Abstract
Macrobrachium candango Mantelatto, Pileggi, Pantaleão, Magalhães, Villalobos and Álvarez, 2021 is an endemic shrimp species to the Brazilian Central-West region (Brasília, Federal District). The present study aims to analyze the relative growth, morphometry, morphological sexual maturity, and fecundity of this species. Specimens were collected from tributaries of the Paranoá Lake hydrographic basin, Brasília, during sporadic periods between 1983 and 2012. Relative growth was analyzed for different structures (carapace, abdomen, second abdominal pleura, merus, carpus, and propodus). The laterality and heterochely patterns were evaluated based on the dimensions of the propodus length. Fecundity was calculated as the average total number of eggs per female and egg size was measured to obtain the volume. There were differences in relative growth (p < 0.05) of the structures analyzed between juveniles and adults, and distinct allometric patterns were observed between the life stages, which could reflect the habit of these animals at each developmental stage. No pattern of laterality and heterochely was observed between the chelipeds of the animals analyzed (p > 0.05). The lack of laterality may indicate that this species directs energy to the smallest propodus when the largest propodus is injured. Egg volume ranged from 4.41 to 7.71 mm3, and fecundity ranged from 38 to 61 eggs, indicating a life cycle with abbreviated larval development. The characteristics presented herein are unprecedented for M. candango and present relevant information needed to assess the conservation status of this species, which is currently threatened.
Keywords
Conservation; fecundity; heterochely; laterality; relative growth
INTRODUCTION
Freshwater shrimps present great examples of adaptation to environments that constantly change or fluctuate, as they present a complex set of characters regarding reproduction, behavior, growth and morphology throughout their lifecycles, that are closely linked to habitat and life history (Holthuis, 1952Holthuis, L.B. 1952. A general revision of the Palaemonidae (Crustacea, Decapoda, Natantia) of the Americas. II. The Subfamily Palaemonidae. Occasional Papers of the Allan Hancock Foundation, 12: 1-396. ; Pereira, 1997Pereira, G. 1997. A cladistic analysis of the freshwater shrimps of the family Palaemonidae (Crustacea, Decapoda, Caridea). Acta Biologica Venezuelica, 17: 1-69.; Bauer, 2004Bauer, R.T. 2004. Remarkable Shrimps: Adaptations and Natural History of the Carideans. Norman, University of Oklahoma Press, 316p.; Anger, 2013Anger, K. 2013. Neotropical Macrobrachium (Caridea: Palaemonidae): on the biology, origin, and radiation of freshwater-invading shrimp. Journal of Crustacean Biology, 33: 151−183.). Among the most complex aspects of the evolutionary process of this taxon is the embryonic development that can be abbreviated or extended, which directly affects the number and size of eggs that will be incubated (Magalhães and Walker, 1988Magalhães, C. and Walker, I. 1988. Larval development and ecological distribution of Central Amazonian palaemonid shrimps (Decapoda: Caridea) . Crustaceana, 55: 279-292. ; Sankoli et al., 1993Sankolli, K.N.; Jalihal, D.R. and Shenoy, S. 1993. Evolution of larval developmental patterns and the process of freshwaterization in the prawn genus Macrobrachium Bate, 1868 (Decapoda, Palaemonidae) . Crustaceana, 65: 365-376.; Odinetz-Collart and Magalhães, 1994Odinetz-Collart, O. and Magalhães, C. 1994. Ecological constraints and life history strategies of palaemonid prawns in Amazonia. Internationale Vereinigung für theoretische und angewandte Limnologie, 25: 2460-2467.; Pereira and Garcia, 1995Pereira, S.G.A. and Garcia, D. 1995. Larval development of Macrobrachium reyesi (Decapoda: Palaemonidae), with a discussion on the origin of abbreviated development in palaemonids. Journal of Crustacean Biology, 15: 117-133.), and represents a phylogenetic premise in some groups (Pileggi and Mantelatto, 2010Pileggi, L.G. and Mantelatto, F.L. 2010. Molecular phylogeny of the freshwater prawn genus Macrobrachium (Decapoda, Palaemonidae), with emphasis on the relationships among selected American species. Invertebrate Systematics, 24: 194-208.).
In Brazil, the family Palaemonidae is widely diverse, with species occupying different environments from the coastal region to inland waters in different watersheds (Coelho and Ramos-Porto, 1984Coelho, P.A. and Ramos-Porto, M. 1984. Camarões de água doce do Brasil: distribuição geográfica. Revista brasileira de Zoologia, 2: 405−410.; Pileggi and Mantelatto, 2010Pileggi, L.G. and Mantelatto, F.L. 2010. Molecular phylogeny of the freshwater prawn genus Macrobrachium (Decapoda, Palaemonidae), with emphasis on the relationships among selected American species. Invertebrate Systematics, 24: 194-208.; Mantelatto et al., 2016Mantelatto, F.L.; Pileggi, L.G.; Magalhães, C.; Carvalho, F.L.; Rocha, S.S.; Mossolin, E.C. and Bueno, S.L.S. 2016. Avaliação dos Camarões Palemonídeos (Decapoda: Palaemonidae). Cap. 20, p. 252-267. In: M.A.A. Pinheiro and H. Boos (Org.), Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010-2014. Porto Alegre, RS, Sociedade Brasileira de Carcinologia - SBC. 466p.). Among the genera of this diverse family, the Brazilian species of Macrobrachium Spence Bate, 1868 received special attention during the last decade with regard to updated taxonomy (Pileggi and Mantelatto, 2012Pileggi, L.G. and Mantelatto, F.L. 2012. Taxonomic revision of doubtful Brazilian freshwater shrimp species of genus Macrobrachium (Decapoda, Palaemonidae). Iheringia, Série Zoologia, 102: 426-437.; Dos Santos et al., 2013Dos Santos, A.; Hayd, L. and Anger, K. 2013. A new species of Macrobrachium Spence Bate, 1868 (Decapoda, Palaemonidae), M. pantanalense, from the Pantanal, Brazil. Zootaxa, 3700: 534−546.; Mantelatto et al., 2016Mantelatto, F.L.; Pileggi, L.G.; Magalhães, C.; Carvalho, F.L.; Rocha, S.S.; Mossolin, E.C. and Bueno, S.L.S. 2016. Avaliação dos Camarões Palemonídeos (Decapoda: Palaemonidae). Cap. 20, p. 252-267. In: M.A.A. Pinheiro and H. Boos (Org.), Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010-2014. Porto Alegre, RS, Sociedade Brasileira de Carcinologia - SBC. 466p.; Rossi et al., 2020Rossi, N.; Magalhães, C.; Mesquita, E.R. and Mantelatto, F.L. 2020. Uncovering a hidden diversity: a new species of freshwater shrimp Macrobrachium (Decapoda: Caridea: Palaemonidae) from Neotropical region (Brazil) revealed by morphological review and mitochondrial genes analyses. Zootaxa, 4732: 177-195.), and most recently a taxonomic revision proposed a new nomenclatural and phylogenetic rearrangement in which all the six American species of the genus Cryphiops Dana, 1852 were accommodated in the genus Macrobrachium (see Mantelatto et al., 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.). Regarding this latter group, the only known species from Brazilian territory is Macrobrachium candangoMantelatto, Pileggi, Pantaleão, Magalhães, Villalobos and Álvarez, 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198. (originally described as Cryphiops brasiliensisGomes Corrêa, 1973Gomes Corrêa, M.M. 1973. Descrição de uma espécie nova do gênero Cryphiops (Decapoda, Natantia, Palaemonidae) . Revista Brasileira de Biologia, 33: 169−173.), which is endemic to the Paranoá Lake hydrographic basin in Brasília, Federal District (Gomes Corrêa, 1973Gomes Corrêa, M.M. 1973. Descrição de uma espécie nova do gênero Cryphiops (Decapoda, Natantia, Palaemonidae) . Revista Brasileira de Biologia, 33: 169−173.; Mantelatto et al., 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.).
The only information available on this species was registered in its description (Gomes Corrêa, 1973Gomes Corrêa, M.M. 1973. Descrição de uma espécie nova do gênero Cryphiops (Decapoda, Natantia, Palaemonidae) . Revista Brasileira de Biologia, 33: 169−173.), which addressed its morphological characters and compared them with those of Macrobrachium caementarius (Molina, 1782) (as Cryphiops caementarius) occurring in coastal areas of Chile. In the same study, some observations about its fecundity were also reported (three ovigerous females incubated between 42 and 63 eggs). Reports from local collections and observations indicate that the abundance of M. candango is naturally low, with a high degree of endemism (Mantelatto et al., 2016Mantelatto, F.L.; Pileggi, L.G.; Magalhães, C.; Carvalho, F.L.; Rocha, S.S.; Mossolin, E.C. and Bueno, S.L.S. 2016. Avaliação dos Camarões Palemonídeos (Decapoda: Palaemonidae). Cap. 20, p. 252-267. In: M.A.A. Pinheiro and H. Boos (Org.), Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010-2014. Porto Alegre, RS, Sociedade Brasileira de Carcinologia - SBC. 466p.), causing the species to be categorized as threatened according to IUCN criteria. Additionally, their habitats have been affected by anthropogenic actions such as pollution and deforestation (Mantelatto et al., 2016Mantelatto, F.L.; Pileggi, L.G.; Magalhães, C.; Carvalho, F.L.; Rocha, S.S.; Mossolin, E.C. and Bueno, S.L.S. 2016. Avaliação dos Camarões Palemonídeos (Decapoda: Palaemonidae). Cap. 20, p. 252-267. In: M.A.A. Pinheiro and H. Boos (Org.), Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010-2014. Porto Alegre, RS, Sociedade Brasileira de Carcinologia - SBC. 466p.; 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.). Shrimps have an important ecological role within aquatic ecosystems (March et al., 2002March, J.G.; Pringle, C.M.; Townsend, M.J. and Wilson, A.I. 2002. Effects of freshwater shrimp assemblages on benthic communities along an altitudinal gradient of a tropical island stream. Freshwater Biology, 47: 377-390.), and in the case of M. candango, which is an endemic species in a region of Brazil, its disappearance from the environment can cause irreversible problems to these ecosystems, influencing the local food chain (March et al., 2002March, J.G.; Pringle, C.M.; Townsend, M.J. and Wilson, A.I. 2002. Effects of freshwater shrimp assemblages on benthic communities along an altitudinal gradient of a tropical island stream. Freshwater Biology, 47: 377-390.; De Souza and Moulton, 2005De Souza, M.L. and Moulton, T.P. 2005. The effects of shrimps on benthic material in a Brazilian island stream. Freshwater Biology, 50: 592−602.). Therefore, due to this degree of endemism, it is necessary to carry out constant monitoring studies addressing M. candango, in order to properly understand its ecological role in the Paranoá Lake ecosystem.
Faced with this scenario we searched for more information by visiting its type locality (ECM and FLM) to collect new specimens. During these field activities, we were positively surprised to find a considerable collection of lots of this species deposited at the Aquatic Crustaceans Collection of the Reserva Ecológica do IBGE (Instituto Brasileiro de Geografia e Estatística), Brasília - Federal District, which serve as the basis for the preparation of this article.
Thus, considering that knowledge about biological characteristics is fundamental for understanding the life cycle, and necessary for defining conservation strategies, particularly for species with low abundance and endemism, the present study aims to investigate basic aspects (relative growth, morphometry, morphological sexual maturity and fecundity) about the biology of M. candango. In the future this information can be used to assess the conservation status of this species.
MATERIAL AND METHODS
Scientific and field collections
The field collection resulted from surveys carried out in 122 lotic stretches of tributaries forming the Paranoá Lake hydrographic basin, collected from 28 sampling points distributed along the four main tributaries of the Paranoá Lake (Fig. 1): Riacho Fundo (three sites, including the type locality), Ribeirão do Torto (three sites), Ribeirão Bananal (five sites), and Ribeirão Gama (17 sites, 11 in the Taquara stream and six in the Roncador stream), which recorded the species in only 23 % of the sampled locations. The previous collection of M. candango deposited at the IBGE’s Aquatic Crustaceans Collection consists of 345 lots containing 932 specimens. Samplings occurred sporadically between 1983 and 2012, specifically in November/1983; February, April and August/1985; November/1986; February and March/1987; May/1988 to April/1989; July/1989; May/1990; October/1992; September/1998; October/2005; September/2009; and May and July/2012. All collections were performed using trawls (mesh size 3 mm).
Brazilian territory and location of the study area in black, amplification of the study area showing streams from the Paranoá Lake basin in Brasília, Federal District, Brazil.
All locations where specimens were present in the streams of Torto and Bananal (Brasilia National Park), Roncador (IBGE Ecological Reserve) and Taquara (IBGE Ecological Reserve, Area of Relevant Ecological Interest Capetinga - Taquara and Ecological Station of the Botanical Garden of Brasilia) are within conservation units. Only the locations in Riacho Fundo, which includes the type locality, are not within an environmental protection area. Only one lot, with one specimen, was collected in a transition zone between the Milho Cozido stream and the Santa Maria Reservoir (Ribeirão do Torto), while all other specimens were captured in lotic environments of the Paranoá Lake tributaries.
The third author (MCLBR) and his team were responsible for all expeditions. Two permanent licenses issued by SUDEPE (Superintendência do Desenvolvimento Pesca; 1983 to 1989) and IBAMA (Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis) in the Federal District (1990 - 2009) allowed the collection of fish and aquatic crustaceans in these respective periods. Those responsible for the Conservation Units also authorized the collections. The surveys carried out in the Brasilia National Park, in 2012, were done in collaboration with CAESB (Companhia de Saneamento Ambiental do Distrito Federal) to prepare the environmental impact study about the Santa Maria Dam construction to renew the environmental license for that enterprise. Therefore, the authorization for scientific activity was issued directly by the ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade), with consent of the Brasilia National Park.
After sampling, the material was screened in the field and shrimps were placed in a solution of clove oil and water for five minutes for euthanasia, and later placed in containers with 70 % alcohol and deposited in the scientific collection of the Reserva Ecológica do IBGE.
Laboratory analysis
The sex of shrimps was checked under a stereomicroscope, following a protocol suggested by Valenti et al. (1989Valenti, W.C.; Lobão, V.L. and Mello, J.T. 1989. Crescimento relativo de Macrobrachium acanthurus (Wiegmann, 1836) (Crustacea, Decapoda, Palaemonidae). Revista brasileira de Zoologia, 6: 1-8.) for the genus Macrobrachium, which requires observing the presence (male) or absence (female) of the male appendage on the second pair of pleopods. Subsequently, they were identified to species level according to Melo (2003Melo, G.A.S. 2003. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. São Paulo, Ed. Loyola, 429p. ) and the length of the following structures were measured with a caliper (0.01 mm): carapace (CL), abdomen (AL), merus (ML), carpus (CaL), and propodus (PrL), along with the width of the second abdominal pleura (PW) (Fig. 2). The merus, carpus, and propodus selected for analysis constitute the cheliped of the second largest pair of pereopods (independent of the side). All animals used in the analyses presented intact structures.
Macrobrachium candangoMantelatto, Pileggi, Pantaleão, Magalhães, Villalobos and Álvarez, 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.. Structures used in morphometric analyses: (CL) carapace length; (AL) abdomen length; (PW) pleura width; (ML) merus length; (CaL) carpus length; (PrL) propodus length. Adapted from Melo (2003Melo, G.A.S. 2003. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. São Paulo, Ed. Loyola, 429p. ).
Relative growth
The comparative analysis of body dimensions was based on the measurements described above. Data normality was verified with the Shapiro-Wilk test (α = 0.05). The non-hierarchical analysis of K-means clustering (Sokal et al., 1979Sokal, R.R.; Rohlf, F.J. and Lahoz-León, M. 1979. Biometría: principios y métodos estadísticos en la investigación biológica. Madrid, Blume, 832p.) was used considering the morphometric relationships measured between the groups of males and females (juveniles and adults). This analysis distributes the data set into groups that are previously established by an interactive process, which minimizes the variance between groups. After determining K-means, a discriminant analysis was performed to refine the data for categorization (Sampedro et al., 1999Sampedro, M.P.; González-Gurriarán, E.; Freire, J. and Muiño, R. 1999. Morphometry and sexual maturity in the spider crab Maja squinado (Decapoda: Majidae) in Galicia, Spain. Journal of Crustacean Biology, 19: 578-592.). Afterwards, the data was submitted to a covariance analysis (ANCOVA) to test the angular and linear coefficients between the groups defined by the analyzes. This showed whether the data for each relationship fit better to a single equation or whether morphometric relationships should be represented by different linear equations.
In the relative growth analysis, changes in the allometric coefficient were verified in the development of body structures related to the independent variable (CL). Scatter plots were constructed from the data set obtained by measuring body structures and adjusted to the allometric equation y = axb (Hartnoll, 1974Hartnoll, R.G. 1974. Variation in growth pattern between some secondary sexual characters in crabs (Decapoda, Brachyura). Crustaceana, 27: 131−136., 1978Hartnoll, R.G. 1978. The determination of relative growth in Crustacea. Crustaceana, 34: 281−293.; Hawkins and Hartnoll, 1982Hawkins, S.J. and Hartnoll, R.G. 1982. The influence of barnacle cover on the numbers, growth and behaviour of Patella vulgata on a vertical pier. Journal of the Marine Biological Association of the United Kingdom, 62: 855−867.). Subsequently, the data was linearized (log y = log a + b × log x) in which "y" is the structure dimension studied, "x" the carapace length, "a" the intersection of the axis "y" and "b" the allometric coefficient (angular) that represents the slope and angle of the line. The allometric constant "b" was calculated for each morphometric relationship and the null hypothesis (H0: b = 1) was tested with the Student test (α = 95 %). The values of the allometric constant correspond to isometric growth (b = 1), positive allometry (b > 1) or negative allometry (b < 1) (Zar, 2010Zar, J.H. 2010. Biostatistical analysis. Pearson Education Upper Saddie River, New Jersey, USA, 944p).
Morphological sexual maturity was estimated from the inflection break of the points resulting from the lines drawn based on the equations of adults and juveniles in the CL x PrL and CL x PW relationships for males and females, respectively. These relationships were chosen because they best reflect the variations that occur during the reproductive phases (juveniles and adults) (Mantelatto and Barbosa, 2005Mantelatto, F.L. and Barbosa, L.R. 2005. Population structure and relative growth of freshwater prawn Macrobrachium brasiliense (Decapoda, Palaemonidae) from São Paulo State, Brazil. Acta Limnologica Brasiliensia, 17: 245-255.; Pescinelli et al., 2018Pescinelli, R.A.; Almeida, A.O. and Costa, R.C. 2018. Population structure, relative growth and morphological sexual maturity of the snapping shrimp Alpheus brasileiro Anker, 2012 (Caridea: Alpheidae) from the south-eastern coast of Brazil. Marine Biology Research, 14: 610-620.).
Laterality and heterochely
The laterality pattern of males and females was investigated using the chi-square test (α = 0.05) (Pearson, 1900Pearson, K. 1900. X. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 50(302): 157-175.), based on the propodus length of both chelipeds (right and left) of the second pair of pereopods.
The heterochely analysis was performed based on the final result of the laterality test, using the Kruskal-Wallis test and its Dunn’s post-hoc test (Daniel, 1990Daniel, W.W. 1990. Kruskal-Wallis one-way analysis of variance by ranks. Applied Nonparametric Statistics, 226−234.). In the heterochely test, the largest propodus x smallest propodus (independent of the side) was compared due to the lack of laterality pattern.
Fecundity
Fecundity was obtained from the total number of eggs adhered to pleopods. Therefore, 15 eggs were removed from the pleopods of each ovigerous female (OF) and the length and width of eggs were measured under a stereomicroscope to calculate egg volume (Anger and Moreira, 1998Anger, K. and Moreira, G.S. 1998. Morphometric and reproductive traits of tropical caridean shrimps. Journal of Crustacean Biology, 18: 823−838.). The egg volume (EV) was calculated as: EV = π * l * h * (h)²; where ‘‘l’’ is the length; ''h'' is width in mm and π = 3.14 (Wehrtmann, 1990Wehrtmann, I.S. 1990. Distribution and reproduction of Ambidexter panamense and Palaemonetes schmitti in Pacific Costa Rica (Crustacea, Decapoda). Revista de Biología Tropical, 38(2A): 327-329.). The developmental stage of the embryos was classified into two stages, being early (eggs with homogeneous color and lacking visible eye pigmentation) and late (embryos with fully developed eyes) (Anger and Moreira, 1998Anger, K. and Moreira, G.S. 1998. Morphometric and reproductive traits of tropical caridean shrimps. Journal of Crustacean Biology, 18: 823−838.). The relationships between CL/PW and CL/number of eggs were investigated using a linear regression. Monthly rainfall data for the years that ovigerous females occurred were obtained from the digital platform HidroWeb of the SNIRH (Sistema Nacional de Informações sobre Recursos Hídricos).
RESULTS
A total of 262 individuals were analyzed, including 157 males (59.9 %) and 105 females (40.1 %). The mean CL for males was 16.44 ± 4.34 mm, ranging from 6.8 to 25.2 mm, and for females was 11.5 ± 2.86 mm, ranging from 4.8 to 17.4 mm, with a significant difference in mean CL between sexes (Mann-Whitney, p < 0.001). To analyze the relative growth of the largest cheliped articles, a total of 166 individuals (102 males and 64 females) with intact appendages were examined.
Relative growth
The ANCOVA analysis (Tab. 1) showed that adult male individuals differed statistically (p < 0.01) from juvenile males in the morphometric relationships related to pleura and chelipeds (CL × PW, CL × PrL, CL × CaL, and CL × ML), however, in the CL × AL morphometric relationship there was no difference between these groups (p > 0.05). The same pattern occurred in females, with statistical difference (p < 0.01) between adult and juvenile females in almost all relationships investigated herein (CL × PW, CL × PrL, CL × CaL, and CL × ML), except for CL × AL (p > 0.05, Tab. 1).
Macrobrachium candangoMantelatto, Pileggi, Pantaleão, Magalhães, Villalobos and Álvarez, 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.. Results of covariance analyses (ANCOVA) with morphometric data. AM: adult males; JM: juvenile males, AF: adult females; JF: juvenile females; CL: carapace length; AL: abdomen length; PW: pleura width; ML: merus length; CaL: carpus length; PrL: propodus length.
Significant correlations were observed in all morphometric relationships (p < 0.01) (Tab. 2). Since the CL × AL relationship did not differ significantly (ANCOVA, p > 0.05) between the demographic categories (adults and juveniles) of both sexes, these were represented by a single equation obtained through linear regression analysis. For males, a negative allometric growth was observed for the CL × PW and CL × AL relationships. Regarding chelipeds (CL × PrL, CL × CaL, and CL × ML), a positive allometric pattern was observed for adult males. For juvenile males, isometry was observed for CL × PrL and negative allometry was observed for the other relationships (CL × CaL and CL × ML).
Macrobrachium candangoMantelatto, Pileggi, Pantaleão, Magalhães, Villalobos and Álvarez, 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.. Regression analysis of morphometric data. The carapace length (CL) was used as an independent variable. CL: carapace length; AL: abdomen length; PW: pleura width; ML: merus length; CaL: carpus length; PrL: propodus length.
For females (adult and juvenile), negative allometry was observed in the relationship CL × AL, while a negative allometry and an isometry were observed for adults and juveniles, respectively, in the CL × PW relationship. Regarding chelipeds (CL × PrL, CL × CaL, and CL x ML), an allometric pattern was observed between adults and juveniles in all relationships, with negative allometric growth (Tab. 2).
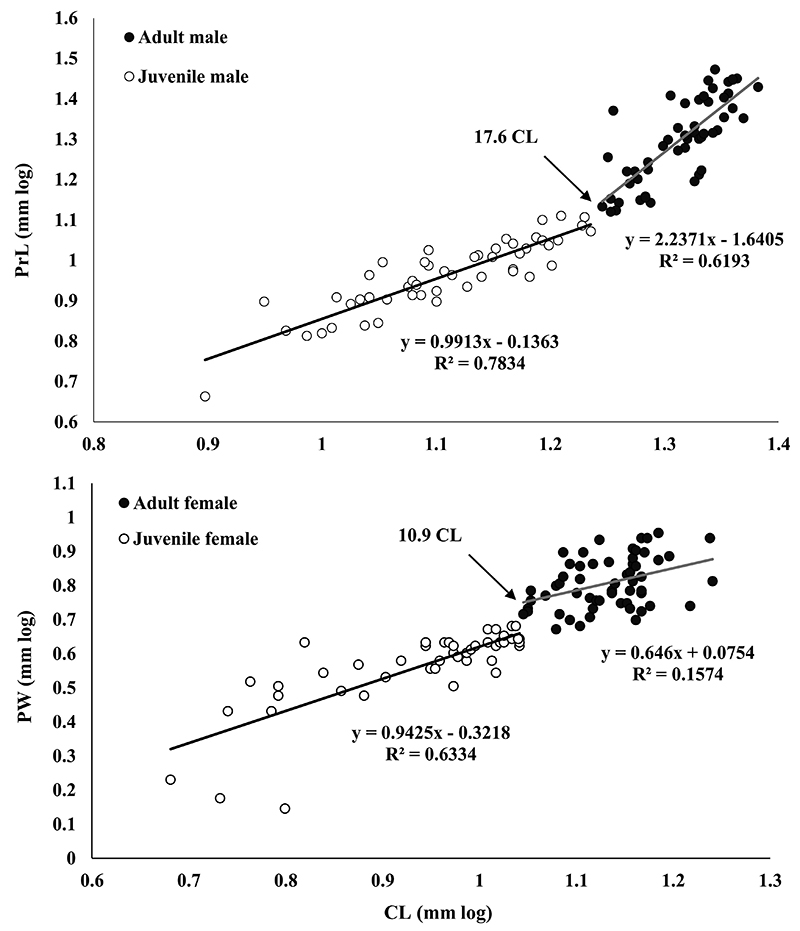
Based on CL × PrL and CL × PW morphometric relationships, the size of morphological sexual maturity was estimated at 17.6 and 10.9 mm CL for males and females, respectively (Fig. 3).
Macrobrachium candangoMantelatto, Pileggi, Pantaleão, Magalhães, Villalobos and Álvarez, 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.. Scatter plots of the selected morphometric relationships in males and females, highlighting the separation of groups (adults and juveniles). The arrows indicate the inflection break and the respective size estimated at the beginning of morphological sexual maturity, which corresponds to the CL of the smallest individual in the interruption of juvenile and adult equations, in relation to CL × PrL (males) and CL × PW (females). (CL) carapace length; (PrL) propodus length; (PW) pleura width.
Laterality and heterochely
There was no defined laterality pattern in males and females (males: χ2 = 0.35 and p = 0.84; females: χ2 = 0.07 and p = 0.96) and, therefore, individuals may present either right or left propodus as most developed. In males, the right and left propodus were more robust in 52.9 % and 47.1 % cases, respectively. In females this proportion was 48.3 % and 51.7 %, respectively.
Due to the lack of laterality pattern, the heterochely analyses compared the largest propodus × smallest propodus. The average length of the largest and smallest propodus of adult males was 20.03 ± 5.1 mm and 16.64 ± 3.86 mm, while in juvenile males it was 9.71 ± 2.25 mm and 8.73 ± 2.1 mm, respectively. The average length of the largest and smallest propodus of adult females was 9.9 ± 1.48 mm and 9.2 ± 1.52 mm, while in juvenile females it was 7.07 ± 0.92 mm and 6.42 ± 0.9 mm, respectively.
Heterochely was not found within the demographic categories (adult and juvenile males; adult and juvenile females), i.e., the length of the largest propodus was not significantly different from the smallest propodus (p > 0.05). However, significant differences in the propodus length were observed between all demographic categories, and sexual dimorphism based on propodus length can be characterized in both life stages. The length of the largest and smallest propodus of adult males differed significantly from all other demographic categories (p < 0.01). The length of the largest propodus of juvenile males and adult females was only significantly different from the propodus (largest and smallest) of juvenile females (p < 0.01), as well as adult males (see Tab. 3).
Macrobrachium candangoMantelatto, Pileggi, Pantaleão, Magalhães, Villalobos and Álvarez, 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.. Dunn’s test results comparing the propodus length (largest × smallest) between adult and juvenile males and females. LPAM: largest propodus of adult males; SPAM: smallest propodus of adult males; LPJM: largest propodus of juvenile males; SPJM: smallest propodus of juvenile males; LPFM: largest propodus of adult females; SPJF: smallest propodus of adult females; LPJF: largest propodus of juvenile females; SPJF: smallest propodus of juvenile females. ns = non-significant.
Reproductive aspects
Fifty OF were used with a mean CL of 13.9 ± 1.08 mm, ranging from 12.2 to 15.3 mm. The average egg volume (EV) was 5.68 ± 0.93 mm3, ranging from 4.41 to 7.71 mm3. The average fecundity was 49 ± 11, ranging from 38 to 61 eggs. Among the 15 OF analyzed, only two females incubated embryos in the late stage of development. The average volume of eggs in the early and late stages was 5.63 ± 0.94 mm3 and 6.03 ± 1.13 mm3, respectively. There was no correlation between CL and number of eggs (p > 0.05), however, there was a positive correlation between PW and number of eggs (p < 0.05). Based on all individuals sampled, a higher abundance of OF was observed in October/2005, followed by September/2009, January/1989, August/1988 and November/1988 (Fig. 4a). In most cases, OF were found in months with a high volume of rainfall, except for August/1988 (Fig. 4b).
Macrobrachium candangoMantelatto, Pileggi, Pantaleão, Magalhães, Villalobos and Álvarez, 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.. A. Number of ovigerous females obtained during the collection period. B. Monthly rainfall variation in the years when ovigerous females were found (1988, 1989, 2005, and 2009). Red arrows indicate in which months the ovigerous females occurred.
DISCUSSION
Forty-nine years after the species was described, detailed information about the morphometry and reproduction of M. candango was gathered from data collected for a 30-year time period and analyzed for the first time herein, revealing information about aspects of its life history.
Morphometric aspects
The development pattern of PW in males and females was similar, except for juvenile females that presented isometric development. This group of juvenile females has this difference possibly due to the need for the pleura to be well developed until its adult phase, which is when this structure will have the function of expanding the size of the hatchery chamber, allowing these females to hatch more eggs (Nagamine and Knight, 1980Nagamine, C. and Knight, A.W.1980. Development, maturation and function of some sexually dimorphic structures of the Malaysian prawn, Macrobrachium rosenbergii (de Man) (Decapoda, Palaemonidae). Crustaceana, 39: 141-152.; Mantelatto and Barbosa, 2005Mantelatto, F.L. and Barbosa, L.R. 2005. Population structure and relative growth of freshwater prawn Macrobrachium brasiliense (Decapoda, Palaemonidae) from São Paulo State, Brazil. Acta Limnologica Brasiliensia, 17: 245-255.; Pralon and Negreiros-Fransozo, 2006Pralon, B.G.N. and Negreiros-Fransozo, M.L. 2006. Population biology of Palaemon (Palaeander) northropi Rankin, 1898 (Crustacea, Decapoda, Palaemonidae) in a tropical South American estuary. Acta Limnologica Brasiliensia, 18: 77-87.; Pantaleão et al., 2012Pantaleão, J.A.F.; Hirose, G.L. and Costa, R.C. 2012. Relative growth, morphological sexual maturity, and size of Macrobrachium amazonicum (Heller 1862) (Crustacea, Decapoda, Palaemonidae) in a population with an entirely freshwater life cycle. Invertebrate Reproduction & Development, 56: 180-190. ; Pescinelli et al., 2018Pescinelli, R.A.; Almeida, A.O. and Costa, R.C. 2018. Population structure, relative growth and morphological sexual maturity of the snapping shrimp Alpheus brasileiro Anker, 2012 (Caridea: Alpheidae) from the south-eastern coast of Brazil. Marine Biology Research, 14: 610-620.). Therefore, this structure develops at the same rate as the carapace during the juvenile phase, but modifies its growth pattern when reaching sexual maturity, which is when the adult female starts to invest more energy in gonadal development (Sampaio et al., 2007Sampaio, C.M.S.; Silva, R.R.; Santos, J.A. and Sales, S.P. 2007. Reproductive cycle of Macrobrachium amazonicum females (Crustacea, Palaemonidae) . Brazilian Journal of Biology, 67: 551-559.).
In the morphometric relationships related to the chelipeds of adult and juvenile males, a distinct pattern was found among these groups, where adult males invest more energy than juveniles in the development of the articles of the largest cheliped. During the life stages of shrimps, chelipeds play important roles in feeding and agonistic behaviors (Nagamine and Knight, 1980Nagamine, C. and Knight, A.W.1980. Development, maturation and function of some sexually dimorphic structures of the Malaysian prawn, Macrobrachium rosenbergii (de Man) (Decapoda, Palaemonidae). Crustaceana, 39: 141-152.; Valenti et al., 1989Valenti, W.C.; Lobão, V.L. and Mello, J.T. 1989. Crescimento relativo de Macrobrachium acanthurus (Wiegmann, 1836) (Crustacea, Decapoda, Palaemonidae). Revista brasileira de Zoologia, 6: 1-8.); thus, the unequal development of this structure between life stages may be related to its different functions (Nagamine and Knight, 1980Nagamine, C. and Knight, A.W.1980. Development, maturation and function of some sexually dimorphic structures of the Malaysian prawn, Macrobrachium rosenbergii (de Man) (Decapoda, Palaemonidae). Crustaceana, 39: 141-152.). Adult individuals require highly developed chelipeds for disputes over females or mating behaviors, on the other hand, juveniles do not dispute sexual partners, possibly this group uses this structure for more general functions such as foraging and for body cleaning (Hartnoll, 1978Hartnoll, R.G. 1978. The determination of relative growth in Crustacea. Crustaceana, 34: 281−293., 1982Hawkins, S.J. and Hartnoll, R.G. 1982. The influence of barnacle cover on the numbers, growth and behaviour of Patella vulgata on a vertical pier. Journal of the Marine Biological Association of the United Kingdom, 62: 855−867.; Mariappan et al., 2000Mariappan, P.; Balasundaram, C. and Schmitz, B. 2000. Decapod crustacean chelipeds: an overview. Journal of Biosciences, 25: 301-313.; Correa and Thiel, 2003Correa, C. and Thiel, M. 2003. Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Revista Chilena de Historia Natural, 76: 187−203.).
For females, the morphometric relationships of the chelipeds showed the same type of development between adults and juveniles (allometrically negative). Again, this result refers to the functional role of these appendages in these categories (Nagamine and Knight, 1980Nagamine, C. and Knight, A.W.1980. Development, maturation and function of some sexually dimorphic structures of the Malaysian prawn, Macrobrachium rosenbergii (de Man) (Decapoda, Palaemonidae). Crustaceana, 39: 141-152.; Mariappan et al., 2000Mariappan, P.; Balasundaram, C. and Schmitz, B. 2000. Decapod crustacean chelipeds: an overview. Journal of Biosciences, 25: 301-313.), because females might use chelipeds differently than males and may not require these appendages to be robust. Therefore, they use less energy to develop this structure, justifying the allometric pattern found, in addition, these results reinforce the sexual dimorphism found in the propodus length between males and females.
Analyzing the average length of the largest and smallest propodus between the demographic categories, significant differences were observed between these groups, with sexual dimorphism between adult and juvenile males and females. Sexual dimorphism based on the length of the chelipeds or propodus is a characteristic that has been widely observed in other shrimp species (Koshy, 1973Koshy, M. 1973. Studies on the sexual dimorphism in the freshwater prawn Macrobrachium dayanum (Henderson, 1893) (Decapoda, Caridea), II. Crustaceana, 24: 110-118.; Nagamine and Knight, 1980Nagamine, C. and Knight, A.W.1980. Development, maturation and function of some sexually dimorphic structures of the Malaysian prawn, Macrobrachium rosenbergii (de Man) (Decapoda, Palaemonidae). Crustaceana, 39: 141-152.; Mariappan and Balasundaram, 1997Mariappan, P. and Balasundaram, C. 1997. Cheliped laterality in freshwater prawn, Macrobrachium nobilii (Henderson and Matthai, 1910). Current Science, 875-877.; Mossolin and Bueno, 2003Mossolin, E.C. and Bueno, S.L. 2003. Relative growth of the second pereiopod in Macrobrachium olfersi (Wiegmann, 1836) (Decapoda, Palaemonidae). Crustaceana, 76: 363-376.; Karplus and Barki, 2019Karplus, I. and Barki, A. 2019. Male morphotypes and alternative mating tactics in freshwater prawns of the genus Macrobrachium: a review. Reviews in Aquaculture, 11: 925-940.), and this unique trait mainly refers to the sexual behavior of these animals, where males use chelipeds to dispute and guard females before and after copulation (Bauer, 2004Bauer, R.T. 2004. Remarkable Shrimps: Adaptations and Natural History of the Carideans. Norman, University of Oklahoma Press, 316p.; Karplus and Barki, 2019Karplus, I. and Barki, A. 2019. Male morphotypes and alternative mating tactics in freshwater prawns of the genus Macrobrachium: a review. Reviews in Aquaculture, 11: 925-940.).
The size of the morphological sexual maturity observed in this study was similar to that observed for Macrobrachium iheringi (Ortmann, 1897) (Nogueira et al., 2019Nogueira, C.S.; Perroca, J.F.; Piantkoski, E.L.; Costa, R.C.; Taddei, F.G. and Fransozo, A. 2019. Relative growth and population dynamics of Macrobrachium iheringi (Decapoda, Palaemonidae). Papéis Avulsos de Zoologia, 59: e20195908.), a species that presents a similar body size variation to that of M. candango. Furthermore, in both species males reach morphological sexual maturity at larger sizes than females, indicating that possibly M. candango has the reproductive strategy called "mate guarding" (Bauer, 2004Bauer, R.T. 2004. Remarkable Shrimps: Adaptations and Natural History of the Carideans. Norman, University of Oklahoma Press, 316p.), as was suggested for M. iheringi (see Nogueira et al., 2019Nogueira, C.S.; Perroca, J.F.; Piantkoski, E.L.; Costa, R.C.; Taddei, F.G. and Fransozo, A. 2019. Relative growth and population dynamics of Macrobrachium iheringi (Decapoda, Palaemonidae). Papéis Avulsos de Zoologia, 59: e20195908.); a result that is correlated with the occurrence of sexual dimorphism in body size and chelipeds, and with different patterns of allometric development between males and females.
The lack of a laterality pattern seems to be a common feature in Macrobrachium species that have heterochely, this was also observed in Macrobrachium olfersii (Wiegmann, 1836) by Mossolin and Bueno (2003Mossolin, E.C. and Bueno, S.L. 2003. Relative growth of the second pereiopod in Macrobrachium olfersi (Wiegmann, 1836) (Decapoda, Palaemonidae). Crustaceana, 76: 363-376.), and in Macrobrachium brasiliense (Heller, 1862) by Nogueira et al. (2020Nogueira, C.S.; Pantaleão, J.A.F.; Almeida, A.C. and Costa, R.C. 2020. Male morphotypes of the freshwater prawn Macrobrachium brasiliense (Decapoda: Caridea: Palaemonidae). Invertebrate Biology, 139(1): e12279.), where the males and females analyzed did not have a constant between which propodus (left or right) would be the most developed. Therefore, it has been suggested that this characteristic (i.e., laterality) may not be genetically predetermined (Mossolin and Bueno, 2003Mossolin, E.C. and Bueno, S.L. 2003. Relative growth of the second pereiopod in Macrobrachium olfersi (Wiegmann, 1836) (Decapoda, Palaemonidae). Crustaceana, 76: 363-376.). In some decapod crustaceans, such as brachyuran crabs, the lack of a laterality pattern is common (Smith and Palmer, 1994Smith, L.D. and Palmer, A.R. 1994. Effects of manipulated diet on size and performance of brachyuran crab claws. Science, 264(5159): 710-712.; Santos et al., 2018Santos, F.M.; Pescinelli, R.A.; Pantaleão, J.A.F. and Costa, R.C. 2018. Relative growth, morphological sexual maturity, heterochely, and handedness in Panopeus occidentalis (Brachyura, Panopeidae). Invertebrate Reproduction & Development, 62: 74-81.). These crabs usually use the largest cheliped as a weapon in agonistic contests. If these individuals lose this appendage during these disputes, the other cheliped (the smallest) will become the largest (Smith and Palmer, 1994Smith, L.D. and Palmer, A.R. 1994. Effects of manipulated diet on size and performance of brachyuran crab claws. Science, 264(5159): 710-712.). Possibly this also occurs in M. candango, since these individuals have a major cheliped, this structure can be used as the main weapon during disputes over resources, therefore, this appendage is more likely to be injured or amputated.
Reproductive features
Based on the low fecundity and large volume of M. candango eggs, it can be inferred that this species has abbreviated larval development, where the embryos develop for a longer time within the egg. After hatching, larvae originate in the final stage of development, leaving few morphological characteristics to be considered juveniles, these characteristics will be developed in two or three larval stages, which precede the juvenile phase (Sankoli et al., 1993Sankolli, K.N.; Jalihal, D.R. and Shenoy, S. 1993. Evolution of larval developmental patterns and the process of freshwaterization in the prawn genus Macrobrachium Bate, 1868 (Decapoda, Palaemonidae) . Crustaceana, 65: 365-376.; Anger, 2013Anger, K. 2013. Neotropical Macrobrachium (Caridea: Palaemonidae): on the biology, origin, and radiation of freshwater-invading shrimp. Journal of Crustacean Biology, 33: 151−183.). Some species of freshwater shrimps exhibit this characteristic, in which low fecundity along with large eggs is correlated to the type of larval development, as recorded, for instance, for M. brasiliense, M. iheringi, Macrobrachium nattereri (Heller, 1862), and Macrobrachium totonacumMejía, Alves and Hartnoll, 2003Mejia-Ortiz, L.M.; Hartnoll, R.G. and Lopez-Mejia, M. 2010. The abbreviated larval development of Macrobrachium totonacum Mejia, Alvarez & Hartnoll, 2003 (Decapoda, Palaemonidae), reared in the laboratory. Crustaceana, 83: 1-16. (Pantaleão et al., 2011Pantaleão, J.A.F.; Gregati, R.A.; Taddei, F.G. and Costa, R.C. 2011. Morphology of the first larval stage of Macrobrachium brasiliense (Heller, 1868) (Caridea: Palaemonidae). Nauplius, 19: 79-85.; Bueno and Rodrigues, 1995Bueno, S.L.S. and Rodrigues, S.A. 1995. Abbreviated larval development of the freshwater prawn, Macrobrachium iheringi (Ortmann, 1897) (Decapoda, Palaemonidae), reared in the laboratory. Crustaceana, 68: 665−686.; Magalhães, 1989Magalhães, C. 1989. The larval development of palaemonid shrimps from the amazon region reared in the laboratory. VI. Abbreviated development of Macrobrachium nattereri (Heller, 1862) (Crustacea, Decapoda). Amazoniana: Limnologia et Oecologia Regionalis Systematis Fluminis Amazonas, 10: 379-392.; Mejía-Ortíz et al., 2010Mejia-Ortiz, L.M.; Hartnoll, R.G. and Lopez-Mejia, M. 2010. The abbreviated larval development of Macrobrachium totonacum Mejia, Alvarez & Hartnoll, 2003 (Decapoda, Palaemonidae), reared in the laboratory. Crustaceana, 83: 1-16., respectively). Molecular analyzes (using mitochondrial 16S and COI genes) indicate that these species are phylogenetically close and are part of the same clade with abbreviated larval development (Pileggi and Mantelatto, 2010Pileggi, L.G. and Mantelatto, F.L. 2010. Molecular phylogeny of the freshwater prawn genus Macrobrachium (Decapoda, Palaemonidae), with emphasis on the relationships among selected American species. Invertebrate Systematics, 24: 194-208.; Mantelatto et al., 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.). Thus, the reproductive and phylogenetic aspects found lead us to infer that M. candango presents abbreviated larval development, a condition that should be investigated in the future with the larval description of the species.
The reproductive period in freshwater shrimps can be characterized in two ways: continuous reproduction throughout the year with reproductive peaks at specific times or the reproductive period is seasonal (Bauer, 1989Bauer, R.T. 1989. Continuous reproduction and episodic recruitment in nine shrimp species inhabiting a tropical seagrass meadow. Journal of Experimental Marine Biology and Ecology, 127: 175−187.). Although we do not have enough evidence to state the type of reproductive period for M. candango due to the sampling method used in the capture of individuals, the majority of ovigerous females (93.3 %) were collected when there was an increase in rainfall, and it is known that there is a strong relationship between this abiotic factor and the seasonal reproductive period in some species of Macrobrachium. This type of reproductive period associated with rainfall has already been observed in some other species of freshwater shrimps, such as M. iheringi and Macrobrachium potiuna (Müller, 1880) (Mattos and Oshiro, 2009Mattos, L.A. and Oshiro, L.M.Y. 2009. Estrutura populacional de Macrobrachium potiuna (Crustacea, Palaemonidae) no Rio do Moinho, Mangaratiba, Rio de Janeiro, Brasil. Biota Neotropica, 9: 81-86.; Nogueira et al., 2019Nogueira, C.S.; Perroca, J.F.; Piantkoski, E.L.; Costa, R.C.; Taddei, F.G. and Fransozo, A. 2019. Relative growth and population dynamics of Macrobrachium iheringi (Decapoda, Palaemonidae). Papéis Avulsos de Zoologia, 59: e20195908., respectively), which could be an indication that M. candango has seasonal reproduction. However, in addition to evidence on the type of larval development, further studies are needed to confirm this hypothesis.
Based on the information obtained in the present study, we can state that M. candango presents similarities with other species of the genus Macrobrachium, e.g., reproductive features such as low fecundity due to abbreviated larval development, along with allometric development patterns. Some characteristics of M. candango categorize it as a species at risk of decreasing its already low natural abundance: endemic, occurring in environments with anthropogenic pressures and low fecundity. Thus, the biological aspects studied herein are important and reinforce the previous recommendation regarding the unrestricted protection of the species (Mantelatto et al., 2016Mantelatto, F.L.; Pileggi, L.G.; Magalhães, C.; Carvalho, F.L.; Rocha, S.S.; Mossolin, E.C. and Bueno, S.L.S. 2016. Avaliação dos Camarões Palemonídeos (Decapoda: Palaemonidae). Cap. 20, p. 252-267. In: M.A.A. Pinheiro and H. Boos (Org.), Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010-2014. Porto Alegre, RS, Sociedade Brasileira de Carcinologia - SBC. 466p.; 2021Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.), including future projects focused on monitoring populations throughout the area of occurrence.
ACKNOWLEDGEMENTS
We express our gratitude to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (304968/2014-5; 302253/2019-0; 471011/2011-8) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Projeto Biota FAPESP - INTERCRUSTA 2018/13685-5; PROTAX 2021/08075-6) for providing research grants to FLM. We give special thanks to IBGE for logistic support and laboratory availability during the visit and field work. The authors also thank Dr. José Wilson Corrêa Rosa (Instituto de Geociências da Universidade de Brasília - UnB) for making the map of occurrence of Macrobrachium candango. CSN would like to thank the ongoing doctoral scholarship provided by FAPESP (2019/00661-3). ECM would like to thank the students Gabriela S. Ribeiro, Lucas R. Rezende, and Michelle F. Agapito for help with data collection. The species collections were compiled in accordance with current applicable state and federal laws of Brazil. We are grateful for the comments made by anonymous referees and assistant editor Dr. Célio Magalhães during the reviewing process.
REFERENCES
- Anger, K. 2013. Neotropical Macrobrachium (Caridea: Palaemonidae): on the biology, origin, and radiation of freshwater-invading shrimp. Journal of Crustacean Biology, 33: 151−183.
- Anger, K. and Moreira, G.S. 1998. Morphometric and reproductive traits of tropical caridean shrimps. Journal of Crustacean Biology, 18: 823−838.
- Bauer, R.T. 1989. Continuous reproduction and episodic recruitment in nine shrimp species inhabiting a tropical seagrass meadow. Journal of Experimental Marine Biology and Ecology, 127: 175−187.
- Bauer, R.T. 2004. Remarkable Shrimps: Adaptations and Natural History of the Carideans. Norman, University of Oklahoma Press, 316p.
- Bueno, S.L.S. and Rodrigues, S.A. 1995. Abbreviated larval development of the freshwater prawn, Macrobrachium iheringi (Ortmann, 1897) (Decapoda, Palaemonidae), reared in the laboratory. Crustaceana, 68: 665−686.
- Coelho, P.A. and Ramos-Porto, M. 1984. Camarões de água doce do Brasil: distribuição geográfica. Revista brasileira de Zoologia, 2: 405−410.
- Correa, C. and Thiel, M. 2003. Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Revista Chilena de Historia Natural, 76: 187−203.
- Daniel, W.W. 1990. Kruskal-Wallis one-way analysis of variance by ranks. Applied Nonparametric Statistics, 226−234.
- De Souza, M.L. and Moulton, T.P. 2005. The effects of shrimps on benthic material in a Brazilian island stream. Freshwater Biology, 50: 592−602.
- Dos Santos, A.; Hayd, L. and Anger, K. 2013. A new species of Macrobrachium Spence Bate, 1868 (Decapoda, Palaemonidae), M. pantanalense, from the Pantanal, Brazil. Zootaxa, 3700: 534−546.
- Franchito, S.H.; Rao, V.B.; Barbieri, P.R. and Santo, C.M. 2008. Rainy-season duration estimated from OLR versus rain gauge data and the 2001 drought in Southeast Brazil. Journal of Applied Meteorology and Climatology, 47: 1493−1499.
- Gomes Corrêa, M.M. 1973. Descrição de uma espécie nova do gênero Cryphiops (Decapoda, Natantia, Palaemonidae) . Revista Brasileira de Biologia, 33: 169−173.
- Hartnoll, R.G. 1974. Variation in growth pattern between some secondary sexual characters in crabs (Decapoda, Brachyura). Crustaceana, 27: 131−136.
- Hartnoll, R.G. 1978. The determination of relative growth in Crustacea. Crustaceana, 34: 281−293.
- Hawkins, S.J. and Hartnoll, R.G. 1982. The influence of barnacle cover on the numbers, growth and behaviour of Patella vulgata on a vertical pier. Journal of the Marine Biological Association of the United Kingdom, 62: 855−867.
- Holthuis, L.B. 1952. A general revision of the Palaemonidae (Crustacea, Decapoda, Natantia) of the Americas. II. The Subfamily Palaemonidae. Occasional Papers of the Allan Hancock Foundation, 12: 1-396.
- Karplus, I. and Barki, A. 2019. Male morphotypes and alternative mating tactics in freshwater prawns of the genus Macrobrachium: a review. Reviews in Aquaculture, 11: 925-940.
- Koshy, M. 1973. Studies on the sexual dimorphism in the freshwater prawn Macrobrachium dayanum (Henderson, 1893) (Decapoda, Caridea), II. Crustaceana, 24: 110-118.
- Magalhães, C. 1989. The larval development of palaemonid shrimps from the amazon region reared in the laboratory. VI. Abbreviated development of Macrobrachium nattereri (Heller, 1862) (Crustacea, Decapoda). Amazoniana: Limnologia et Oecologia Regionalis Systematis Fluminis Amazonas, 10: 379-392.
- Magalhães, C. and Walker, I. 1988. Larval development and ecological distribution of Central Amazonian palaemonid shrimps (Decapoda: Caridea) . Crustaceana, 55: 279-292.
- Mantelatto, F.L. and Barbosa, L.R. 2005. Population structure and relative growth of freshwater prawn Macrobrachium brasiliense (Decapoda, Palaemonidae) from São Paulo State, Brazil. Acta Limnologica Brasiliensia, 17: 245-255.
- Mantelatto, F.L.; Pileggi, L.G.; Magalhães, C.; Carvalho, F.L.; Rocha, S.S.; Mossolin, E.C. and Bueno, S.L.S. 2016. Avaliação dos Camarões Palemonídeos (Decapoda: Palaemonidae). Cap. 20, p. 252-267. In: M.A.A. Pinheiro and H. Boos (Org.), Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010-2014. Porto Alegre, RS, Sociedade Brasileira de Carcinologia - SBC. 466p.
- Mantelatto, F.L.; Pileggi, L.G.; Pantaleão, J.A.F.; Magalhães, C.; Villalobos, J.L. and Alvarez, F. 2021. Multigene phylogeny and taxonomic revision of American shrimps of the genus Cryphiops Dana, 1852 (Decapoda, Palaemonidae) implies a proposal for reversal of precedence with Macrobrachium Spence Bate, 1868. ZooKeys, 1047: 155-198.
- March, J.G.; Pringle, C.M.; Townsend, M.J. and Wilson, A.I. 2002. Effects of freshwater shrimp assemblages on benthic communities along an altitudinal gradient of a tropical island stream. Freshwater Biology, 47: 377-390.
- Mariappan, P. and Balasundaram, C. 1997. Cheliped laterality in freshwater prawn, Macrobrachium nobilii (Henderson and Matthai, 1910). Current Science, 875-877.
- Mariappan, P.; Balasundaram, C. and Schmitz, B. 2000. Decapod crustacean chelipeds: an overview. Journal of Biosciences, 25: 301-313.
- Mattos, L.A. and Oshiro, L.M.Y. 2009. Estrutura populacional de Macrobrachium potiuna (Crustacea, Palaemonidae) no Rio do Moinho, Mangaratiba, Rio de Janeiro, Brasil. Biota Neotropica, 9: 81-86.
- Mejia-Ortiz, L.M.; Hartnoll, R.G. and Lopez-Mejia, M. 2010. The abbreviated larval development of Macrobrachium totonacum Mejia, Alvarez & Hartnoll, 2003 (Decapoda, Palaemonidae), reared in the laboratory. Crustaceana, 83: 1-16.
- Melo, G.A.S. 2003. Manual de identificação dos Crustacea Decapoda de água doce do Brasil. São Paulo, Ed. Loyola, 429p.
- Mossolin, E.C. and Bueno, S.L. 2003. Relative growth of the second pereiopod in Macrobrachium olfersi (Wiegmann, 1836) (Decapoda, Palaemonidae). Crustaceana, 76: 363-376.
- Nagamine, C. and Knight, A.W.1980. Development, maturation and function of some sexually dimorphic structures of the Malaysian prawn, Macrobrachium rosenbergii (de Man) (Decapoda, Palaemonidae). Crustaceana, 39: 141-152.
- Nogueira, C.S.; Pantaleão, J.A.F.; Almeida, A.C. and Costa, R.C. 2020. Male morphotypes of the freshwater prawn Macrobrachium brasiliense (Decapoda: Caridea: Palaemonidae). Invertebrate Biology, 139(1): e12279.
- Nogueira, C.S.; Perroca, J.F.; Piantkoski, E.L.; Costa, R.C.; Taddei, F.G. and Fransozo, A. 2019. Relative growth and population dynamics of Macrobrachium iheringi (Decapoda, Palaemonidae). Papéis Avulsos de Zoologia, 59: e20195908.
- Odinetz-Collart, O. and Magalhães, C. 1994. Ecological constraints and life history strategies of palaemonid prawns in Amazonia. Internationale Vereinigung für theoretische und angewandte Limnologie, 25: 2460-2467.
- Pantaleão, J.A.F.; Gregati, R.A.; Taddei, F.G. and Costa, R.C. 2011. Morphology of the first larval stage of Macrobrachium brasiliense (Heller, 1868) (Caridea: Palaemonidae). Nauplius, 19: 79-85.
- Pantaleão, J.A.F.; Hirose, G.L. and Costa, R.C. 2012. Relative growth, morphological sexual maturity, and size of Macrobrachium amazonicum (Heller 1862) (Crustacea, Decapoda, Palaemonidae) in a population with an entirely freshwater life cycle. Invertebrate Reproduction & Development, 56: 180-190.
- Pearson, K. 1900. X. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 50(302): 157-175.
- Pereira, G. 1997. A cladistic analysis of the freshwater shrimps of the family Palaemonidae (Crustacea, Decapoda, Caridea). Acta Biologica Venezuelica, 17: 1-69.
- Pereira, S.G.A. and Garcia, D. 1995. Larval development of Macrobrachium reyesi (Decapoda: Palaemonidae), with a discussion on the origin of abbreviated development in palaemonids. Journal of Crustacean Biology, 15: 117-133.
- Pescinelli, R.A.; Almeida, A.O. and Costa, R.C. 2018. Population structure, relative growth and morphological sexual maturity of the snapping shrimp Alpheus brasileiro Anker, 2012 (Caridea: Alpheidae) from the south-eastern coast of Brazil. Marine Biology Research, 14: 610-620.
- Pileggi, L.G. and Mantelatto, F.L. 2010. Molecular phylogeny of the freshwater prawn genus Macrobrachium (Decapoda, Palaemonidae), with emphasis on the relationships among selected American species. Invertebrate Systematics, 24: 194-208.
- Pileggi, L.G. and Mantelatto, F.L. 2012. Taxonomic revision of doubtful Brazilian freshwater shrimp species of genus Macrobrachium (Decapoda, Palaemonidae). Iheringia, Série Zoologia, 102: 426-437.
- Pralon, B.G.N. and Negreiros-Fransozo, M.L. 2006. Population biology of Palaemon (Palaeander) northropi Rankin, 1898 (Crustacea, Decapoda, Palaemonidae) in a tropical South American estuary. Acta Limnologica Brasiliensia, 18: 77-87.
- Rossi, N.; Magalhães, C.; Mesquita, E.R. and Mantelatto, F.L. 2020. Uncovering a hidden diversity: a new species of freshwater shrimp Macrobrachium (Decapoda: Caridea: Palaemonidae) from Neotropical region (Brazil) revealed by morphological review and mitochondrial genes analyses. Zootaxa, 4732: 177-195.
- Sampaio, C.M.S.; Silva, R.R.; Santos, J.A. and Sales, S.P. 2007. Reproductive cycle of Macrobrachium amazonicum females (Crustacea, Palaemonidae) . Brazilian Journal of Biology, 67: 551-559.
- Sampedro, M.P.; González-Gurriarán, E.; Freire, J. and Muiño, R. 1999. Morphometry and sexual maturity in the spider crab Maja squinado (Decapoda: Majidae) in Galicia, Spain. Journal of Crustacean Biology, 19: 578-592.
- Sankolli, K.N.; Jalihal, D.R. and Shenoy, S. 1993. Evolution of larval developmental patterns and the process of freshwaterization in the prawn genus Macrobrachium Bate, 1868 (Decapoda, Palaemonidae) . Crustaceana, 65: 365-376.
- Santos, F.M.; Pescinelli, R.A.; Pantaleão, J.A.F. and Costa, R.C. 2018. Relative growth, morphological sexual maturity, heterochely, and handedness in Panopeus occidentalis (Brachyura, Panopeidae). Invertebrate Reproduction & Development, 62: 74-81.
- Smith, L.D. and Palmer, A.R. 1994. Effects of manipulated diet on size and performance of brachyuran crab claws. Science, 264(5159): 710-712.
- Sokal, R.R.; Rohlf, F.J. and Lahoz-León, M. 1979. Biometría: principios y métodos estadísticos en la investigación biológica. Madrid, Blume, 832p.
- Valenti, W.C.; Lobão, V.L. and Mello, J.T. 1989. Crescimento relativo de Macrobrachium acanthurus (Wiegmann, 1836) (Crustacea, Decapoda, Palaemonidae). Revista brasileira de Zoologia, 6: 1-8.
- Wehrtmann, I.S. 1990. Distribution and reproduction of Ambidexter panamense and Palaemonetes schmitti in Pacific Costa Rica (Crustacea, Decapoda). Revista de Biología Tropical, 38(2A): 327-329.
- Zar, J.H. 2010. Biostatistical analysis. Pearson Education Upper Saddie River, New Jersey, USA, 944p
Publication Dates
-
Publication in this collection
21 Mar 2022 -
Date of issue
2022
History
-
Received
09 July 2021 -
Accepted
21 Oct 2021