 9(6):995-1003.
17 Buzdar AU. Pharmacology and pharmacokinetics of the newer generation aromatase inhibitors. Clin Cancer Res. 2003; 9(1 Pt 2):468S-72S.-1818 Johnston SR, Dowsett M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer. 2003; 3(11):821-31. Inhibition of the aromatase enzyme occurs particularly through competitive binding of aromatase to the heme group of cytochrome P450, decreasing estrogen biosynthesis in the peripheral tissues of the body and in the breast.1919 Sanford M, Plosker GL. Anastrozole: a review of its use in postmenopausal women with early-stage breast cancer. Drugs. 2008; 68(9):1319-40.
Anastrozole has significant effects on breast cancer treatment and, therefore, it is currently used as first-line treatment in estrogen receptor (ER)-positive postmenopausal women, particularly to treat locally advanced or metastatic breast cancer. Furthermore, it is also indicated for early cancer treatment, tumor chemoprevention and postmenopausal women using TAM, especially if the drug is used during a prolonged period of time and has been indicated in the disease's recurrence, i.e., as another therapeutic endocrine option.99 Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract. 2007; 61(12):2051-63.,1010 Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ; Panel members. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009; 20(8):1319-29.
Prolonged use of anastrozole has no effects on the concentrations of steroid hormones cortisol, aldosterone, androstenedione and 16-hydroxyprogesterone, confirming that it is highly selective for the inhibition of aromatase without interfering in other pathways of adrenal stereoidogenesis. The lack of alterations in luteinizing hormone and folllicle-stimulating hormone demonstrates that anastrozole has no estrogenic, progestational or androgenic activity, and it does not affect the synthesis of gonadotropins.88 Di Nardo G, Gilardi G. Human aromatase: perspectives in biochemistry and biotechnology. Biotechnol Appl Biochem. 2013; 60(1):92-101.,2020 Bhatnaga AS. The discovery and mechanism of action of letrozole. Breast Cancer Res Treat. 2007; 105(Suppl 1):7-17.
Pharmacokinetic properties of anastrozole
Some studies were conducted in an attempt to identify the daily dose required for anastrozole to promote aromatase inhibition and decrease estrogen synthesis. The results showed that a daily dose of 1 mg of anastrozole was the minimum capable of consistently supressing estrone and estradiol at the limit detectable by radioimmunoassay.1818 Johnston SR, Dowsett M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer. 2003; 3(11):821-31.,2121 Milani M, Jha G, Potter DA. Anastrozole use in early stage breast cancer of postmenopausal women. Clin Med Ther. 2009; 31(1):141-56. However, recent studies have reported that a daily dose of 1 mg may not benefit all breast cancer patients because interindividual variability may alter the efficacy and tolerability of anastrozole,2222 Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010; 70(8):3278-86.,2323 Ingle JN, Kalari KR, Buzdar AU, Robson ME, Goetz MP, Desta Z, et al. Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids. 2015; 99(Pt A):32-8. interfering in its pharmacodynamic and or pharmacokinetic properties.2424 Edavana VK, Dhakal IB, Williams S, Penney R, Boysen G, Yao-Borengasser A, et al. Potential role of UGT1A4 promoter SNPs in anastrozole pharmacogenomics. Drug Metab Dispos. 2013; 41(4):870-7.
Administered orally during fasting, anastrozole is rapidly absorbed. After meals, however, it has a slower absorption rate. In the recommended dose of 1 mg, anastrozole achieves maximum plasma concentrations within 2 hours after its administration and, after seven days, approximately 90 to 95% of its plasma concentrations are obtained. Less than 10% of anastrozole is excreted in the form of unaltered drug, while 60% are excreted as metabolites.1717 Buzdar AU. Pharmacology and pharmacokinetics of the newer generation aromatase inhibitors. Clin Cancer Res. 2003; 9(1 Pt 2):468S-72S.,1818 Johnston SR, Dowsett M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer. 2003; 3(11):821-31.
Anastrozole is metabolized in the liver, involving N-dealkylation, hydroxylation and glucuronidation reactions, leading to a mean plasmatic half-life of 50 hours, which indicates that the administration of a single daily dose of the drug is adequate. The three main metabolites of anastrozole observed in the plasma and urine of human patients are: triazol, hydroxy-anastrozole glucuronide and anastrozole glucuronide. Triazole is the main metabolite; however, it is inactive and does not suppress, along with two other metabolites, the activity of aromatase. The excretion of these metabolites is mainly through urine.2222 Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010; 70(8):3278-86.,2525 Kamdem LK, Liu Y, Stearns V, Kadlubar SA, Ramirez J, Jeter S, et al. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br J Clin Pharmacol. 2010; 70(6):854-69.
The main side effects of anastrozole use include hot flashes (35%), asthenia (17%), headache (13%) and edema (10%). Nausea is the most common gastrointestinal side effect (19%), while diarrhea, constipation, abdominal pain and anorexia are less frequently reported (8%).2626 Wilkinson K. Anastrozole (Arimidex). Clin J Oncol Nurs. 2004; 8(1):87-8. Nevertheless, in addition to these effects, studies have recently identified the presence of muscle and joint pain, as well as a significant increase in the loss of bone mass, leading to increased incidence of osteopenia and osteoporosis.2323 Ingle JN, Kalari KR, Buzdar AU, Robson ME, Goetz MP, Desta Z, et al. Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids. 2015; 99(Pt A):32-8.,2727 Abubakar MB, Wei K, Gan SH. The influence of genetic polymorphisms on the efficacy and side effects of anastrozole in postmenopausal breast cancer patients. Pharmacogenet Genomics. 2014; 24(12):575-81.
Metastatic setting
AIs, including anastrozole, are commonly used for first-line treatment of hormone receptor-positive postmenopausal women with metastatic breast cancer.2828 Zucchini G, Geuna E, Milani A, Aversa C, Martinello R, Montemurro F. Clinical utility of exemestane in the treatment of breast cancer. Int J Womens Health. 2015; 7:551-63. The combination strategy of AIs and the selective ER degrader fulvestrant has been investigated. The Fulvestrant and Anastrozole Combination Therapy (FACT) trial was a randomized study involving 514 postmenopausal women allocated into a treatment group with anastrozole and another group that received a combination of fulvestrant and anastrozole. This trial did not reveal significant differences in median time of disease progression and overall survival when both groups were compared.2929 Bergh J, Jönsson PE, Lidbrink EK, Trudeau M, Eiermann W, Brattström D, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012; 30(16):1919-25. In contrast, the Southwestern Oncology Group (SWOG) S226 trial had a design similar to the FACT trial and included 707 patients, who demonstrated not only a significant improvement in disease-free survival (DFS) but also advantages in overall survival (OS). This study involved a large number of patients with recurrent metastasis who had not received previous endocrine therapy and who seemed to have more benefits from the combination of fulvestrant and anastrozole.3030 Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012; 367(5):435-44.
The Faslodex Versus Exemestane With/Without Arimidex (SoFEA) trial aimed to evaluate endocrine resistance in postmenopausal patients through the combination of fulvestrant and anastrozole versus fulvestrant versus exemestane, and did not demostrate any benefits to the groups of patients treated with fulvestrant and exemestane alone. These data suggest that treatment with fulvestrant combined with anastrozole may offer advantages to postmenopausal women with metastatic recurrence and there seems to be no significant benefit from the combination of fulvestrant and anastrozole in patients with endocrine resistance who had previously received endocrine therapy.3131 Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, et al.; SoFEA investigators. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013; 14(10):989-98.
Adjuvant setting
The use of AIs has been widely investigated in postmenopausal women with early breast cancer.3232 Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, et al. ; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010; 28(23):3784-96. Several strategies have been investigated comparing the benefits of AIs and TAM in the adjuvant setting.3333 Chumsri S, Howes T, Bao T, Sabnis G, Brodie A. Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol. 2011; 125(1-2):13-22. For initial treatment strategy for breast cancer, both anastrozole and letrozol have demonstrated a significant improvement in DFS after 5 years of study compared to the use of TAM alone or combined with anastrozole during the same time period. Nevertheless, a significant improvement in OS was not observed in these studies, probably due to crossover of a considerable number of patients after publication of the studies. Despite this fact, anastrozole and letrozol seemed to be better tolerated, with fewer serious adverse events related to treatment, in comparison to TAM. Contrary to the benefit derived from the combination of AIs and the selective ER modulator, the administration of anastrozole combined with TAM in the ATAC study demonstrated damaging effects on DFS compared to the sole use of anastrozole.3434 Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al.; ATAC/LATTE investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010; 11(12):1135-41.
In an attempt to change strategies, some clinical trials compared 5 years of TAM with the sequential TAM treatment for 2-3 years followed by AIs.3535 BIG 1-98 Collaborative Group, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thürlimann B, Paridaens R, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009; 361(8):766-76.
36 Kaufmann M, Jonat W, Hilfrich J, Eidtmann H, Gademann G, Zuna I, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated aftertreatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol. 2007; 25(19):2664-70.
37 Boccardo F, Rubagotti A, Guglielmini P, Fini A, Paladini G, Mesiti M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol. 2006; 17(Suppl 7):vii10-4.-3838 Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al.; Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004; 350(11):1081-92. All these trials demonstrated a significant improvement in DFS among patients who received sequential treatment compared to TAM alone. Furthermore, a meta-analysis conducted on adjuvant breast cancer treatments indicated that the AIs anastrozole, letrozol and exemestane consistently presented lower recurrence rates compared to the use of TAM, either as initial therapy or after 2-3 years of TAM therapy. These studies provide clear evidence that AIs (anastrozole, letrozol and exemestane) achieve a significant reduction in the recurrence rates of early breast cancer.3131 Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, et al.; SoFEA investigators. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013; 14(10):989-98.
The Breast International Group (BIG) 1-98 trial was the only study that directly compared a sequential AI treatment followed by TAM, the inverse sequence, and the initial treatment with AIs and TAM only. There was no statistically significant difference between both arms of sequential treatment. Nevertheless, a higher number of relapses occurred during the first years of TAM treatment followed by AI, particularly in patients with lymph node involvement. Therefore, this study suggests that patients at high-risk or those with axillary nodal involvement should initially use anastrozole or letrozol; in case of intolerance due to the use of these AIs, a change to TAM after 2-3 years on AIs may be considered, since there was no statistically significant difference in DFS among patients who received 5 years of AI treatment compared with 2-3 years of AI treatment followed by TAM.3535 BIG 1-98 Collaborative Group, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thürlimann B, Paridaens R, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009; 361(8):766-76.
Neoadjuvant setting
The use of anastrozole in the neoadjuvant setting for the treatment of early-stage breast cancer was investigated by a study that compared anastrozole and TAM as neoadjuvant therapy for breast cancer in premenopausal women receiving goserelin. It was observed that responsiveness improved after 24 weeks of treatment in women using anastrozole. This study suggests that the combination of anastrozole and goserelin represents an alternative to neoadjuvant treatment and an option for premenopausal women with early stage breast cancer.3939 Masuda N, Sagara Y, Kinoshita T, Iwata H, Nakamura S, Yanagita Y, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. 2012; 13(4):345-52.
Chemoprevention
The side effects of AIs are limited compared to those of TAM (risk of endometrial cancer and venous thromboembolism), and effectiveness of AIs in breast cancer treatment has been demonstrated. Therefore, there has been great interest in the use of AIs for breast cancer chemoprevention. However, in the configuration of the chemoprevention programs, AIs have not yet been approved.4040 Sestak I. Preventative therapies for healthy women at high risk of breast cancer. Cancer Manag Res. 2014; 17(6):423-30.,4141 Chumsri S. Clinical utilities of aromatase inhibitors in breast cancer. Int J Womens Health. 2015; 7:493-9. In recent years, two clinical trials have reported their main results on cancer prevention using AIs: the Mammary Prevention 3 Study (MAP3) and the International Breast Cancer Intervention Study-II (IBIS-II).
The MAP3 trial involved the randomization of 4,500 postmenopausal women at risk of developing breast cancer, who were allocated into two groups, exemestane and placebo, for 5 years. After a mean 35 month follow-up period, a 65% reduction in invasive breast cancers was observed in women using exemestane and no effect was observed in ER-negative women. No significant differences in side effects were found between groups, suggesting a good risk-benefit profile. However, the limitation of this study was the short follow-up period, which was only 35 months. Due to this, the MAP3 trial does not allow for any conclusions on the long term safety and efficacy of this drug.4242 Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al.; NCIC CTG MAP.3 Study Investigators. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011; 364(25): 2381-91.
The aim of the IBIS-II trial was to evaluate the efficacy and safety of anastrozole for breast cancer prevention in high-risk postmenopausal women. The IBIS-II assessed only anastrozole versus placebo due to more effective action of anastrozole in breast cancer chemoprevention of postmenopausal women compared to tamoxifen.4343 Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al.; IBIS-II investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014; 383(9922):1041-8. For that trial, postmenopausal women (n=3,864) were randomized into a group using 1 mg of anastrozole daily or a group treated with a placebo. After a median follow-up period of 5 years, it could be observed that anastrozole significantly reduced the diagnosis of invasive breast cancer, and ductal carcinoma in situ, similarly to the results reported in the MAP3 trial. Also similarly to the MAP3 trial, it was found that anastrozole had no effect on ER-negative women. The strengths of this study were the large number of cancers reported and the mean follow-up period of 5 years. All women in the study continue the long-term follow-up in a blinded fashion. This is important because the long-term global efficacy of anastrozole and other AIs has not yet been established for healthy postmenopausal women who are at increased risk of developing breast cancer.4343 Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al.; IBIS-II investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014; 383(9922):1041-8.
The decline in invasive breast carcinoma observed with the use of exemestane and anastrozole was greater than that observed with TAM or any other SERM, and these results indicate that both drugs are attractive options for the prevention of breast cancer in postmenopausal women at increased risk of developing the disease.4040 Sestak I. Preventative therapies for healthy women at high risk of breast cancer. Cancer Manag Res. 2014; 17(6):423-30.
Animal models
The chemopreventive effects of anastrozole have also been investigated in animal models. Significant improvement in tumor supression was observed after induction of premenopausal breast carcinogenesis in rats. The incidence of tumor supression was 40% with the use of a dose of 0.5 mg/kg of anastrozole. In addition, there were no adverse effects on the genital system, lipid and bone metabolism of the rats. Nevertheless, an increase in body weight was observed.4444 Kubatka P, Sadlonová V, Kajo K, Nosál'ová G, Ostatníková D, Adamicová K. Chemopreventive effects of anastrozole in a premenopausal breast cancer model. Anticancer Res. 2008; 28(5A):2819-23.
A similar study evaluated the side effects of anastrozole as a chemopreventive agent in the induction of premenopausal breast carcinogenesis in rats. In rats given a dose of 0.5 mg/kg of anastrozole, there were no macroscopic alterations in the uterus and vagina, histological exam showed no atrophic changes in the endometrium and vaginal epithelium, although the myometrium was significantly thicker. Changes in lipid metabolism and serum levels of sex hormones were not observed. However, a significant increase in cortical bone thickness and body weight was found.4545 Sadlonova V, Kubatka P, Kajo K, Ostatnikova D, Nosalova G, Adamicova K, et al. Side effects of anastrozole in the experimental pre-menopausal mammary carcinogenesis. Neoplasma. 2009; 56(2):124-9.
Conclusion
Anastrozole is one of the third-generation AIs, a highly competitive and selective inhibitor of aromatase, which is the enzyme responsible for the conversion of androgens into estrogen in postmenopausal women. Large studies have demonstrated the effective use of a daily dose of 1 mg of anastrozole in these women. Anastrozole has a significant action in metastatic disease, as well as in the adjuvant treatment and chemoprevention of breast cancer. However, recent studies have shown that interindividual variability may affect pharmacodynamic and pharmacokinetic properties while using this dose in some women. Anastrozole may be used as an option for efficient and tolerable endocrine treatment in all stages of cancer in the majority of postmenopausal women.
-
Study conducted at Universidade Federal do Piauí, Teresina, PI, Brazil
References
-
1DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014; 64(1):52-62.
-
2Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136(5):E359-86.
-
3Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004; 6(5):213-8.
-
4Key T, Appleby P, Barnes I, Reeves G; Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002; 94(8):606-16.
-
5Lake DE, Hudis C. Aromatase inhibitors in breast cancer: an update. Cancer Control. 2002; 9(6):490-8.
-
6Freedman OC, Verma S, Clemons MJ. Pre-menopausal breast cancer and aromatase inhibitors: treating a new generation of women. Breast Cancer Res Treat. 2006; 99(3):241-7.
-
7Geisler J, Lønning PE. Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res. 2005; 11(8):2809-21.
-
8Di Nardo G, Gilardi G. Human aromatase: perspectives in biochemistry and biotechnology. Biotechnol Appl Biochem. 2013; 60(1):92-101.
-
9Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. Int J Clin Pract. 2007; 61(12):2051-63.
-
10Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ; Panel members. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009; 20(8):1319-29.
-
11Lombardi P. Exemestane, a new steroidal aromatase inhibitor of clinical relevance. Biochim Biophys Acta. 2002; 1587(2-3):326-37.
-
12Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003; 348(24):2431-42.
-
13Gobbi S, Rampa A, Belluti F, Bisi A. Nonsteroidal aromatase inhibitors for the treatment of breast cancer: an update. Anticancer Agents Med Chem. 2014; 14(1):54-65.
-
14Pan K, Chlebowski RT. Adjuvant endocrine therapy of perimenopausal and recently postmenopausal women with hormone receptor-positive breast cancer. Clin Breast Cancer. 2014; 14(3):147-53.
-
15Haynes BP, Dowsett M, Miller WR, Dixon JM, Bhatnagar AS. The pharmacology of letrozole. J Steroid Biochem Mol Biol. 2003; 87(1):35-45.
-
16Kelly CM, Buzdar AU. Anastrozole. Expert Opin Drug Saf. 2010; 9(6):995-1003.
-
17Buzdar AU. Pharmacology and pharmacokinetics of the newer generation aromatase inhibitors. Clin Cancer Res. 2003; 9(1 Pt 2):468S-72S.
-
18Johnston SR, Dowsett M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer. 2003; 3(11):821-31.
-
19Sanford M, Plosker GL. Anastrozole: a review of its use in postmenopausal women with early-stage breast cancer. Drugs. 2008; 68(9):1319-40.
-
20Bhatnaga AS. The discovery and mechanism of action of letrozole. Breast Cancer Res Treat. 2007; 105(Suppl 1):7-17.
-
21Milani M, Jha G, Potter DA. Anastrozole use in early stage breast cancer of postmenopausal women. Clin Med Ther. 2009; 31(1):141-56.
-
22Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010; 70(8):3278-86.
-
23Ingle JN, Kalari KR, Buzdar AU, Robson ME, Goetz MP, Desta Z, et al. Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids. 2015; 99(Pt A):32-8.
-
24Edavana VK, Dhakal IB, Williams S, Penney R, Boysen G, Yao-Borengasser A, et al. Potential role of UGT1A4 promoter SNPs in anastrozole pharmacogenomics. Drug Metab Dispos. 2013; 41(4):870-7.
-
25Kamdem LK, Liu Y, Stearns V, Kadlubar SA, Ramirez J, Jeter S, et al. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br J Clin Pharmacol. 2010; 70(6):854-69.
-
26Wilkinson K. Anastrozole (Arimidex). Clin J Oncol Nurs. 2004; 8(1):87-8.
-
27Abubakar MB, Wei K, Gan SH. The influence of genetic polymorphisms on the efficacy and side effects of anastrozole in postmenopausal breast cancer patients. Pharmacogenet Genomics. 2014; 24(12):575-81.
-
28Zucchini G, Geuna E, Milani A, Aversa C, Martinello R, Montemurro F. Clinical utility of exemestane in the treatment of breast cancer. Int J Womens Health. 2015; 7:551-63.
-
29Bergh J, Jönsson PE, Lidbrink EK, Trudeau M, Eiermann W, Brattström D, et al. FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol. 2012; 30(16):1919-25.
-
30Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012; 367(5):435-44.
-
31Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L, et al.; SoFEA investigators. Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol. 2013; 14(10):989-98.
-
32Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, et al. ; American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010; 28(23):3784-96.
-
33Chumsri S, Howes T, Bao T, Sabnis G, Brodie A. Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol. 2011; 125(1-2):13-22.
-
34Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al.; ATAC/LATTE investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010; 11(12):1135-41.
-
35BIG 1-98 Collaborative Group, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thürlimann B, Paridaens R, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009; 361(8):766-76.
-
36Kaufmann M, Jonat W, Hilfrich J, Eidtmann H, Gademann G, Zuna I, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated aftertreatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol. 2007; 25(19):2664-70.
-
37Boccardo F, Rubagotti A, Guglielmini P, Fini A, Paladini G, Mesiti M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol. 2006; 17(Suppl 7):vii10-4.
-
38Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al.; Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004; 350(11):1081-92.
-
39Masuda N, Sagara Y, Kinoshita T, Iwata H, Nakamura S, Yanagita Y, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. 2012; 13(4):345-52.
-
40Sestak I. Preventative therapies for healthy women at high risk of breast cancer. Cancer Manag Res. 2014; 17(6):423-30.
-
41Chumsri S. Clinical utilities of aromatase inhibitors in breast cancer. Int J Womens Health. 2015; 7:493-9.
-
42Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al.; NCIC CTG MAP.3 Study Investigators. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011; 364(25): 2381-91.
-
43Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al.; IBIS-II investigators. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014; 383(9922):1041-8.
-
44Kubatka P, Sadlonová V, Kajo K, Nosál'ová G, Ostatníková D, Adamicová K. Chemopreventive effects of anastrozole in a premenopausal breast cancer model. Anticancer Res. 2008; 28(5A):2819-23.
-
45Sadlonova V, Kubatka P, Kajo K, Ostatnikova D, Nosalova G, Adamicova K, et al. Side effects of anastrozole in the experimental pre-menopausal mammary carcinogenesis. Neoplasma. 2009; 56(2):124-9.
Publication Dates
-
Publication in this collection
Apr 2017
History
-
Received
06 July 2016 -
Accepted
19 Oct 2016


 Source: adapted from Freedman et al.
Source: adapted from Freedman et al. Source: adapted from Di Nardo and Gilardi.
Source: adapted from Di Nardo and Gilardi.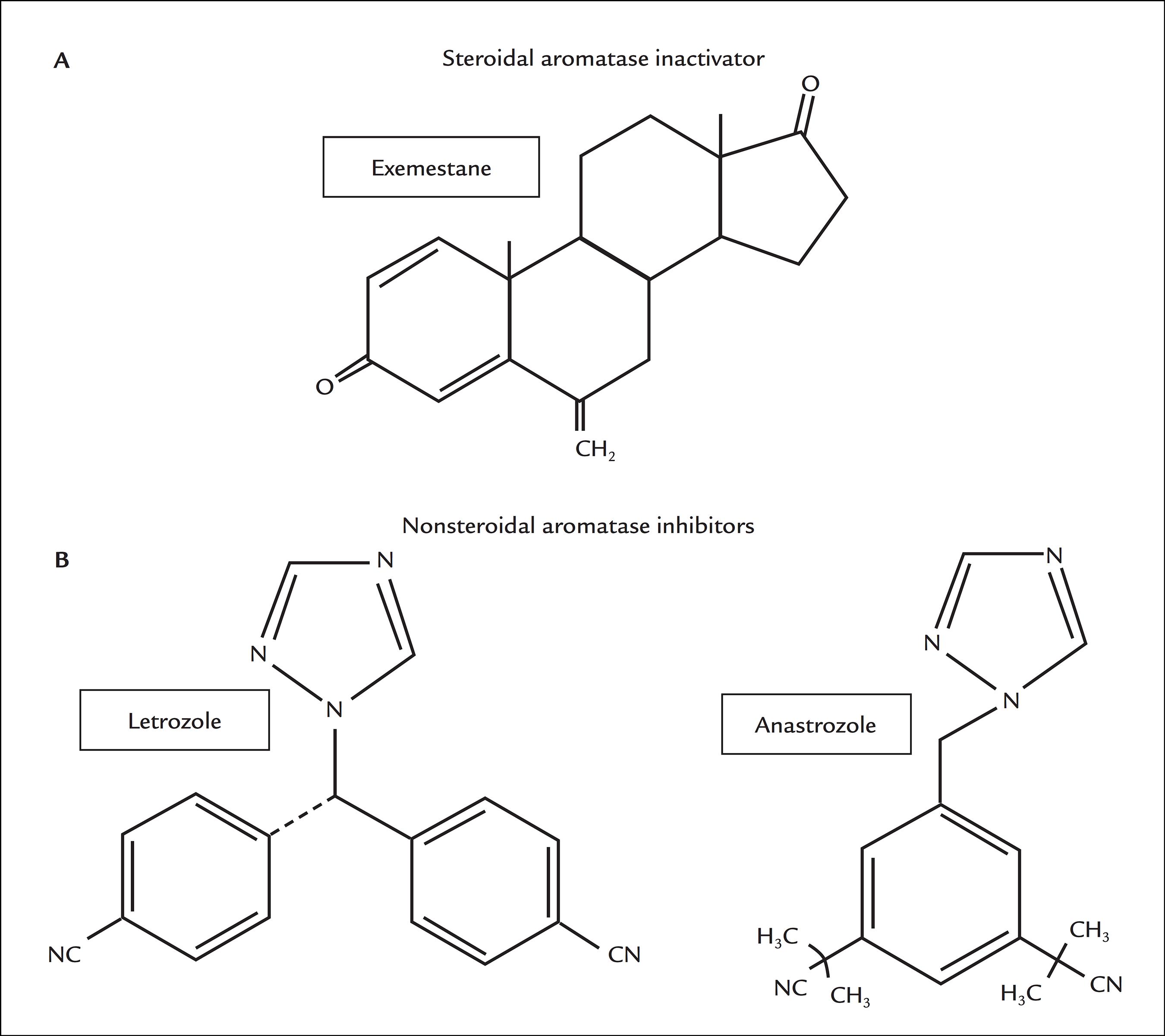 Source: adapted from Geisler and Lønning.
Source: adapted from Geisler and Lønning. Source: adapted from Johnston and Dowsett.
Source: adapted from Johnston and Dowsett.