Abstract
Mantis shrimps are prominent predatory crustaceans. Their larvae, although morphologically very differently-appearing from their adult counterparts, are already predators; yet, unlike the adults they are not benthic. Instead they are part of the plankton preying on other planktic organisms. Similar to some types of lobsters and crab-like crustaceans the planktic larvae of mantis shrimps can grow quite large, reaching into the centimeter range. Nonetheless, our knowledge on mantis shrimp larvae is still rather limited. Recently new types of giant mantis shrimp larvae with “extreme morphologies” have been reported. Here we describe another type that qualifies to be called “extreme”. Comparative measurements of certain morphological structures on selected known larvae support the exceptionality of the new specimen. It differs in several aspects from the original four types of extreme mantis shrimp larvae described by C. Haug et al. (2016). With this fifth type we expand the known morphological diversity of mantis shrimp larvae and also contribute to our still very incomplete, although growing, knowledge of this life phase.
Key Words
Stomatopoda; Verunipeltata; giant larva; erichthus; plankton
Introduction
Plankton is the entirety of organisms in the water column that are unable to swim against the current (e.g., Lalli and Parsons, 1997Lalli, C.M. and Parsons, T.R. 1997. Biological Oceanography. An introduction. 2nd edition, Oxford, Elsevier Butterworth-Heinemann. ). The metazoan fraction of the plankton - zooplankton - is largely dominated by crustaceans, especially copepods (e.g., Sommer and Stibor, 2002Sommer, U. and Stibor, H. 2002. Copepoda - Cladocera - Tunicata: The role of three major mesozooplankton groups in pelagic food webs. Ecological Research, 17: 161-174.), but more precisely, by larval forms of crustaceans. Among these forms are, besides copepod larvae, larval stages of crabs, lobsters, shrimps, prawns, but also those of mantis shrimps (Stomatopoda, Verunipeltata). Adult mantis shrimps are fierce predators in the benthos. Yet, their larvae live among the plankton, but are likewise predators, preying on other planktic organisms.
Although the general assumption is that being part of the plankton immediately means being small (but see Lalli and Parsons, 1997Lalli, C.M. and Parsons, T.R. 1997. Biological Oceanography. An introduction. 2nd edition, Oxford, Elsevier Butterworth-Heinemann. , their fig. 1.2), crustacean larvae include exceptions to this assumption with larval forms reaching into the centimeter range. Among these exceptions are larval forms of different lobsters (polychelidan lobsters, e.g., Martin, 2014Martin, J.W. 2014. Polychelida. p. 279-282. In: Martin, J.W.; Olesen, J. and Høeg, J.T. (eds), Atlas of Crustacean Larvae. Baltimore, The Johns Hopkins University Press .; Torres et al., 2014Torres, A.P.; Palero, F.; Santos, A.; Abelló, P.; Blanco, E.; Boné, A. and Guerao, G. 2014. Larval stages of the deep-sea lobster Polycheles typhlops (Decapoda, Polychelida) identified by DNA analysis: morphology, systematic, distribution and ecology. Helgoland Marine Research, 68: 379-397.; J.T. Haug et al., 2015Haug, J.T.; Audo, D.; Haug, C.; Abi Saad, P.; Petit, G. and Charbonnier, S. 2015. Unique occurrence of polychelidan lobster larvae in the fossil record and its evolutionary implications. Gondwana Research, 28: 869-874.; Eiler and J.T. Haug, 2016Eiler, S.M. and Haug, J.T. 2016. Larval development of fossil polychelidan crustaceans, exemplified by the 150 million years old species Palaeopentacheles roettenbacheri. Neues Jahrbuch fur Geologie und Paläontologie, Abhandlungen, 279: 295-310.; achelatan lobsters, e.g., Palero et al., 2014Palero, F.; Clark, P.F. and Guerao, G. 2014. Achelata. p. 272-278. In: Martin, J.W.; Olesen, J. and Høeg, J.T. (eds), Atlas of Crustacean Larvae. Baltimore, The Johns Hopkins University Press .; J.T. Haug and C. Haug, 2016Haug, J.T. and Haug, C. 2016. “Intermetamorphic” developmental stages in 150 million-year-old achelatan lobsters - The case of the species tenera Oppel, 1862. Arthropod Structure and Development, 45: 108-121.), larvae of crab-like crustaceans (Martin and Ormsby, 1991Martin J. and Ormsby, B.1991. A large brachyuran-like larva of the Hippidae (Crustacea: Decapoda: Anomura) from the Banda Sea, Indonesia: the largest known zoea. Proceedings of the Biological Society of Washington, 104: 561-568.; Rudolf et al., 2016Rudolf, N.R.; Haug, C. and Haug, J.T. 2016. Functional morphology of giant mole crab larvae: a possible case of defensive enrollment. Zoological Letters, 2: art. 17.), but also the later larval stages of mantis shrimps.
The latter have generally been differentiated into two types: erichthus and alima (for a more detailed overview on the morphology and development of these larvae, see Ahyong et al., 2014Ahyong, S.T.; Haug, J.T. and Haug, C. 2014. Stomatopoda. p. 185-189. In: Martin, J.W.; Olesen, J., and Høeg, J.T. (eds), Atlas of Crustacean Larvae. Baltimore, The Johns Hopkins University Press. and C. Haug et al., 2016Haug, J.T.; Rudolf, N.R.; Wagner, P.; Gundi, P.T.; Fetzer, L.-L. and Haug, C. 2016. An intermetamorphic larval stage of a mantis shrimp and its contribution to the 'missing-element problem' of stomatopod raptorial appendages. Annual Research and Review in Biology, 10: 1-19.). This differentiation dates back to the work of Giesbrecht (1910Giesbrecht, W. 1910. Stomatopoden, Erster Theil. Fauna und Flora des Golfes von Neapel, Monographie, 33: 1-39.). The work of Giesbrecht (1910Giesbrecht, W. 1910. Stomatopoden, Erster Theil. Fauna und Flora des Golfes von Neapel, Monographie, 33: 1-39.), despite its age, is in fact still a key publication in the field of mantis shrimp larvae (Ahyong et al., 2014Ahyong, S.T.; Haug, J.T. and Haug, C. 2014. Stomatopoda. p. 185-189. In: Martin, J.W.; Olesen, J., and Høeg, J.T. (eds), Atlas of Crustacean Larvae. Baltimore, The Johns Hopkins University Press.). Since then mantis shrimp larvae have been treated astonishingly rarely in a comparable way in the literature (notable exceptions are Alikunhi, 1952Alikunhi, K.H. 1952. An account of the stomatopod larvae of the Madras plankton. Records of the Indian Museum, 49: 239-319., Townsley, 1953Townsley, S.J. 1953. Adult and larval stomatopod crustaceans occurring in Hawaiian waters. Pacific Science, 7: 399-437. or Shanbhogue, 1975Shanbhogue, S.L. 1975. Descriptions of stomatopod larvae from the Arabian Sea with a list of stomatopod larvae and adults from the Indian Ocean and a key for their identification Part 1. Journal of the Marine Biological Association of India, 17: 196-238.). It might therefore not be surprising that, although we know of about 500 species of mantis shrimps, we only know the larval series of about half a dozen of these (Gohar and Al-Kholy, 1957Gohar, H.A.F. and Al-Kholy, A.A. 1957. The larval stages of three stomatopod Crustacea. Publications of the Marine Biological Station, Al-Ghardaqa, Red Sea, 9: 85-130.; Manning and Provenzano, 1963Manning, R.B. and Provenzano, A.J. 1963. Studies on development of stomatopod Crustacea I. Early larval stages of Gonodactylus oerstedii Hansen. Bulletin of Marine Science, 13: 467-487.; Pyne, 1972Pyne, R.R. 1972. Larval development and behaviour of the mantis shrimp, Squilla armata Milne Edwards (Crustacea: Stomatopoda). Journal of the Royal Society of New Zealand, 2: 121-146.; Provenzano and Manning, 1978Provenzano, A.J. and Manning, R.B. 1978. Studies on development of stomatopod Crustacea II. The later larval stages of Gonodactylus oerstedii Hansen reared in the laboratory. Bulletin of Marine Science, 28: 297-315.; Morgan and Provenzano, 1979Morgan, S.G. and Provenzano, A.J. 1979. Development of pelagic larvae and postlarva of Squilla empusa (Crustacea, Stomatopoda) with an assessment of larval characters within the Squillidae. Fishery Bulletin, 77: 61-90.; Greenwood and Williams, 1984Greenwood, J.G. and Williams, B.G. 1984. Larval and early post-larval stages in the abbreviated development of Heterosquilla tricarinata (Claus, 1871) (Crustacea, Stomatopoda). Journal of Plankton Research, 6: 615-635.; Hamano and Matsuura, 1987Hamano, T. and Matsuura, S. 1987. Egg size, duration of incubation, and larval development of the Japanese mantis shrimp in the laboratory. Nippon Suisan Gakkaishi, 53: 23-39.; Morgan and Goy, 1987Morgan, S.G. and Goy, J.G. 1987. Reproduction and larval development of the mantis shrimp Gonodactylus bredini (Crustacea: Stomatopoda) maintained in the laboratory. Journal of Crustacean Biology, 7: 595-618.; Diaz and Manning, 1998Diaz, G.A. and Manning, R.B. 1998. The last pelagic stage and juvenile of Lysiosquilla scabricauda (Lamarck, 1818) (Crustacea, Stomatopoda). Bulletin of Marine Science, 63: 453-457.; see discussion in J.T. Haug et al., 2016Haug, J.T.; Rudolf, N.R.; Wagner, P.; Gundi, P.T.; Fetzer, L.-L. and Haug, C. 2016. An intermetamorphic larval stage of a mantis shrimp and its contribution to the 'missing-element problem' of stomatopod raptorial appendages. Annual Research and Review in Biology, 10: 1-19.) and isolated stages of a few more. Even with modern molecular methods it is still frustratingly difficult to identify larvae to species (e.g., Tang et al., 2010Tang, R.W.; Yau, C. and Ng, W.C. 2010. Identification of stomatopod larvae (Crustacea: Stomatopoda) from Hong Kong waters using DNA barcodes. Molecular Ecology Resources, 10: 439-448.; but see Feller et al., 2013Feller, K.D.; Cronin, T.W.; Ahyong, S.T. and Porter, M.L. 2013. Morphological and molecular description of the late-stage larvae of Alima Leach, 1817 (Crustacea: Stomatopoda) from Lizard Island, Australia. Zootaxa, 3722: 22-32.).
The fact that our knowledge of mantis shrimp larvae is rather limited combined with the large size and predatory nature of these larvae leaves us with a strange situation. The large number of mantis shrimp larvae found in plankton samples (pers. obs.) points to relatively high abundances. Hence, there appears to be a rather large fraction of the plankton that is predatory on smaller plankton, and is at the same time the food source for even larger predators (e.g., Brock, 1985Brock, R.E. 1985. Preliminary study of the feeding habits of pelagic fish around Hawaiian fish aggregation devices or can fish aggregation devices enhance local fishery productivity? Bulletin of Marine Science, 37: 40-49). When trying to understand food web interactions in the oceans, the lack of knowledge about the biology of mantis shrimp larvae is quite unfortunate.
Recent work on historical material of stomatopod larvae provided important new findings especially widening the known morphological diversity of mantis shrimps larvae. C. Haug and J.T. Haug (2014Haug, C. and Haug, J.T. 2014. Defensive enrolment in mantis shrimp larvae (Malacostraca: Stomatopoda). Contributions to Zoology, 83: 185-194.) reported morphological adaptations of certain erichthus-type larvae that allow these forms to perform defensive enrolment, comparable to well known terrestrial modern arthropods such as pill bugs and pill millipedes, or the extinct marine trilobites.
J.T. Haug et al. (2016Haug, J.T.; Rudolf, N.R.; Wagner, P.; Gundi, P.T.; Fetzer, L.-L. and Haug, C. 2016. An intermetamorphic larval stage of a mantis shrimp and its contribution to the 'missing-element problem' of stomatopod raptorial appendages. Annual Research and Review in Biology, 10: 1-19.) reported an unusual intermediate stage of a small-sized stomatopod larva that is morphologically in the transition between the early larval phase (antizoea) and later larval phase (erichthus). This specimen provided important new reference points for understanding the composition of the raptorial appendages of modern mantis shrimps.
C. Haug et al. (2016Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.) reported four different morphotypes of new erichthus-type larvae with rather unusual or “extreme” morphologies. In all these forms the shield has very prominent modifications in the one or other direction. C. Haug et al. (2016Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.) suggested that most of these modifications could be understood as adaptations, allowing these forms to still float in the plankton while achieving comparably large body sizes.
These examples show that also old plankton samples in museum collections can provide valuable contributions to our overall knowledge of biology in general and more specifically to mantis shrimp larvae. Here we report another specimen of a mantis shrimp larva found in a museum collection. The specimen also falls into the “extreme morphology category” but differs from the four so far known types, hence again expanding the morphological diversity of these larval forms.
Material and Methods
Material
The single known specimen central to this study comes from the collection of the Museum für Naturkunde Berlin. The specimen was in a plankton sample together with mainly large-sized phyllosoma-type larvae of achelatan lobsters. The sample had been collected south of Mauritius at a depth of c. 20 m during the German South Pole Expedition (Dt. Südpolar-Expedition) on May 14, 1903.
The specimen is partly damaged (indicated by crack lines). Due to the already fragile condition of the specimen and its origin from a historical expedition, it had to be handled carefully. Hence, not all appendages could be positioned optimally without damaging them, and also some dirt could not be removed without further damaging the specimen and was therefore left in place. The specimen is deposited under collection number 30023A (an older number associated with the specimen is 19353).
Comparative material for measurements came from the Natural History Museum of Denmark (NHMD, originally ZMUC-CRU for crustacean collection of the Zoological Museum of the University of Copenhagen). Some specimens have been presented earlier (for details, check C. Haug and J.T. Haug, 2014Haug, C. and Haug, J.T. 2014. Defensive enrolment in mantis shrimp larvae (Malacostraca: Stomatopoda). Contributions to Zoology, 83: 185-194., C. Haug et al., 2016 Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.and J.T. Haug et al., 2016Haug, J.T. and Haug, C. 2016. “Intermetamorphic” developmental stages in 150 million-year-old achelatan lobsters - The case of the species tenera Oppel, 1862. Arthropod Structure and Development, 45: 108-121.).
Documentation methods
Specimens were kept in their original storage liquid (70% EtOH). They were placed in plastic containers. For positioning the specimens glass blocks and large-sized coverslips were used. Metal nuts and washers were used to fix the coverslips with their weight.
Specimens were documented with a Canon Rebel T3i camera. Depending on the size, either a Canon EF-S 18-55mm lens (sometimes with distance rings) or a MP-E 65 mm macro lens was used. Illumination was provided either by a MeiKe FC 100 LED ring light, a Canon MT 24-EX Macro Twin Flash or two Yongnuo Speedlite Flashes with remote controls. Light was always cross-polarised, i.e. polarisation filters on the light sources and a perpendicularly oriented filter in front of the lens were used. Cross-polarised light reduces reflections and enhances the colour contrast (e.g., C. Haug et al., 2011Haug, C.; Mayer, G.; Kutschera, V.; Waloszek, D.; Maas, A. and Haug, J.T. 2011. Imaging and documenting gammarideans. International Journal of Zoology, art. 380829, DOI 10.1155/2011/380829
https://doi.org/10.1155/2011/380829...
). For overcoming limitations of depth of field stacks of images with differing focal planes were recorded. Limitations of field of view were overcome by documenting adjacent areas of the specimen with detail images (e.g., J.T. Haug et al., 2008Haug, J.T.; Haug, C. and Ehrlich, M. 2008. First fossil stomatopod larva (Arthropoda: Crustacea) and a new way of documenting Solnhofen fossils (Upper Jurassic, Southern Germany). Palaeodiversity, 1: 103-109.; 2011Haug, J.T.; Haug, C.; Kutschera, V.; Mayer, G.; Maas, A.; Liebau, S.; Castellani, C.; Wolfram, U.; Clarkson, E.N.K. and Waloszek, D. 2011. Autofluorescence imaging, an excellent tool for comparative morphology. Journal of Microscopy, 244: 259-272.). Finally in cases of complex three-dimensional structures specimens were documented as stereo images, by moving the camera to some degree left and right (e.g., C. Haug et al., 2011Haug, C.; Mayer, G.; Kutschera, V.; Waloszek, D.; Maas, A. and Haug, J.T. 2011. Imaging and documenting gammarideans. International Journal of Zoology, art. 380829, DOI 10.1155/2011/380829
https://doi.org/10.1155/2011/380829...
). Specimens were documented from at least three sides (dorsal, ventral, lateral) and where possible also in anterior and posterior view.
Image processing
Stacks of images were fused with the freely available program CombineZP. Adjacent image details were stitched to panoramas with Adobe Photoshop CS 3 or Adobe Photoshop Elements 11. Red-cyan stereo anaglyphs were assembled in Adobe Photoshop CS2. Further processing such as removing dirt particles and back ground with a lasso tool, optimisation of the histogram, saturation and sharpness was performed in Adobe Photoshop CS2.
Measurements
Different body dimensions were measured which appear to represent the major determinants of the general body shape, especially of the shield, which corresponds to a large part of the larval body. The measured dimensions are height of the shield (including spines), length of the shield (from insertion area of rostrum to posterior end of midline, hence excluding postero-lateral spines), maximum width of the shield (excluding spines), maximum width of the pleon, and total length (from insertion area of rostrum to posterior end of telson including telson spines) (Fig. 1; Tab. 1). Measuring was performed with the measuring tool in Adobe Acrobat Reader DC. The tool also allows measuring non-straight edges. Scatter plots were made in OpenOffice Calc and redrawn in Adobe Photoshop CS2.
Measurements exemplified on specimen NHMD-232175. A. Ventral view. B. dorsal view. C. Lateral view. Abbreviations: h(s) = height of shield (including spines); l(s) = length of shield (from insertion area of rostrum to posterior end of midline, hence excluding postero-lateral spines); l(t) = total length (from insertion area of rostrum to posterior end of telson including telson spines); w(p) = maximum width pleon; w(s) = maximum width shield (excluding spines).
Calculated ratios of the relative measurements of the specimens (see Fig. 1 for details); abbreviations: h(s) = height of shield; l(s) = length of shield ; l(t) = total length; w(p) = maximum width pleon; w(s) = maximum width shield. In addition to the repository numbers of the specimens in museum collections, also the original numbers (where present) and the reference to the station the specimens had been collected are given.
Results
Description of the specimen
The specimen is about 23 mm long and 16 mm wide (Figs. 2, 3). The body is organised into 20 segments, the ocular segment and 19 post-ocular segments. The segments form distinct tagmata (see C. Haug et al., 2012Haug, C.; Sallam, W.S.; Maas, A.; Waloszek, D.; Kutschera, V. and Haug, J.T. 2012. Tagmatization in Stomatopoda - reconsidering functional units of modern-day mantis shrimps (Verunipeltata, Hoplocarida) and implications for the interpretation of fossils. Frontiers in Zoology, 9: art. 31.):
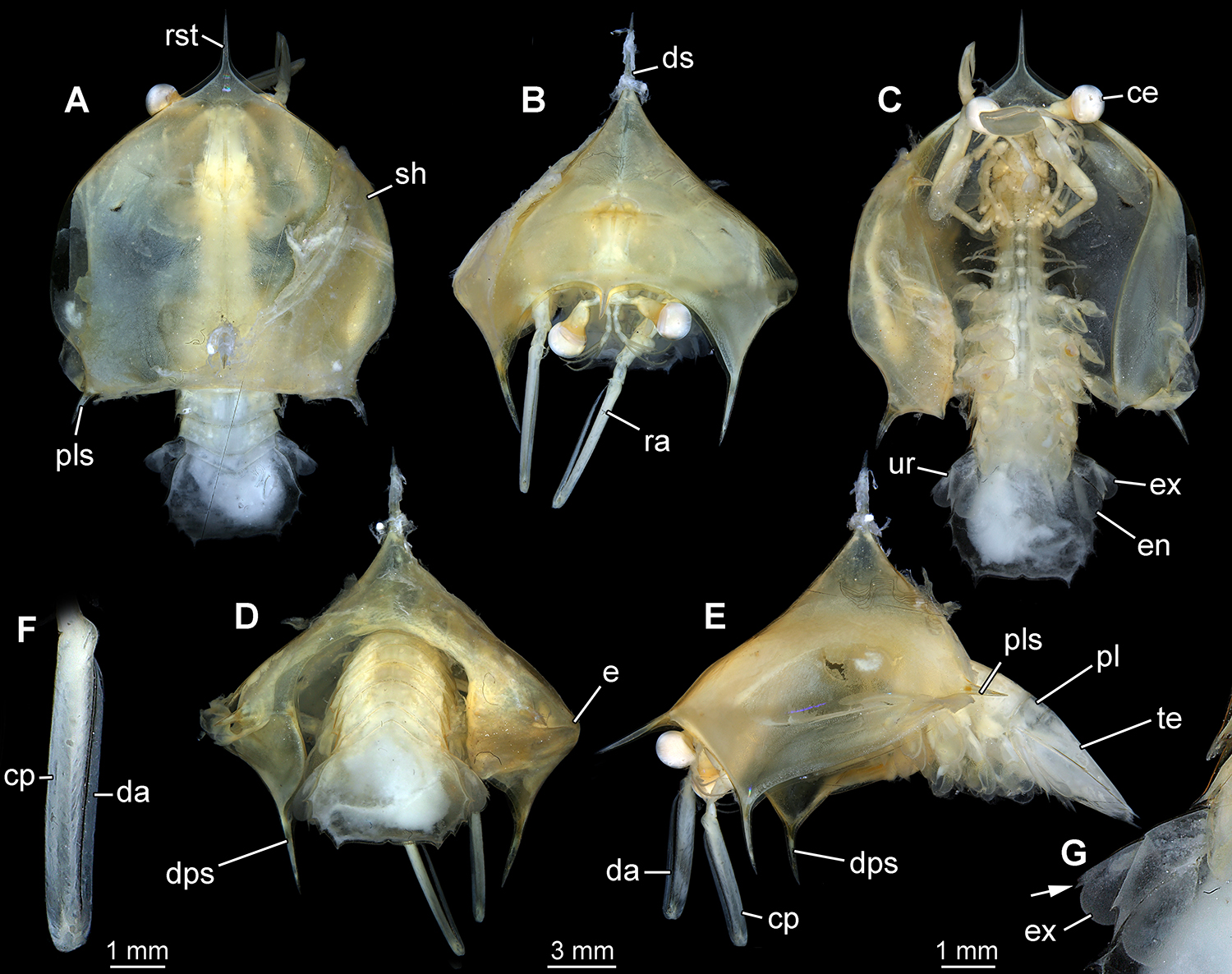
New morphotype 5, “spiked helmet”, specimen 30023A. A. Dorsal view. B. Anterior view. C. Ventral view. D. Posterior view. E. Lateral view on left side. F. Close-up of raptorial appendage. G. Close-up of uropod; note single tooth-like spine on the exopod (arrow). Abbreviations: ce = compound eye; cp = carpopropodus; da = dactylus; dps = downward pointing spine; ds = dorsal spine; e = edge; en = endopod; ex = exopod; pl = pleon; pls = postero-lateral spine; ra = raptorial appendage (appendage of post-ocular segment 7); rst = rostrum; sh = shield; te = telson; ur = uropod.
New morphotype 5, “spiked helmet”, specimen 30023A, stereo images. A. Dorsal view. B. Anterior view. C. Ventral view. D. Posterior view. E. Lateral view on left side. All images are red-cyan stereo-anaglyphs, please use red-cyan glasses to view.
Tagma I, sensorial unit, includes ocular segment and post-ocular segments 1 and 2 with compound eyes, antennulae and antennae. Tagma II, anterior food-processing unit, includes post-ocular segments 3-5 with mandibles, maxillulae and maxillae. Tagma I and II represent the head region.
Tagma III, posterior food-processing unit, includes post-ocular segments 6-9 and part of post-ocular segment 10 with maxillipeds 1-5. Tagma IV, anterior locomotory unit, includes the other parts of post-ocular segment 10 (see C. Haug et al., 2012Haug, C.; Sallam, W.S.; Maas, A.; Waloszek, D.; Kutschera, V. and Haug, J.T. 2012. Tagmatization in Stomatopoda - reconsidering functional units of modern-day mantis shrimps (Verunipeltata, Hoplocarida) and implications for the interpretation of fossils. Frontiers in Zoology, 9: art. 31. for details) and post-ocular segments 11-13 with thoracopods 6-8 (walking legs). Tagma III and IV represent the thorax region (thorax I of Walossek and Müller, 1998Walossek, D. and Müller, K.J. 1998. Cambrian 'Orsten'-type arthropods and the phylogeny of Crustacea. p. 139-153. In: Fortey, R.A. and Thomas, R.H. (eds), Arthropod Relationships. London, Chapman and Hall.).
Post-ocular segments 14-18 with pleopods 1-5 form tagma V, the posterior locomotory unit. Post-ocular segment 19 with uropods and the telson form the tail fan. Tagma V and the tail fan represent the pleon region.
From the posterior part of the head region dorsally a very prominent shield arises, dominating the overall body shape. The shield appears large and massive, and concerning its length, width and height exceeds the “normal” condition for most mantis shrimp larvae, in which the shield does usually not protrude that far from the body in any direction (though there is admittedly a rather large degree of variation between different forms of larvae). In the specimen described here, the shield encloses the anterior part of the body; only pleomeres 4-6 and the tail fan extend from under the shield.
In dorsal view, the contour of the shield is roughly heart-shaped with the tip pointing forward. The anterior tip is drawn out into a short spine directing anteriorly (rostrum). Also the postero-lateral corners of the shield bear one short spine each pointing postero-laterally (Figs. 2A, 3A). The length of all spines of the shield is difficult to determine as they are not clearly set off from the shield, but rather arise from a broad base gently tapering distally.
From anterior or posterior view, the outline seems triangular with one short dorsal spine pointing upwards and two additional short spines pointing downwards from the two outer corners (Figs. 2B, D, 3B, D). In lateral view it becomes apparent that these downward-pointing spines arise from the shield quite far anteriorly (Figs. 2E, 3E).
Ventrally, the shield forms a wide gape, and laterally distinct pouches (Fig. 2C, 3C). Anterior, posterior and lateral view reveal a distinct edge (although not forming a crest) running from the rostrum to the postero-lateral spines. This edge represents the widest dimensions of the shield. In posterior view a distinct notch is apparent, leaving space for the pleon to stick out (Figs. 2D, 3D).
The specimen bears one pair of compound eyes and 19 pairs of appendages (Fig. 4). The eyes are pear shaped, with the distal wider region forming the facetted cornea, and the tapering more proximal region forming the stalks. The eye stalks sit on short bars arising laterally from the ocular segment.
Ventral details of the new larval mantis shrimp specimen 30023A. A. Macro-photograph. B. Colour-coded version of A for identifying the outlines of the structures. Abbreviations: a7-14 = appendage of post-ocular segment 7-14 (= maxillipeds 2-5, thoracopods 1-3, pleopod 1); an = antenna; co = cornea; en = endopod; es = eye stalk; ex = exopod; gi = gill.
The appendage of post-ocular segment 1, the antennula, has three large peduncle elements from which one flagellum and a fourth peduncle element arise distally, the latter carrying two additional flagella. The proximal flagellum appears not yet subdivided into flagellomeres, unlike the distal two flagella (Figs. 2B, 3B).
The appendage of post-ocular segment 2, the antenna, is not accessible in all details. Flagellum and exopod (scaphocerite) seem well developed.
Appendages of post-ocular segments 3-6 (mouth parts and cleaning appendage/maxilliped 1) are not properly accessible; only their general presence can be confirmed, but no further details can be described.
The appendage of post-ocular segment 7, the major raptorial appendage or maxilliped 2, is well developed and the most prominent appendage. Only distal elements are accessible. The penultimate element (carpo-propodus) is quite slender and straight, almost 8x as long as wide. Also the ultimate element (dactylus) is very elongate slender and almost straight. It appears needle-like and is about 24x as long as wide (Fig. 2F).
The appendages of post-ocular segments 8-10, maxillipeds 3-5, are only partly accessible. They are subsimilar in their general organisation, but differ in their size. Maxilliped 3 is the largest, maxilliped 4 is about 1/6 shorter than maxilliped 3 (though exact measurements are difficult with the appendages in situ), and maxilliped 5 is about 2/3 the length of maxilliped 4. Principle arrangement appears similar to that occurring in adult mantis shrimps, with six elements along the proximal-distal axis of which the distal two form a claw, though the differentiation of the elements is only properly visible in the distal ones. The dactyli are slender and almost straight, being about 5-6x as long as wide. The carpo-propodi of maxillipeds 3 and 4 appear to be subquadrate, which would indicate that this specimen is a lysiosquilloid larva (Shane Ahyong, pers. comm., 2018). Proximally, epipods are already visible, but not fully developed yet.
The appendages of post-ocular segments 11-13, thoracopods 6-8, the walking appendages, are relatively small compared to the other appendages when taking into account the length proportions of adult appendages. The future adult morphology is already recognizable, with coxa, basipod, endopod and bipartite exopod, but size and pronounced subdivision are still underdeveloped.
The appendages of post-ocular segments 14-18, the pleopods, are differentiated into the proximal basipod bearing endopod and exopod. Some first gill structures are visible arising from the exopods proximo-anteriorly (probably more medially, but this area is difficult to see; Fig. 4). The gills are composed of finger-like structures, but the exact number of these cannot be determined.
The appendage of post-ocular segment 19, the uropod, is subdivided into the proximal basipod bearing endopod and exopod. The basipod bears a prominent anteriorly arising spine, which possesses two tips. The endopod is ovate in anterior view (of the appendage) and bears setae along the distal margin. The exopod is also ovate in anterior view and bears a prominent spine at the position where in the adult the joint to the distal paddle-like exopod part appears; however it is not yet divided off (Fig. 2G).
The telson is very broad, about 1.6 times as wide as long at its maximum width and length. It bears four pairs of spines along the lateral margins (hence eight spines in total). The spines of the most posterior pair are at far distance from each other, about 2/3 of the maximum width of the telson. At the postero-median edge of the telson a distinct indent is apparent (Fig. 2C).
Scatter plots
Plotting the height to length ratio of the shield against the width to length ratio of the shield results in a graph with different larval morphotypes occupying different areas (Fig. 5A): in the upper left area occur larvae with a relatively high but narrow shield (both compared to the shield length), in the lower left area those with a low and narrow shield; in the upper right area are larvae with a high and broad shield, while in the lower right area larvae with a low but broad shield occur.
Scatter plots of measured dimensions and images of different specimens. A. Plot of height to length ratio of the shield against width to length ratio of the shield. B. Plot of shield width to pleon width ratio against shield length to total length ratio. a. new morphotype 5 “spiked helmet” (30023A); b. erichthus-type with neither “normal” nor “extreme” shield dimensions (NHMD-232172); c. early erichthus-type larva (NHMD-232163); d. elongate erichthus-type larva of the “Jurassic” type (NHMD-232169); e. extreme larva of morphotype 4 “gnome hat larva” (NHMD-86465, originally labeled as ZMUC-CRU-8661); f. extreme larva of morphotype 1 “balloon larva” (NHMD-86466, originally labeled as ZMUC-CRU-8662); g. extreme larva of morphotype 3 “flying saucer” (NHMD-86470, originally labeled as ZMUC-CRU-8666); h. extreme larva of morphotype 2 “spiny balloon” (NHMD-86469, originally labeled as ZMUC-CRU-8665); i. early erichthus-type larva (NHMD-232162); j. early erichthus-type larva with a relatively narrower and slightly shorter shield than i (NHMD-232179); k. elongate erichthus-type larva of the “Jurassic” type (NHMD-232167); l. erichthus-type with shield slightly narrower and shorter than in new morphotype 5 (NHMD-232170); m. erichthus-type with relatively narrow but long shield (NHMD-232175).
In this plot, the majority of the individuals is concentrated in the lower left area of the graph. These are cases in which the shield is relatively low and narrow, hence those larvae with rather streamlined shield morphology. The upper left area of the plot is mostly unoccupied, which means that there were almost no larvae with a relatively high but narrow shield included into the analysis (if this is due to a sampling bias or to a real lack of this morphotype cannot be judged here). A certain scattering occurs in the center of the graph, but most of the larval morphotypes described as extreme plot in the (upper) right area of the graph. Only the spiny balloon (morphotype 2) occurs relatively close to the scattering in the center of the graph, but at its right margin, directing towards the morphotypes described as extreme.
The new larva plots together with the extreme morphotypes, indicating its specific morphology. Still it occurs relatively separate from the other morphotypes, demonstrating that the morphology is different from already known ones. Its closest neighbor is the gnome hat larva (morphotype 4) which mainly has a slightly higher and wider shield (compared to the shield length) than the new larva. Also the balloon larva (morphotype 1) plots relatively close to the new larva, its shield being about the same width but less high (compared to the shield length).
Plotting the shield width to pleon width ratio against the shield length to total length ratio results in another graph, in which different larval morphotypes occupy different areas (Fig. 5B): in the upper left area are larvae with a relatively broad but short shield (compared to pleon width resp. total body length), in the lower left area those with a relatively narrow and short shield; in the upper right area are larvae with a relatively broad and long shield, while in the lower right area larvae with a relatively narrow but long shield appear.
This plot provides a different pattern than the previous one. Also here the morphotypes described as extreme as well as the new larva plot largely in the upper right area of the graph, but also some other larvae plot in this area. The other data points appear rather scattered. The new larva plots closest to a larva which had not been noticed as possessing a very specific morphology previously. However, the gnome hat larva (morphotype 4), which had occurred closest to the new larva in the previous plot (Fig. 5A), could not be included into this graph as the total length could not be measured with the larva being tightly enrolled.
Discussion
Another extreme larva
The new larval specimen possesses a rather unusual morphology for a mantis shrimp larva. The principle organisation is that of an erichthus-type. The specimen is rather large with almost 25 mm overall length and appears quite massive considering width and height. Most prominent is the shield. In this aspect it is comparable to the four extreme larvae described by C. Haug et al. (2016Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.). The scatter plots, especially the one only including shield dimensions, further support this qualitative notion. The larva plots outside the denser occupied area that roughly represents the “normal” mantis shrimp larvae. It plots closer to the extreme larvae of C. Haug et al. (2016Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.) but is clearly separated far enough to be recognised as being something different and new. We therefore suggest to recognise it as a new but different type of extreme larva, shortly referred to as morphotype 5 (in amendment to the four morphotypes described in C. Haug et al., 2016Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.). Due to the highly domed shield with the prominent central spine, we use the nickname “spiked helmet - Pickelhaube” in reference to the helmets worn especially by German military in the 19th and 20th century.
Developmental state
The morphology of the posterior thoracopods and the uropods are very informative concerning the estimation of the developmental state of a mantis shrimp larva. Compared to other larvae we can immediately identify that it is most likely not the ultimate larval stage before the metamorphosis to the juvenile. The future walking appendages are already present, but are still very short and appear rather undifferentiated, i.e. the joints are indicated by faint folds, but appear not yet fully functional. There are late larvae that possess already further differentiated and also relatively longer appendages (e.g., Ahyong et al., 2014Ahyong, S.T.; Haug, J.T. and Haug, C. 2014. Stomatopoda. p. 185-189. In: Martin, J.W.; Olesen, J., and Høeg, J.T. (eds), Atlas of Crustacean Larvae. Baltimore, The Johns Hopkins University Press.; C. Haug et al., 2016Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.). Similarly the uropod is not yet fully differentiated. The exopod only has a single prominent spine indicating a future joint, but it is still not functional (Fig. 2G). Among the extreme larvae, morphotype 3 (flying saucer) for example already has a fully functional joint with a divided off distal paddle-like area of the exopod, and the proximal part of the exopod bears already numerous prominent tooth-like spines.
It seems therefore very likely that another larval stage would follow this stage. We can estimate its maximum size based on the fact that size gain between moults among crustaceans can be up to about 30% (see discussion in Kutschera et al., 2012Kutschera, V.; Maas, A.; Waloszek, D.; Haug, C. and Haug, J.T. 2012. Re-study of larval stages of Amphionides reynaudii (Malacostraca: Eucarida) with modern imaging techniques. Journal of Crustacean Biology, 32: 916-930.). This would mean this stage would supposedly measure 30 mm in total length. While extreme larvae of morphotype 2-4 seem to represent most likely final larval stages, we know morphotype 1 from at least two larval stages. Here already the smaller stage possesses an extreme morphology, in this aspect comparable to the new morphotype 5. This further supports that extreme morphologies are not necessarily restricted to last larval stages but may at least occur also in the penultimate larval stage.
Comparison to the other extreme larvae
In principle the new larva shows a kind of mixture of characters seen in some of the other extreme larvae. Yet, it possesses only few similarities with morphotype 3 (flying saucer) besides the more general aspects shared among all the extreme larvae: 1) prominent shield, that is quite long and wide; 2) the shield forms distinct lateral pouches more or less along the entire shield, as the lateral rims of the shield are folded further ventrally also forming a recognisable outline of the ventral gape; 3) a distinct edge running from the base of the rostrum to the postero-lateral spines.
A distinct difference to morphotype 3 is the major raptorial appendage. In morphotype 3 the carpopropodus is not elongate and rectangular (as in morphotypes 1 and 5), but rather bulbous and rounded. The dactylus is distinctly curved in morphotype 3, unlike the very straight dactylus in morphotype 5.
As pointed out, morphotype 5 is similar to morphotype 1 (balloon larva) in reaching an extreme morphology already in the penultimate larval stage, although it remains unclear what the developmental pattern is in the other types. In the scatter plot only including shield dimensions (Fig. 5A) morphotype 1 is indeed relatively close to the new morphotype 5, but still clearly differing from it. Morphotype 5 is clearly not as strongly rounded and hence not as balloon-shaped as morphotype 1. Also, although in morphotype 1 spines are present, they are not as prominent as in the new morphotype 5. A further similarity between morphotype 1 and 5 is the morphology of the major raptorial appendage. In both forms the carpopropodus is very straight and slender, almost rectangular in outline. The dactylus is also very straight and appears almost dagger-like.
Morphotype 1 lacks downward pointing spines, present in morphotype 5. The overall arrangement of spines in morphotype 5 is more similar to that in morphotype 4 (gnome hat larva). Also in the scatter plot only including shield dimensions (Fig. 5A) morphotype 5 and morphotype 4 are closest neighbors (though still with a distinct distance). Morphotype 4 lacks a distinctly drawn out rostrum, which is well developed in morphotype 5. Yet, this could reflect an ontogenetic effect: the shorter rostrum could be already an adult condition in an ultimate larval stage, while the distinct rostrum in morphotype 4 is still present in the supposedly penultimate stage, but would be reduced in the following stage.
Morphotype 1 and 4 both have a tightly constricted ventral gape, which is different in the new morphotype. In this aspect the new morphotype is more similar to morphotype 2 (spiny balloon), where the gape is wider and less constricted. A tightly constricted ventral gape has been discussed as a possible adaptation for defensive enrollment (C. Haug and J.T. Haug, 2014Haug, C. and Haug, J.T. 2014. Defensive enrolment in mantis shrimp larvae (Malacostraca: Stomatopoda). Contributions to Zoology, 83: 185-194.; Rudolf et al., 2016Rudolf, N.R.; Haug, C. and Haug, J.T. 2016. Functional morphology of giant mole crab larvae: a possible case of defensive enrollment. Zoological Letters, 2: art. 17.). However, the new specimen does not contribute any further information on functional aspects.
As C. Haug et al. (2016Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.) have pointed out there are other larval forms that have some of these characters. For example, some smaller-sized larvae possess already (?) smaller pouches around the bases of the postero-lateral spines. This already gives them a quite special appearance but not as much as for the five extreme morphotypes. There are also some large larval mantis shrimps with a very wide and prominent shield that even have an edge extending forward from the postero-dorsal spines. Yet this edge does not extend towards the rostrum and also there are no pouches. This type of morphology does not give a comparably extreme expression and leaves the five extreme morphotypes as something distinct and special.
Morphological diversity of mantis shrimp larvae
C. Haug et al. (2016Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.) have argued that the extreme larval types indicate a still unrecognised diversity among mantis shrimp larvae. The fact that the newly discovered forms, including the here described morphotype 5, are all of large size is especially important in this aspect. Growing to this size means these larvae spend a considerable time in the plankton (under the assumption that the rate of development is not increased significantly). This further means they can be potentially transported quite far. So far we do not know much about the recruitment of mantis shrimp populations, but such long distance transport could indicate quite complex recruitment strategies for at least some mantis shrimp species.
Also concerning food web reconstruction the large larvae are of interest. Growing to these astonishing sizes means that the larvae have to consume quite some biomass from other plankton organisms. On the other hand, these large larvae are a potential food source, for example, for larger fishes. A higher diversity of these larval forms, as further presented here, also means that more mantis shrimp species fulfill such an ecological role, further stressing the importance of such large-sized planktotrophic planktic predators in the oceanic food web.
Acknowledgements
The study has been kindly supported by numerous people and institutions. We would like to express our thanks to all of them. Shane Ahyong, Sydney, and an anonymous reviewer provided helpful comments, which improved the manuscript significantly. All people who attended the excursion to Berlin helped with general work in the collections in the Museum für Naturkunde Berlin. Special thanks go to Christine Kiesmüller who pointed out the new specimen to us. The curator of the crustacean collection, Oliver C. Coleman, kindly hosted and supported us during our excursion. Lehre@LMU and the Kommission für Studienzuschüsse supported the excursion to Berlin; Lehre@LMU also provided funding for FB. The research visit to the Zoological Museum of the University of Copenhagen by CH was supported with a grant from the European Commission’s (FP 6) Integrated Infrastructure Initiative program SYNTHESYS (DK-TAF-2591). Jørgen Olesen and Tom Schiøtte hosted and supported CH during her visit. Gideon T. Haug assisted with documentation during the visit in Copenhagen. The study is part of the project “Palaeo-Evo-Devo of Malacostraca” kindly funded by the German Research Foundation (DFG HA 6300/3-2). We also thank all people involved in providing low cost, open access and open source software.
References
- Ahyong, S.T.; Haug, J.T. and Haug, C. 2014. Stomatopoda. p. 185-189. In: Martin, J.W.; Olesen, J., and Høeg, J.T. (eds), Atlas of Crustacean Larvae. Baltimore, The Johns Hopkins University Press.
- Alikunhi, K.H. 1952. An account of the stomatopod larvae of the Madras plankton. Records of the Indian Museum, 49: 239-319.
- Brock, R.E. 1985. Preliminary study of the feeding habits of pelagic fish around Hawaiian fish aggregation devices or can fish aggregation devices enhance local fishery productivity? Bulletin of Marine Science, 37: 40-49
- Diaz, G.A. and Manning, R.B. 1998. The last pelagic stage and juvenile of Lysiosquilla scabricauda (Lamarck, 1818) (Crustacea, Stomatopoda). Bulletin of Marine Science, 63: 453-457.
- Eiler, S.M. and Haug, J.T. 2016. Larval development of fossil polychelidan crustaceans, exemplified by the 150 million years old species Palaeopentacheles roettenbacheri Neues Jahrbuch fur Geologie und Paläontologie, Abhandlungen, 279: 295-310.
- Feller, K.D.; Cronin, T.W.; Ahyong, S.T. and Porter, M.L. 2013. Morphological and molecular description of the late-stage larvae of Alima Leach, 1817 (Crustacea: Stomatopoda) from Lizard Island, Australia. Zootaxa, 3722: 22-32.
- Giesbrecht, W. 1910. Stomatopoden, Erster Theil. Fauna und Flora des Golfes von Neapel, Monographie, 33: 1-39.
- Gohar, H.A.F. and Al-Kholy, A.A. 1957. The larval stages of three stomatopod Crustacea. Publications of the Marine Biological Station, Al-Ghardaqa, Red Sea, 9: 85-130.
- Greenwood, J.G. and Williams, B.G. 1984. Larval and early post-larval stages in the abbreviated development of Heterosquilla tricarinata (Claus, 1871) (Crustacea, Stomatopoda). Journal of Plankton Research, 6: 615-635.
- Hamano, T. and Matsuura, S. 1987. Egg size, duration of incubation, and larval development of the Japanese mantis shrimp in the laboratory. Nippon Suisan Gakkaishi, 53: 23-39.
- Haug, C.; Ahyong, S.T.; Wiethase, J.H.; Olesen, J. and Haug, J.T. 2016. Extreme morphologies of mantis shrimp larvae. Nauplius, 24: e2016020.
- Haug, J.T.; Audo, D.; Haug, C.; Abi Saad, P.; Petit, G. and Charbonnier, S. 2015. Unique occurrence of polychelidan lobster larvae in the fossil record and its evolutionary implications. Gondwana Research, 28: 869-874.
- Haug, C. and Haug, J.T. 2014. Defensive enrolment in mantis shrimp larvae (Malacostraca: Stomatopoda). Contributions to Zoology, 83: 185-194.
- Haug, J.T. and Haug, C. 2016. “Intermetamorphic” developmental stages in 150 million-year-old achelatan lobsters - The case of the species tenera Oppel, 1862. Arthropod Structure and Development, 45: 108-121.
- Haug, J.T.; Haug, C. and Ehrlich, M. 2008. First fossil stomatopod larva (Arthropoda: Crustacea) and a new way of documenting Solnhofen fossils (Upper Jurassic, Southern Germany). Palaeodiversity, 1: 103-109.
- Haug, J.T.; Haug, C.; Kutschera, V.; Mayer, G.; Maas, A.; Liebau, S.; Castellani, C.; Wolfram, U.; Clarkson, E.N.K. and Waloszek, D. 2011. Autofluorescence imaging, an excellent tool for comparative morphology. Journal of Microscopy, 244: 259-272.
- Haug, C.; Mayer, G.; Kutschera, V.; Waloszek, D.; Maas, A. and Haug, J.T. 2011. Imaging and documenting gammarideans. International Journal of Zoology, art. 380829, DOI 10.1155/2011/380829
» https://doi.org/10.1155/2011/380829 - Haug, J.T.; Rudolf, N.R.; Wagner, P.; Gundi, P.T.; Fetzer, L.-L. and Haug, C. 2016. An intermetamorphic larval stage of a mantis shrimp and its contribution to the 'missing-element problem' of stomatopod raptorial appendages. Annual Research and Review in Biology, 10: 1-19.
- Haug, C.; Sallam, W.S.; Maas, A.; Waloszek, D.; Kutschera, V. and Haug, J.T. 2012. Tagmatization in Stomatopoda - reconsidering functional units of modern-day mantis shrimps (Verunipeltata, Hoplocarida) and implications for the interpretation of fossils. Frontiers in Zoology, 9: art. 31.
- Kutschera, V.; Maas, A.; Waloszek, D.; Haug, C. and Haug, J.T. 2012. Re-study of larval stages of Amphionides reynaudii (Malacostraca: Eucarida) with modern imaging techniques. Journal of Crustacean Biology, 32: 916-930.
- Lalli, C.M. and Parsons, T.R. 1997. Biological Oceanography. An introduction. 2nd edition, Oxford, Elsevier Butterworth-Heinemann.
- Manning, R.B. and Provenzano, A.J. 1963. Studies on development of stomatopod Crustacea I. Early larval stages of Gonodactylus oerstedii Hansen. Bulletin of Marine Science, 13: 467-487.
- Martin, J.W. 2014. Polychelida. p. 279-282. In: Martin, J.W.; Olesen, J. and Høeg, J.T. (eds), Atlas of Crustacean Larvae. Baltimore, The Johns Hopkins University Press .
- Martin J. and Ormsby, B.1991. A large brachyuran-like larva of the Hippidae (Crustacea: Decapoda: Anomura) from the Banda Sea, Indonesia: the largest known zoea. Proceedings of the Biological Society of Washington, 104: 561-568.
- Morgan, S.G. and Goy, J.G. 1987. Reproduction and larval development of the mantis shrimp Gonodactylus bredini (Crustacea: Stomatopoda) maintained in the laboratory. Journal of Crustacean Biology, 7: 595-618.
- Morgan, S.G. and Provenzano, A.J. 1979. Development of pelagic larvae and postlarva of Squilla empusa (Crustacea, Stomatopoda) with an assessment of larval characters within the Squillidae. Fishery Bulletin, 77: 61-90.
- Palero, F.; Clark, P.F. and Guerao, G. 2014. Achelata. p. 272-278. In: Martin, J.W.; Olesen, J. and Høeg, J.T. (eds), Atlas of Crustacean Larvae. Baltimore, The Johns Hopkins University Press .
- Provenzano, A.J. and Manning, R.B. 1978. Studies on development of stomatopod Crustacea II. The later larval stages of Gonodactylus oerstedii Hansen reared in the laboratory. Bulletin of Marine Science, 28: 297-315.
- Pyne, R.R. 1972. Larval development and behaviour of the mantis shrimp, Squilla armata Milne Edwards (Crustacea: Stomatopoda). Journal of the Royal Society of New Zealand, 2: 121-146.
- Rudolf, N.R.; Haug, C. and Haug, J.T. 2016. Functional morphology of giant mole crab larvae: a possible case of defensive enrollment. Zoological Letters, 2: art. 17.
- Shanbhogue, S.L. 1975. Descriptions of stomatopod larvae from the Arabian Sea with a list of stomatopod larvae and adults from the Indian Ocean and a key for their identification Part 1. Journal of the Marine Biological Association of India, 17: 196-238.
- Sommer, U. and Stibor, H. 2002. Copepoda - Cladocera - Tunicata: The role of three major mesozooplankton groups in pelagic food webs. Ecological Research, 17: 161-174.
- Tang, R.W.; Yau, C. and Ng, W.C. 2010. Identification of stomatopod larvae (Crustacea: Stomatopoda) from Hong Kong waters using DNA barcodes. Molecular Ecology Resources, 10: 439-448.
- Torres, A.P.; Palero, F.; Santos, A.; Abelló, P.; Blanco, E.; Boné, A. and Guerao, G. 2014. Larval stages of the deep-sea lobster Polycheles typhlops (Decapoda, Polychelida) identified by DNA analysis: morphology, systematic, distribution and ecology. Helgoland Marine Research, 68: 379-397.
- Townsley, S.J. 1953. Adult and larval stomatopod crustaceans occurring in Hawaiian waters. Pacific Science, 7: 399-437.
- Walossek, D. and Müller, K.J. 1998. Cambrian 'Orsten'-type arthropods and the phylogeny of Crustacea. p. 139-153. In: Fortey, R.A. and Thomas, R.H. (eds), Arthropod Relationships. London, Chapman and Hall.
Publication Dates
-
Publication in this collection
08 Oct 2018 -
Date of issue
2018
History
-
Received
11 Oct 2017 -
Accepted
24 Apr 2018