SUMMARY
OBJECTIVE
It has been observed that celiac disease (CD) is not restricted to a single type characterized by diarrhea but also has atypical, asymptomatic (silent), and latent forms. The prevalence of this autoimmune disease, which affects approximately 1% of the world, is estimated to be around 3%, including atypical and asymptomatic cases. In our study, we aimed to evaluate adult celiac patients.
METHODS
Between December 2008-2015, patients diagnosed with CD over the age of 18 years old were included in the study. Patients’ symptoms at admission, frequency and type of anemia, transaminase levels, and celiac antibody positivity, and autoimmune diseases diagnosed at follow up were evaluated retrospectively.
RESULTS
Of 195 patients, 151 (77.4%) were female. The mean age of the patients was 35.73 ± 12.19 years (range, 18-71 years). A hundred patients (51.3%) had gastrointestinal symptoms. At the time of admission, 118 patients (60.5%) had anemia, and 52 (26.7%) had hypertransaminasemia. During the mean follow-up period of 58 months (36-120 months), 84 (43.1%) of the patients presented at least one autoimmune disease, and this rate was 96.6% in individuals diagnosed above the age of 50 years.
CONCLUSION
In adult CD, resistant anemia, dyspepsia, and hypertransaminasemia are very common findings at the time of diagnosis, and the association with other autoimmune diseases, especially Hashimoto’s thyroiditis, is high.
Celiac Disease; Adult; Autoimmune diseases; Anemia
RESUMO
OBJETIVOS
Observou-se que a doença celíaca (DC) não se restringe a um único tipo caracterizado por diarreia, mas também tem formas atípicas, assintomáticas (silenciosas) e latentes. Estima-se que a prevalência desta doença autoimune, que afeta aproximadamente 1% da população do mundo, seja em torno de 3%, incluindo casos atípicos e assintomáticos. Em nosso estudo, objetivou-se avaliar pacientes celíacos adultos.
MÉTODOS
Entre dezembro de 2008 e 2015, pacientes diagnosticados como DC com idade acima de 18 anos foram incluídos no estudo. Os sintomas dos pacientes na admissão, frequência e tipo de anemia, níveis de transaminases e positividade de anticorpos celíacos e doenças autoimunes diagnosticadas no seguimento foram avaliados retrospectivamente.
RESULTADOS
Dos 195 pacientes, 151 (77,4%) eram do sexo feminino. A média de idade dos pacientes foi de 35,73±12,19 anos (variação de 18 a 71 anos). Cem pacientes (51,3%) foram encaminhados com sintomas gastrointestinais. No momento da internação, 118 pacientes (60,5%) apresentavam anemia e 52 (26,7%) apresentavam hipertransaminemia. Durante o período médio de acompanhamento de 58 meses (36-120 meses), 84 (43,1%) pacientes estavam acompanhados por pelo menos uma doença autoimune, e essa taxa foi de 96,6% em indivíduos diagnosticados acima dos 50 anos de idade.
CONCLUSÃO
No adulto DC, anemia resistente, dispepsia e hipertransaminasemia são achados muito comuns no momento do diagnóstico e a associação com outras doenças autoimunes, especialmente tireoidite de Hashimoto, é alta.
Doença celíaca; Adulto; Doenças autoimunes; Anemia
INTRODUCTION
Celiac disease (CD) is an immune-mediated persistent gluten intolerance that develops against gluten proteins such as barley, wheat, and rye in genetically predisposed individuals. Gluten proteins induce T-cell-associated inflammation in the small intestine and cause an autoimmune response to their own proteins, such as tissue transglutaminase.11. Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2(9):647-55. This causes villous atrophy, crypt hypertrophy, and intraepithelial lymphocytosis. With the developing technology and laboratory methods, it was observed that CD is not restricted to a single form characterized by diarrhea; atypical, asymptomatic (silent), and latent forms of the disease have also been discovered. In recent studies, it was observed that its incidence and prevalence have increased in both sexes and all age groups and that it is an autoimmune disease that affects approximately 1% of the world population.22. West J, Fleming KM, Tata LJ, Card TR, Crooks CJ. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol. 2014;109(5):757-68. Since this ratio shows a biopsy-diagnosed population, it is suggested that the prevalence can be around 3% when atypical and asymptomatic cases are included.33. Mooney PD, Leeds JS, Libzo N, Sidhu R, Evans KE, Hall EJ, et al. Case-finding for coeliac disease in secondary care: a prospective multicentre UK study. Dig Liver Dis. 2014;46(1):32-5.
Atypical and asymptomatic cases are frequently seen in patients diagnosed in adulthood. In the classical form seen in adults, only half of the cases have diarrhea, bloating, and abdominal discomfort. Diarrhea is sudden and is often chronic. There are no gastrointestinal symptoms in the atypical form. Hematological, psychiatric, endocrine, renal, neurological, rheumatologic, dermatological, and cardiovascular symptoms are common in the atypical form.44. Paez MA, Gramelspacher AM, Sinacore J, Winterfield L, Venu M. Delay in diagnosis of celiac disease in patients without gastrointestinal complaints. Am J Med. 2017;130(11):1318-23. However, most of these patients show severe mucosal damage and have a celiac-specific antibody pattern.
In our study, we aimed to perform a retrospective evaluation to determine their reason for admission, the prevalence of anemia, hypertransaminasemia at the time of diagnosis, and the frequency of autoimmune disease diagnosed in follow up or at the diagnosis in patients who were diagnosed with CD at our center over the age of 18 and were followed up for at least 3 years.
MATERIAL AND METHODS
Study group
Patients diagnosed with CD, with celiac antibodies positivity and duodenal biopsy between December 2008 and December 2015 were included in the study. CD was confirmed by the presence of partial, subtotal or total villous atrophy on duodenal biopsies, associated with increased intraepithelial lymphocyte counts and crypt hyperplasia, as defined by the modified Marsh criteria.33. Mooney PD, Leeds JS, Libzo N, Sidhu R, Evans KE, Hall EJ, et al. Case-finding for coeliac disease in secondary care: a prospective multicentre UK study. Dig Liver Dis. 2014;46(1):32-5.
Inclusion Criteria
Patients who had transaminase levels, complete blood count (CBC), anemia panel (iron, total iron-binding capacity, ferritin, B12, folic acid), thyroid function tests (free triiodothyronine (fT3), free thyroxine (fT4), thyroid-stimulating hormone (TSH), Anti-M antibodies, Anti-T antibodies) and celiac antibodies (anti-tissue transglutaminase immunoglobulin Ig A, anti-gliadin antibody IgA and IgG, anti-endomysial Ig A and G) at the time of diagnosis were included the study.
Exclusion Criteria
Patients diagnosed at under 18 years old, patients who had missing laboratory data and celiac antibodies at admission, patients whose duodenum and bulbous biopsies did not support CD were not included in the study.
The study was approved by the local ethical committee of the Tepecik Education and Research Hospital (No: 2019-0014).
Evaluation of patients in the study group
Symptoms of patients at admission, frequency, and type of anemia, transaminase levels, positivity, and levels of celiac antibodies and autoimmune diseases diagnosed during follow up were evaluated retrospectively.
Statistical analysis
Statistical analysis of the study was performed using SPSS 22.0 (IBM Statistical Package for Social Sciences software version 22). Continuous variables were expressed as mean ± standard deviation (SD) or median (minimum-maximum) and categorical variables as percentages. The Chi-square test was used to compare categorical values between groups, and the Mann-Whitney U test was used to compare continuous variables between groups. P < 0.05 was considered statistically significant.
RESULTS
Demographic characteristics of patients and distribution by age groups
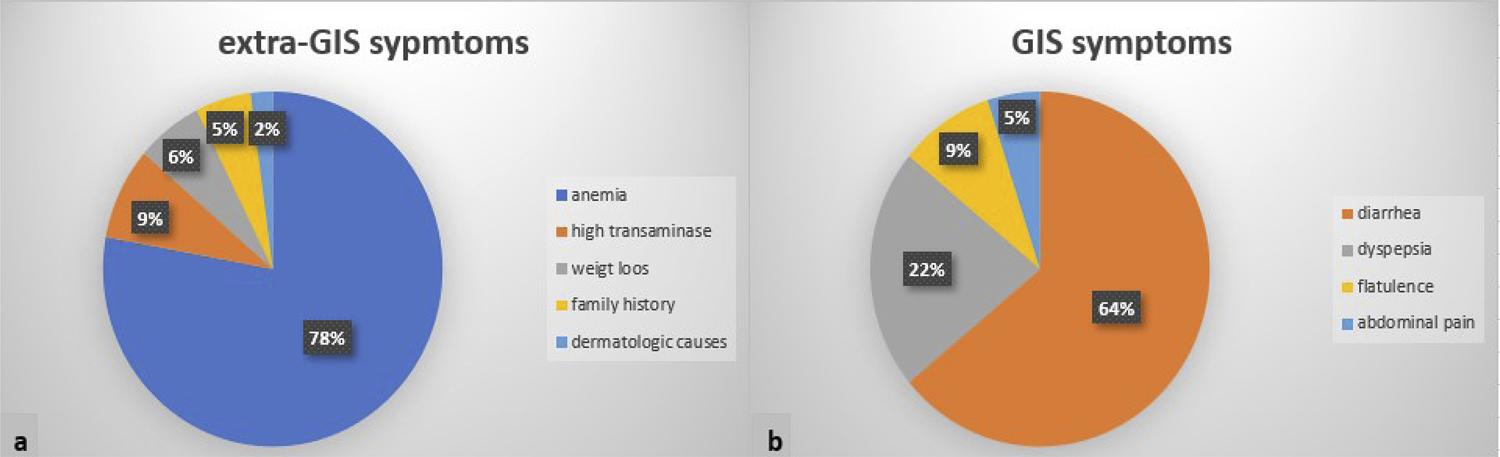
A total of 195 patients (151 females, 44 males) were included in the study. The mean age of the patients was 35.73 ± 12.19 years (range, 18-71 years). A hundred of these patients (51.3%) were referred to the gastroenterology outpatient clinic because of gastrointestinal (GIS) symptoms, 90 (46.2%) for extra-GIS symptoms, and 5 (2.6%) due to family history. The distribution of patients according to age groups and their reasons for referral to the gastroenterology outpatient clinic are summarized in Table 1. The distribution of the groups at admission are summarized in Figure 1a and b.
Serology positivity at admission
At the time of diagnosis, 185 (94.8%) of the cases had anti-tissue transglutaminase IgA, 180 (92.4%) had the anti-endomysium antibody, and 176 (90.2%) had anti-gliadin antibody positivity.
Rates of anemia and hypertransaminasemia in patients at admission
A hundred and eighteen patients (60.5%) had anemia (hemoglobin level; female: <12, male: <13 g/L) at admission. A total of 53.3% of patients had iron deficiency anemia (IDA) (normal values of ferritin : 10-120 ng/mL, normal values of transferrin saturation: 20-40 ug/dL), 38.4% had folic acid deficiency (normal values of folic acid: 4.6-18.7 pg/mL), 25% had B12 deficiency (normal values of B12: 191-663 pg/mL) and 10.2% had chronic disease anemia. Fifty-two patients (26.7%) had hypertransaminasemia (AST or ALT level > 35 U/L) at admission. The rates of anemia and hypertransaminasemia at the time of admission according to age groups are summarized in Figure 2a and b. In the subgroup analysis, no statistically significant difference was found between the groups in terms of anemia and hypertransaminasemia when the patients were classified as < 40 and ≥ 40 years old (Table 2).
A) DISTRIBUTION OF ANEMIA RATES ACCORDING TO AGE GROUPS B) DISTRIBUTION OF HYPERTRANSAMINASEMIA ACCORDING TO AGE GROUPS.
THE RATES OF ANEMIA AND HYPERTRANSAMINASEMIA AT DIAGNOSIS AND THYROID AND AUTOIMMUNE DISEASES IN FOLLOW-UP AT PATIENTS < 40 AND ≥ 40 YEARS OLD.
Rates of autoimmune diseases diagnosed during follow-up
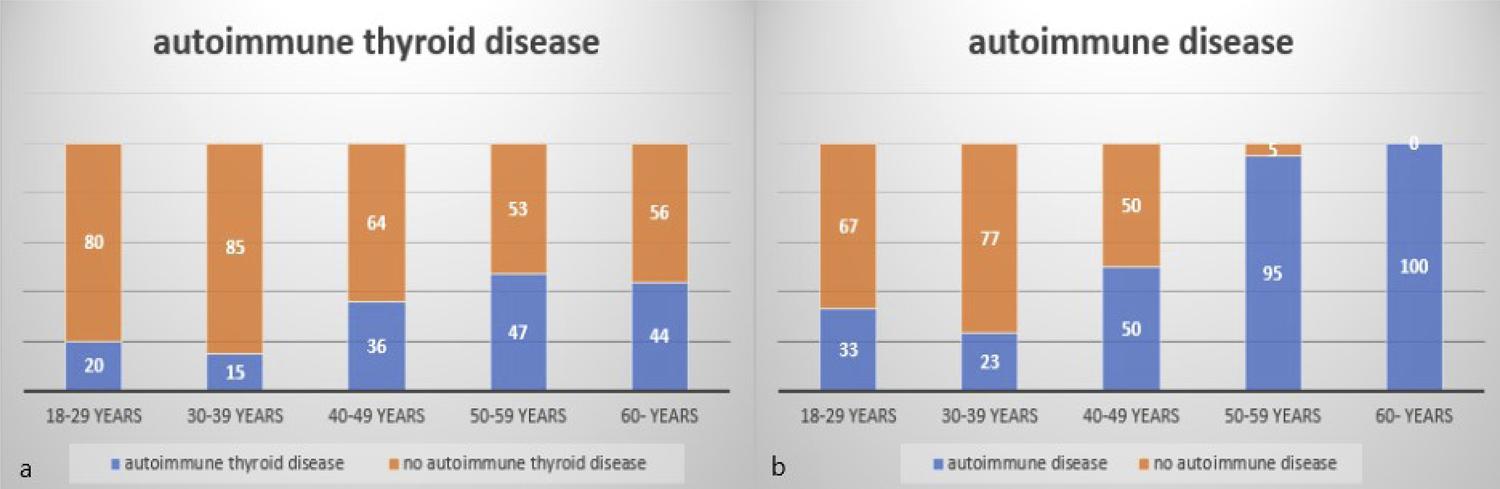
The mean follow-up period of 195 patients with CD diagnosed between December 2008 and December 2015 was 58 months (36-120 months). During the follow-up period, it was found that 84 patients (43.1%) presented at least one autoimmune disease, and the most common was autoimmune thyroid disease (25.6%) (Table 3). The total number of autoimmune diseases was 94. The distribution of autoimmune thyroid diseases and other autoimmune diseases according to age groups is summarized in Figure 3a and b. In the subgroup analysis, it was found that there was a statistically significant difference between the groups in terms of autoimmune thyroid diseases and other autoimmune diseases when the patients were classified as < 40 and ≥ 40 years old (Table 2).
A) DISTRIBUTION OF PATIENTS WITH AUTOIMMUNE THYROID DISEASES ACCORDING TO AGE GROUPS DURING FOLLOW-UP B) DISTRIBUTION OF PATIENTS DIAGNOSED WITH AUTOIMMUNE DISEASE ACCORDING TO AGE GROUPS DURING FOLLOW-UP.
DISCUSSION
As with other autoimmune diseases, CD is 2 to 3 times more common in females, affecting approximately 1% of the community.55. Bardella MT, Fredella C, Saladino V, Trovato C, Cesana BM, Quatrini M, et al. Gluten intolerance: gender- and age-related differences in symptoms. Scand J Gastroenterol. 2005;40(1):15-9. In our study, most patients were female (female/male; 2.9), similar to the literature. The prevalence of CD diagnosed with biopsy is reported between 0.4-0.9% in our country.66. Çaltepe G. The hidden danger: silent celiac disease. Turk J Gastroenterol. 2018;29(5):530-1.,77. Sezgin O, Sarıtaş B, Aydın İ, Şaşmaz T, Linke ES. Celiac disease prevalence in Turkey: a population based cross-sectional study. Acta Med Mediterr. 2016;32:463-71. Developing diagnostic methods showed that the disease indeed resembles an iceberg and can be seen in individuals with atypical symptoms and in asymptomatic individuals, as well as in those with typical symptoms. In studies, especially in an adult group, it is found that the delays in diagnosis are quite frequent because of the frequency of atypical symptoms.44. Paez MA, Gramelspacher AM, Sinacore J, Winterfield L, Venu M. Delay in diagnosis of celiac disease in patients without gastrointestinal complaints. Am J Med. 2017;130(11):1318-23.,88. Grode L, Bech BH, Jensen TM, Humaidan P, Agerholm IE, Plana-Ripoll O, et al. Prevalence, incidence, and autoimmune comorbidities of celiac disease: a nation-wide, population-based study in Denmark from 1977 to 2016. Eur J Gastroenterol Hepatol. 2018;30(1):83-91.,99. Zipser RD, Patel S, Yahya KZ, Baisch DW, Monarch E. Presentations of adult celiac disease in a nationwide patient support group. Dig Dis Sci. 2003;48(4):761-4.In the study of Volta et al.1010. Volta U, Caio G, Stanghellini V, De Giorgio R. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol. 2014;14:194., 86% of adult patients diagnosed in the last 5 years were found to be with non-classical and subclinical phenotypes. In the literature, we see that atypical and silent forms are more prominent than the classical symptoms in the cases diagnosed in adulthood in the last decades.44. Paez MA, Gramelspacher AM, Sinacore J, Winterfield L, Venu M. Delay in diagnosis of celiac disease in patients without gastrointestinal complaints. Am J Med. 2017;130(11):1318-23.,1010. Volta U, Caio G, Stanghellini V, De Giorgio R. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol. 2014;14:194. In our study, we found that almost half of the patients presented with extra-GIS symptoms. In addition, one-fifth of the cases referred for GIS symptoms were individuals with dyspeptic symptoms. In the subgroup analysis, more than 55% of patients, especially in the age groups of 30-50 and over 60, were referred with extra-GIS symptoms. The majority of these cases consisted of patients with resistant anemia and hypertransaminasemia. This shows that screening tests, such as anti-tissue transglutaminase Ig A, are important in individuals with resistant anemia, dyspepsia, and hypertransaminasemia, especially in the adult age group without typical GIS symptoms.
CD is also highly associated with other autoimmune diseases.88. Grode L, Bech BH, Jensen TM, Humaidan P, Agerholm IE, Plana-Ripoll O, et al. Prevalence, incidence, and autoimmune comorbidities of celiac disease: a nation-wide, population-based study in Denmark from 1977 to 2016. Eur J Gastroenterol Hepatol. 2018;30(1):83-91.,1111. Bibbò S, Pes GM, Usai-Satta P, Salis R, Soro S, Quarta Colosso BM, et al. Chronic autoimmune disorders are increased in coeliac disease: a case-control study. Medicine (Baltimore). 2017;96(47):e8562.
12. Collin P, Salmi J, Hällström O, Reunala T, Pasternack A. Autoimmune thyroid disorders and coeliac disease. Eur J Endocrinol. 1994;130(2):137-40.
13. Kalkan Ç, Karakaya F, Soykan I. Similarities and differences between older and young adult patients with celiac disease. Geriatr Gerontol Int. 2017;17(11):2060-7.-1414. Lundin KE, Wijmenga C. Coeliac disease and autoimmune disease-genetic overlap and screening. Nat Rev Gastroenterol Hepatol. 2015;12(9):507-15. In the large patient cohort study by Grode et al.88. Grode L, Bech BH, Jensen TM, Humaidan P, Agerholm IE, Plana-Ripoll O, et al. Prevalence, incidence, and autoimmune comorbidities of celiac disease: a nation-wide, population-based study in Denmark from 1977 to 2016. Eur J Gastroenterol Hepatol. 2018;30(1):83-91., the disease was associated with 31 different autoimmune comorbid diseases. Studies have shown that CD is more common in patients with type 1 Diabetes Mellitus, thyroiditis, and psoriasis because of shared immune-associated pathogenesis.1111. Bibbò S, Pes GM, Usai-Satta P, Salis R, Soro S, Quarta Colosso BM, et al. Chronic autoimmune disorders are increased in coeliac disease: a case-control study. Medicine (Baltimore). 2017;96(47):e8562.,1414. Lundin KE, Wijmenga C. Coeliac disease and autoimmune disease-genetic overlap and screening. Nat Rev Gastroenterol Hepatol. 2015;12(9):507-15. The association of endocrine and dermatological events is more common in the diagnosis of CD in adulthood. The most common concomitant autoimmune disease was Hashimato’s thyroiditis.1515. Demirezer Bolat A, Akin FE, Tahtaci M, Tayfur Yürekli Ö, Köseoglu H, Erten Ş, et al. Risk factors for polyautoimmunity among patients with celiac disease: a cross-sectional survey. Digestion. 2015;92(4):185-91. In the literature, the association of thyroid diseases with CD was reported as 2-24.3%.44. Paez MA, Gramelspacher AM, Sinacore J, Winterfield L, Venu M. Delay in diagnosis of celiac disease in patients without gastrointestinal complaints. Am J Med. 2017;130(11):1318-23.,1111. Bibbò S, Pes GM, Usai-Satta P, Salis R, Soro S, Quarta Colosso BM, et al. Chronic autoimmune disorders are increased in coeliac disease: a case-control study. Medicine (Baltimore). 2017;96(47):e8562.,1212. Collin P, Salmi J, Hällström O, Reunala T, Pasternack A. Autoimmune thyroid disorders and coeliac disease. Eur J Endocrinol. 1994;130(2):137-40. The reason for this prevalence appears to be multi genetic loci such as HLA-DR3, HLA-DQ2, common to autoimmune diseases.1616. Houlston RS, Tomlinson IP, Ford D, Seal S, Marossy AM, Ferguson A, et al. Linkage analysis of candidate regions for coeliac disease genes. Hum Mol Genet. 1997;6(8):1335-9. In our study, autoimmune diseases were observed in 43.1% of the cases, and autoimmune thyroid diseases were the most common with 25.6%, due to the mean follow-up period of 58 months after diagnosis. In the subgroup analysis, it was determined that there was a statistically significant difference in autoimmune disease (28.7% versus 71.2%; p < 0.001) and autoimmune thyroid diseases (17.8% versus 40.9%; p < 0.001) in individuals between < 40 and ≥ 40 years old.
In addition, it was found that anemia is quite a common finding, especially in patients with CD. In studies, the prevalence of anemia is 12-85% in patients with CD.44. Paez MA, Gramelspacher AM, Sinacore J, Winterfield L, Venu M. Delay in diagnosis of celiac disease in patients without gastrointestinal complaints. Am J Med. 2017;130(11):1318-23.,1717. Bottaro G, Cataldo F, Rotolo N, Spina M, Corazza GR. The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol. 1999;94(3):691-6.,1818. Harper JW, Holleran SF, Ramakrishnan R, Bhagat G, Green PH. Anemia in celiac disease is multifactorial in etiology. Am J Hematol. 2007;82(11):996-1000. In particular, IDA is quite common due to the pathophysiology of the disease. In one meta-analysis, one of 31 patients with IDA was reported to have CD.1919. Mahadev S, Laszkowska M, Sundström J, Björkholm M, Lebwohl B, Green PHR, et al. Prevalence of celiac disease in patients with iron deficiency anemia: a systematic review with meta-analysis. Gastroenterology. 2018;155(2):374-82. IDA was observed in 26%, folate deficiency in 12%, B12 deficiency in 5% of 405 patients according to Harper et al.’s1818. Harper JW, Holleran SF, Ramakrishnan R, Bhagat G, Green PH. Anemia in celiac disease is multifactorial in etiology. Am J Hematol. 2007;82(11):996-1000.study. IDA and folic acid deficiency associated primarily with the effect of CD on the proximal small bowel mucosa are common findings, and B12 deficiency absorbed from terminal ileum is also another cause. Several hypotheses have tried to explain B12 deficiency in CD. Although it cannot be fully elucidated, one of the probabilities is the association with autoimmune gastric atrophy, which is another autoimmune disease. This may also be due to the disruption of B12 and salivary R protein (haptocorrin) complexes due to concomitant pancreatic insufficiency and associated disruption of pancreatic proteases.2020. Allen RH, Seetharam B, Allen NC, Podell ER, Alpers DH. Correction of cobalamin malabsorption in pancreatic insufficiency with a cobalamin analogue that binds with high affinity to R protein but not to intrinsic factor. In vivo evidence that a failure to partially degrade R protein is responsible for cobalamin malabsorption in pancreatic insufficiency. J Clin Invest. 1978;61(6):1628-34. Another possibility is that villous involvement in celiac disease is not limited to the proximal small bowel but also exists in the ileum.2121. Dickey W. Low serum vitamin B12 is common in coeliac disease and is not due to autoimmune gastritis. Eur J Gastroenterol Hepatol. 2002;14(4):425-7. In studies, B12 deficiency was reported in 11-41% of CD.44. Paez MA, Gramelspacher AM, Sinacore J, Winterfield L, Venu M. Delay in diagnosis of celiac disease in patients without gastrointestinal complaints. Am J Med. 2017;130(11):1318-23.,2121. Dickey W. Low serum vitamin B12 is common in coeliac disease and is not due to autoimmune gastritis. Eur J Gastroenterol Hepatol. 2002;14(4):425-7. In our study, we found that more than half of the patients were anemic (60.1%) in all age groups at the time of admission. In accordance with the literature, it was observed that IDA (53.3%) and folic acid deficiency (38.4%) were the most frequent, and B12 deficiency (25.6%) was found in one-fourth of the cases. In the subgroup analysis, the rates of anemia were found to be similar in individuals < 40 and ≥ 40 years old (62% versus 57.6%; p = 0.327).
Hypertransaminasemia is a very common finding in treatment-naive patients and is seen in approximately 40% of adult cases and 60% of pediatric cases at the time of diagnosis.2222. Rubio-Tapia A, Murray JA. Liver involvement in celiac disease. Minerva Med. 2008;99(6):595-604. In addition, approximately 9% of individuals with chronic unexplained hypertransaminasemia can have clinical or serological evidence of CD.2323. Bardella MT, Vecchi M, Conte D, Del Ninno E, Fraquelli M, Pacchetti S, et al. Chronic unexplained hypertransaminasemia may be caused by occult celiac disease. Hepatology. 1999;29(3):654-7. Studies have shown that the risk of late-stage liver disease is 2 to 6 times greater, and the risk of death due to liver cirrhosis is 8 times higher than in the normal population.2424. Ludvigsson JF, Elfström P, Broomé U, Ekbom A, Montgomery SM. Celiac disease and risk of liver disease: a general population-based study. Clin Gastroenterol Hepatol. 2007;5(1):63-9.However, the relationship between the disease and liver damage cannot be clearly identified; in individuals susceptible, following gluten exposure to the abnormal intestinal permeability due to a predisposition of common genetic factors, such as HLA associated with autoimmunity, cytokines, autoantibodies, and various biological mediators are thought to play a role in the pathogenesis.2525. Peláez-Luna M, Schmulson M, Robles-Díaz G. Intestinal involvement is not sufficient to explain hypertransaminasemia in celiac disease? Med Hypotheses. 2005;65(5):937-41. In our study, close to one-fourth of the cases (26.7%) had hypertransaminasemia at the admission. In addition, 2 patients (1%) diagnosed with cirrhosis were also diagnosed with CD based on the results of celiac antibodies and duodenal biopsy, although they were asymptomatic. In the subgroup analysis, hypertransaminasemia was found to be similar in individuals < 40 and ≥ 40 years old (26.4% versus 27.3%; p = 0.51).
Since our study is a retrospective study, it has some limitations. First, there is a lack of data for many patients regarding the duration of symptoms in the past during their referral to the outpatient clinic. This prevents us from having data about the delay of the diagnosis. Secondly, due to the lack of detailed data on the other causes of anemia, such as diet, menstrual cycle-related excessive bleeding, anemia rates reflect the overall rates of anemia seen in CD at the time of diagnosis. Thirdly, it is useful to note that the cases with hypertransaminasemia at the time of diagnosis had not only CD but should also be associated with other diseases that cause hypertransaminasemia, such as hypothyroidism, as well as concomitant autoimmune liver disease.
CONCLUSIONS
In conclusion, CD patients may not present typical symptoms and signs in adulthood. Especially in individuals with unexplained resistant anemia, dyspepsia, and hypertransaminasemia, screening tests for CD are important. The disease is frequently associated with other autoimmune diseases due to its autoimmune character. We think that, especially in cases diagnosed above the age of 40 years, it is necessary to conduct an examination and screening tests, especially for a second comorbid such as endocrine and rheumatic autoimmune diseases.
REFERENCES
-
1Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2(9):647-55.
-
2West J, Fleming KM, Tata LJ, Card TR, Crooks CJ. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol. 2014;109(5):757-68.
-
3Mooney PD, Leeds JS, Libzo N, Sidhu R, Evans KE, Hall EJ, et al. Case-finding for coeliac disease in secondary care: a prospective multicentre UK study. Dig Liver Dis. 2014;46(1):32-5.
-
4Paez MA, Gramelspacher AM, Sinacore J, Winterfield L, Venu M. Delay in diagnosis of celiac disease in patients without gastrointestinal complaints. Am J Med. 2017;130(11):1318-23.
-
5Bardella MT, Fredella C, Saladino V, Trovato C, Cesana BM, Quatrini M, et al. Gluten intolerance: gender- and age-related differences in symptoms. Scand J Gastroenterol. 2005;40(1):15-9.
-
6Çaltepe G. The hidden danger: silent celiac disease. Turk J Gastroenterol. 2018;29(5):530-1.
-
7Sezgin O, Sarıtaş B, Aydın İ, Şaşmaz T, Linke ES. Celiac disease prevalence in Turkey: a population based cross-sectional study. Acta Med Mediterr. 2016;32:463-71.
-
8Grode L, Bech BH, Jensen TM, Humaidan P, Agerholm IE, Plana-Ripoll O, et al. Prevalence, incidence, and autoimmune comorbidities of celiac disease: a nation-wide, population-based study in Denmark from 1977 to 2016. Eur J Gastroenterol Hepatol. 2018;30(1):83-91.
-
9Zipser RD, Patel S, Yahya KZ, Baisch DW, Monarch E. Presentations of adult celiac disease in a nationwide patient support group. Dig Dis Sci. 2003;48(4):761-4.
-
10Volta U, Caio G, Stanghellini V, De Giorgio R. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol. 2014;14:194.
-
11Bibbò S, Pes GM, Usai-Satta P, Salis R, Soro S, Quarta Colosso BM, et al. Chronic autoimmune disorders are increased in coeliac disease: a case-control study. Medicine (Baltimore). 2017;96(47):e8562.
-
12Collin P, Salmi J, Hällström O, Reunala T, Pasternack A. Autoimmune thyroid disorders and coeliac disease. Eur J Endocrinol. 1994;130(2):137-40.
-
13Kalkan Ç, Karakaya F, Soykan I. Similarities and differences between older and young adult patients with celiac disease. Geriatr Gerontol Int. 2017;17(11):2060-7.
-
14Lundin KE, Wijmenga C. Coeliac disease and autoimmune disease-genetic overlap and screening. Nat Rev Gastroenterol Hepatol. 2015;12(9):507-15.
-
15Demirezer Bolat A, Akin FE, Tahtaci M, Tayfur Yürekli Ö, Köseoglu H, Erten Ş, et al. Risk factors for polyautoimmunity among patients with celiac disease: a cross-sectional survey. Digestion. 2015;92(4):185-91.
-
16Houlston RS, Tomlinson IP, Ford D, Seal S, Marossy AM, Ferguson A, et al. Linkage analysis of candidate regions for coeliac disease genes. Hum Mol Genet. 1997;6(8):1335-9.
-
17Bottaro G, Cataldo F, Rotolo N, Spina M, Corazza GR. The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol. 1999;94(3):691-6.
-
18Harper JW, Holleran SF, Ramakrishnan R, Bhagat G, Green PH. Anemia in celiac disease is multifactorial in etiology. Am J Hematol. 2007;82(11):996-1000.
-
19Mahadev S, Laszkowska M, Sundström J, Björkholm M, Lebwohl B, Green PHR, et al. Prevalence of celiac disease in patients with iron deficiency anemia: a systematic review with meta-analysis. Gastroenterology. 2018;155(2):374-82.
-
20Allen RH, Seetharam B, Allen NC, Podell ER, Alpers DH. Correction of cobalamin malabsorption in pancreatic insufficiency with a cobalamin analogue that binds with high affinity to R protein but not to intrinsic factor. In vivo evidence that a failure to partially degrade R protein is responsible for cobalamin malabsorption in pancreatic insufficiency. J Clin Invest. 1978;61(6):1628-34.
-
21Dickey W. Low serum vitamin B12 is common in coeliac disease and is not due to autoimmune gastritis. Eur J Gastroenterol Hepatol. 2002;14(4):425-7.
-
22Rubio-Tapia A, Murray JA. Liver involvement in celiac disease. Minerva Med. 2008;99(6):595-604.
-
23Bardella MT, Vecchi M, Conte D, Del Ninno E, Fraquelli M, Pacchetti S, et al. Chronic unexplained hypertransaminasemia may be caused by occult celiac disease. Hepatology. 1999;29(3):654-7.
-
24Ludvigsson JF, Elfström P, Broomé U, Ekbom A, Montgomery SM. Celiac disease and risk of liver disease: a general population-based study. Clin Gastroenterol Hepatol. 2007;5(1):63-9.
-
25Peláez-Luna M, Schmulson M, Robles-Díaz G. Intestinal involvement is not sufficient to explain hypertransaminasemia in celiac disease? Med Hypotheses. 2005;65(5):937-41.
Publication Dates
-
Publication in this collection
27 Feb 2020 -
Date of issue
2020
History
-
Received
01 July 2019 -
Accepted
29 July 2019