Abstract
Supercritical CO2 (SCO2) can be utilized to extract oils from a number of plant materials as a nontoxic alternative to hexane, and there is industrial interest in using SCO2 extraction to obtain high-quality oils for cosmetics and other high-value applications. A possible substrate is rosehip (Rosa aff. rubiginosa) seed. The scope of our work was to select SCO2 extraction conditions and to compare cold-pressed, hexane-extracted and SCO2-extracted rosehip oil. We used a fractional factorial experimental design with extraction temperature (T, 40-60 °C), extraction pressure (p, 300-500 bar) and dynamic extraction time (t, 90-270 min) as independent variables and yield and color as response variables. Samples of 100 g flaked rosehip seeds were extracted with 21 g CO2/min, following a static extraction (15 min adjustment) period. Resulting data were analyzed using response surface methodology. Extracted oil (4.7-7.1% in our experimental region) increased slightly with p and more pronouncedly with T and specially t. On the other hand, the photometric color index was independent of t but worsened (increased) as a result of an increase in either p or specially T. We extracted five batches of 250 g seeds with 21 g CO2/min at 40 °C and 300 bar for 270 min and compared the oil with samples obtained by solvent extraction (a batch of 2.5 kg of laminated seeds was treated with 10 L hexane and rotaevaporated until there was virtually no residual hexane) and cold pressing, by determining color, fatty acid composition, iodine index and saponification index. It was concluded that SCO2 allows an almost complete recovery of rosehip oil (6.5% yield), which is of a better quality than the oil extracted with hexane. Yield was higher than it was when using a cold-pressing process (5.0% yield).
Supercritical carbon dioxide; rosehip seed; oil extraction; response surface methodology
COMPARISION OF CONVENTIONAL AND SUPERCRITICAL CO2EXTRACTED ROSEHIP OIL
J.M. del Valle1,4* * To whom correspondence should be addressed , S. Bello1, J. Thiel2, A. Allen3 and L. Chordia3
1Dept. Chemical & Bioprocess Engineering, Pont. Univ. Católica de Chile, Vicuña Mackenna 4860,
San Joaquín, Santiago, Chile.
2Novbeltec S.A., Lago Llanquihue 3172, Lo Espejo, Santiago, Chile.
3Thar Designs, 730 William Pitt Way, Pittsburgh, PA, USA.
4Fax: (562) 686-5803. E-mail: delvalle@ing.puc.cl.
(Received: March 9, 2000 ; Accepted: June 5, 2000)
Abstrac - Supercritical CO2 (SCO2) can be utilized to extract oils from a number of plant materials as a nontoxic alternative to hexane, and there is industrial interest in using SCO2 extraction to obtain high-quality oils for cosmetics and other high-value applications. A possible substrate is rosehip (Rosa aff. rubiginosa) seed. The scope of our work was to select SCO2 extraction conditions and to compare cold-pressed, hexane-extracted and SCO2-extracted rosehip oil. We used a fractional factorial experimental design with extraction temperature (T, 40-60 °C), extraction pressure (p, 300-500 bar) and dynamic extraction time (t, 90-270 min) as independent variables and yield and color as response variables. Samples of 100 g flaked rosehip seeds were extracted with 21 g CO2/min, following a static extraction (15 min adjustment) period. Resulting data were analyzed using response surface methodology. Extracted oil (4.7-7.1% in our experimental region) increased slightly with p and more pronouncedly with T and specially t. On the other hand, the photometric color index was independent of t but worsened (increased) as a result of an increase in either p or specially T. We extracted five batches of 250 g seeds with 21 g CO2/min at 40 °C and 300 bar for 270 min and compared the oil with samples obtained by solvent extraction (a batch of 2.5 kg of laminated seeds was treated with 10 L hexane and rotaevaporated until there was virtually no residual hexane) and cold pressing, by determining color, fatty acid composition, iodine index and saponification index. It was concluded that SCO2 allows an almost complete recovery of rosehip oil (6.5% yield), which is of a better quality than the oil extracted with hexane. Yield was higher than it was when using a cold-pressing process (5.0% yield).
Keywords: Supercritical carbon dioxide, rosehip seed, oil extraction, response surface methodology.
INTRODUCTION
In the past, supercritical CO2 (SCO2) has been utilized as a nontoxic alternative to hexane and other organic solvents for the extraction of oil from corn germ (Christianson et al., 1984), cottonseed (Snyder et al., 1984; Kuk & Hron, 1994), peanut (Snyder et al., 1984; Goodrum & Kilgo, 1987), rapeseed (Stahl et al., 1980; Fattori et al., 1987, 1988; Temelli, 1992), soybean (Stahl et al., 1980; Friedrich & List, 1982; Friedrich et al., 1982; Snyder et al., 1984), sunflower seed (Stahl et al., 1980) and other oil-bearing materials (Favati et al., 1991; Molero Gómez et al., 1996; Roy et al., 1996; Alexander et al., 1997; Illés et al., 1997). Early reviews on this subject can be found in articles by Mangold (1982) and Friedrich and Pryde (1984) with seminal work carried out in Germany and the USA, respectively. Comprehensive and recent sources on most aspects of SCO2 extraction processes for oilseeds are the book edited by King and List in 1996 and Kings review of 1997. Although this technology is not yet commercial for triglycerides, there is industrial interest in using it to obtain high-quality oils and derivatives for cosmetics and other high-value applications (Calame & Steiner, 1982; Sankar, 1994; Moyler, 1996).
A possible oily substrate for SCO2 extraction is rosehip (Rosa aff. rubiginosa) seed, which is produced competitively in Chile (Muñoz, 1982). Rosehip is considered to be an important source of dietary fiber and nutrients (vitamin C), flavonoids, carotenoid pigments, etc. (Muñoz et al., 1981). The main commercial product is the waxy skin, whereas the seed is a low-value by-product that may be used as animal feed or fuel (Peña, 1978). However, it is interesting to evaluate the extraction of seed oil for its cosmetic properties. Rosehip oil is considered to be an important natural source of unsaturated fatty acids, which may attenuate aging and regenerate human skin, diminishing expression lines, eliminating grooves and improving the appearence of scars (Pareja & Kehl, 1990). The oil is commonly extracted using organic solvents, mainly hexane, diethyl ether, ethylic ether and petroleum ether (Pareja & Kehl, 1990). It is reddish yellow in color, with a characteristic odor and flavor, and is very susceptible to oxidative rancidity reactions (Illés et al., 1997; Omarova et al., 1997). Yield from dry seeds ranges from 6 to 8% (w/w) (Valladares et al., 1985).
Conventional solvent extraction produces low-quality oil that requires extensive purification operations (solvent removal, degumming, neutralizing, decoloring, deodorizing, etc.) (Wan & Wakelyn, 1997). Alternative deoiling processes, such as pressing, are customary only for seeds containing · 20% oil (Aguilera & Stanley, 1999). Thus, it is expected that supercritical fluid extraction (SFE) may be an alternative for high-value, low-throughput applications such as rosehip extraction. To the best of the knowledge of the authors, the only published works on this subject are those of Illés et al. (1997) and Omarova et al. (1997). Illés et al. (1997) compared SCO2, supercritical propane (C3H8) and a 1:2 (molar) CO2/C3H8 mixture as solvents for rosehip seeds. Yield increased from 5.71% (SCO2 at 35 °C and 250 bar) to 6.59% (C3H8 at 28 °C and 80 bar) and to 6.75% (CO2/C3H8 mixture at 28 °C and 120 bar). Their results indicated that supercritical fluids extracted micronutrients much more effectively than hexane. Omarova et al. (1997) obtained a total yield of 2% when extracting rosehip fruit with SCO2 at 26 °C and 630 bar. Mostly lipids (88.2%) and water (9.0%) and minor amounts of pigments (anthocyanins, xanthophylls, carotenoids) and aqueo- and liposoluble vitamins (carotenes pro-vitamin A, ascorbic acid vitamin C, tocopherols vitamin E) constituted this extract.
The objetive of this work was to evaluate the effects of extraction temperature, extraction pressure and extraction time on the yield and color quality of rosehip oil and to compare it with products obtained by solvent extraction and cold pressing.
MATERIALS AND METHODS
Materials
Flaked rosehip seed samples were provide by Novbeltec S.A. (Santiago, Chile). Samples were kept in sealed plastic bags in a refrigerator up to the time of analysis. Cold-pressed oil, obtained using their proprietary process, was also provided by Novbeltec S.A. Liquid carbon dioxide with a purity of · 99.8% was provided in 22-27 kg cylinders by AGA S.A. (Santiago, Chile). Technical grade hexane was purchased from Idapquim (Santiago, Chile).
Extraction Processes
Conventional Extraction. A batch of 2.5 kg flaked seeds was treated with 10 L hexane in a pilot plant Soxhlet extractor (Quickfit and Quarz Ltd., London, UK) so as to achieve total oil exhaustion (20 h, in cycles of about 1 h). Solvent was removed from the miscella in a Heindolph Elektro GmbH & Co. (Kelheim, Germany) Lovarota rotaevaporator until there was virtually no residual hexane.
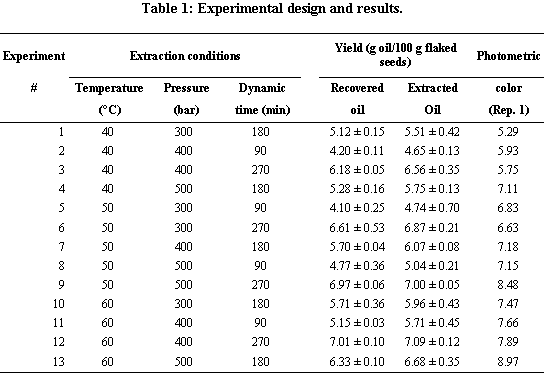
SFE Extraction. Experiments were carried out using Thar Designs (Pittsburgh, PA) SFE-1L process development unit (PDU). This fully computerized system allows a precise and independent automatic control of extraction and collection temperature and pressure (·690 bar) and solvent and cosolvent flow rate (·200 g CO2/min). For the screening experiments, 100 g flaked seed samples were placed in a 200 mL extraction vessel and treated with 21 g CO2/min, following a static extraction (15 min adjustment) period. Oil was recovered in 25 mL cyclone separators. A fractional factorial experimental design (Table 1) with three independent variables and three levels was utilized: extraction temperature ranged from 40 to 60 °C, extraction pressure from 300 to 500 bar, and dynamic extraction time from 90 to 270 min. Both the yield and the photometric color of recovered oil were evaluated.
Temperature control was achieved by placing the extractor vessel and cyclone separator in separate convection ovens. The extraction oven was set to the required extraction temperature for 30 min to achieve temperature equilibration prior to initiating the static extraction period. Temperature was automatically controlled by reading the temperature of the loaded CO2 stream leaving the extractor, and adjusting the oven heater. The collection oven was set at 60 °C in all experiments.
For the static extraction period, the CO2 pump was operated in the pressure control mode and the on/off valve at the exit of the extraction oven was maintained in the off position. During the dynamic extraction period, the main pump was set to the flow control mode, the aforementioned on/off valve was set to the on position and the extraction pressure was maintained by the back pressure regulator (BPR) located in the collection oven. After 90 min of dynamic extraction (in experiments of >90 min duration), oil collection was switched from one cyclone separator to the other with a multiposition valve located behind the BPR. In this way we obtained complete information and some duplicates on the effect of extraction temperature and pressure on oil collected during the 90 min extraction period.
Two similar systems were utilized for each replicate. One was located in the Extraction Laboratory of Biological Materials (LEMaB) of the main author (Replicate 1) in Santiago, Chile and the other at the facilities of Thar Designs in Pittsburgh, PA (Replicate 2). The main difference was that temperature control in Replicate 2 was achieved by circulating water from a thermostated bath at the required extraction and collection temperature through heat jackets on extraction and cyclone vessels. Once the experiment was concluded, each cyclone separator was washed out with ·10 ml hexane, and the collected miscella was placed in a sealed vial and kept refrigerated up to the time of analysis. Just prior to quantitation of recovered oil and color, hexane was removed by placing the vials on a vacuum oven set at ca. 60 °C and 0.27 bar (absolute pressure).
For oil comparison purposes, five additional batches of 250 g flaked seeds were placed in a 500 mL jacketed extraction vessel and processed with 21 g CO2/min at 300 bar for 270 min. Oil was recovered in a 200 mL jacketed cyclone separator. The extraction vessel was heated by circulating water at 40 °C from a thermostated bath, whereas the cyclone vessel was tempered by circulating tap water (ca. 15 °C) through their jackets.
Analytical Methods
Moisture and Oil Content. Moisture was determined gravimetrically by drying samples of ca. 3 g flaked seeds (weighed accurately) in an oven at 105 °C to a constant final weight (24 h). Oil content was determined gravimetrically by total extraction of ca. 10 g samples (weighed accurately) with hexane in a Soxhlet apparatus (24 h) placed in a water bath at ca. 75 °C, followed by solvent removal of recovered miscella in a laboratory rotavaporator (bath set at ca. 60 °C) operated under vacuum (0.27 bar). Both determinations were done in duplicate.
Oil Yield Calculations. Recovered oil was reported in g oil/100 g flaked seeds. We also estimated extracted oil by subtracting from the initial content (ca. 7.12 g oil/100 g flaked seeds) the residual oil content in treated samples determined using Soxhlet extraction. Care was taken to homogenize all solids recovered from the extraction vessel prior to sampling. To account for differences in sample moisture brought about by SFE extraction, both the initial and residual oil contents were expressed per gram of nonfat dry solids prior to subtraction, and the difference was multiplied by a factor of ca. 0.847 (corresponding to the amount of nonfat dry solids in 1 g of flaked seeds). Thus, for each replicate we were able to assess both recovered oil and extracted oil.
Determination of Photometric Color. Oil color was assessed in a Shimadzu (Kyoto, Japan) spectrophotometer model UV-160. Filtered oil samples at ca. 20 °C were placed in 0.7 ml cubettes 10 mm in width. Absorbance values at wavelengths of 460 (A460), 550 (A550), 620 (A620) and 670 nm (A670) were measured and recorded. Photometric color (PhC) was assessed in triplicate according to equation 1 (Mehlenbacher, 1979):
PhC = 1.29 A460 + 69.7 A550 + 41.2 A620 56.4 A670
(1)
It is interesting to point out that readings of PhC correlated well with sensory Wesson values, which give an indication of reddish pigmentation in oils (Mehlenbacher, 1979).
Characterization of oil samples. Official methods of the Americal Oil Chemists Society (AOCS, 1989) were utilized to characterize oil samples. Determinations included iodine value (Official Method Cd 1-25) and saponification value (Official Method Cd 3-25), wich were both performed in duplicate. The fatty acids profile was determined using capillary gas liquid chromatography (GLC) of methyl esters of fatty acids according to AOCS Official Method Ce 1e-91 (1989), which uses an Erba Serie 8000 GLC equipped with a flame ionization detector (FID) and a fused silica capillary column SGE BPX-70 (50 m x 0.32 mm) with a film thickness of 0.25 µm. The injection and detection ports were kept at 250 and 270 °C, respectively. The temperature program for the oven was 8 min at 185 °C, followed by 7 min at 205 °C, and finally, 5 min at 230 °C.
RESULTS AND DISCUSSION
Effects of Extraction Temperature, Extraction Pressure and Treatment Time on Oil Extraction and Oil Recovery
The fractional factorial experimental design utilized in this research allowed a reduction in the required experimental work and still gave a clear picture of the effect of independent variables on yield and quality of rosehip oil extracted with SCO2. To facilitate the statistical analysis, independent variables were first recoded according to equation 2 so that they adopted values of -1 (minimum value utilized in our experimental design), 0 (intermediate value) or +1 (maximum value), where

(2a)

(2b)

(2c)
where T is the extraction temperature (°C); p, the extraction pressure (bar); and t, the dynamic extraction time (min). Experimental values of oil yield (Y) and photometric color (PhC) (Table 1) were fitted to an empirical second order polynomial equation (eqn. 3) with  ,
,  and
and  as variables. In general terms:
as variables. In general terms:

(3)
where response variable R corresponded to either Y or PhC; x0 = 1; x1 is the independent variable  ; x2 is the independent variable
; x2 is the independent variable  ; and x3 is the independent variable
; and x3 is the independent variable  .
.
Table 2 presents a statistical analysis of our oil yield data using response surface methodology. It indicates that replicate does not have a significant effect (p ·10%) on oil yield. However, it suggests that the method utilized to determine yield does have a significant effect (p < 5%) on the amount of oil extracted. In fact, we determined that estimates of extracted oil (EO) were consistently higher than those of recovered oil (RO) for the same experiments (Fig. 1). The explanation for these differences is that some unrecovered oil remained in equipment lines, both between the BPR and the cyclone inlet and in the cyclone outlet line, as a result of a sharp decrease in solubility associated with a pressure decrease in the BPR. However, this unrecovered oil is accounted for in the determination of extracted oil. Figure 1, however, demonstrates that differences between EO and RO, although statistically significant were fairly small (0.05-0.95 g oil/100 g flaked rosehip seed). We decided to utilize EO in the remainder of this manuscript because it gives a more precise indication of all the oil that was extracted by SCO2.
After redoing the statistical analysis to consider only yield data generated using EO, it was demonstrated that replicate does not have a significant effect (p · 10%) on EO (Table 2). This is very important because it demonstrates that similar results can be obtained by two groups utilizing different equipment configurations and that Thar Designs SFE-1L PDU has a very reproducible control of main process variables (mainly extraction temperature and pressure).
Table 2 also demonstrated that both extraction temperature and processing time have a significant effect (p · 1%) on EO, whereas extraction pressures between 300 and 500 bar do not (p > 10%). Furthermore, Table 2 demonstrates that second-order terms do not have a significant effect (p > 10%) on EO. Finally, it was concluded that  ,
,  and
and  have a significant effect (p · 1%) on EO. In conclusion, its appear as if a very simple response surface equation, considering only the linear effects of
have a significant effect (p · 1%) on EO. In conclusion, its appear as if a very simple response surface equation, considering only the linear effects of  and
and  , could represent the changes in EO with extraction variables.
, could represent the changes in EO with extraction variables.
Statistical analysis notwithstanding, we feel that the best response surface equation should consider the linear effects of all three variables ( ,
,  ,
,  ) as well as the interaction terms
) as well as the interaction terms  *
*  and
and  *
*  (Table 3). This is based on the fact that the standard deviation (SD) of discrepancies between model predictions and experimental values was minimal (0.296) after eliminating the terms
(Table 3). This is based on the fact that the standard deviation (SD) of discrepancies between model predictions and experimental values was minimal (0.296) after eliminating the terms  2,
2,  *
*  ,
,  2 and
2 and  2 (in this order). This is due to a more pronounced decline in degrees of freedom than the sum of squares of deviations between predicted and experimental values. The elimination of the least significant remaining term (
2 (in this order). This is due to a more pronounced decline in degrees of freedom than the sum of squares of deviations between predicted and experimental values. The elimination of the least significant remaining term ( *
*  ) causes an increase in SD to 0.299. These values are reasonable in that they are comparable to the experimentally assessed average SD corresponding to variations between Replicate 1 and Replicate 2 (0.280).
) causes an increase in SD to 0.299. These values are reasonable in that they are comparable to the experimentally assessed average SD corresponding to variations between Replicate 1 and Replicate 2 (0.280).
The selected response surface equation
suggests a yield of 5.97% oil for a 3 h extraction with SCO2 at 50 °C and 400 bar. Under these conditions, yield would increase by 0.37% as a result of an increase in temperature of 10 °C, by 0.18% as a result of an increase in pressure of 100 bar and by 0.93% as a result of an increase in extraction time of 1.5 h. There is also a positive  *
*  interaction (increases in yield with extraction temperature are more pronounced at high than at low pressure) and a negative
interaction (increases in yield with extraction temperature are more pronounced at high than at low pressure) and a negative  *
*  interaction (increases in yield with temperature are less pronounced as extraction progresses).
interaction (increases in yield with temperature are less pronounced as extraction progresses).
The response surface is plotted in Figure 2. It is clear that for relatively short extraction times of 1.5 h (Fig. 2A), it is possible to increase yield by increasing extraction pressure or, more effectively, by increasing extraction temperature. These effects are not as clear for long extraction times of 4.5 h when, independently of extraction conditions, yields are close to the maximal 7.12% value (Fig. 2B). In agreement with these plots, experimental values of yield ranged from 4.7% (1.5 h extraction with SCO2 at 40 °C and 400 bar) to 7.1% (4.5 h extraction with SCO2 at 60 °C and 400 bar) (Table 1).
Experimental variations in yield as a function of extraction temperature and extraction pressure can be compared values for oil solubility found in the literature. Pseudosolubility values (cps, in g oil/ml CO2) were estimated by dividing the total amount of oil recovered in Replicate 1 during the first 1.5 h of extraction (RO90, in grams) by the total volume of SCO2 that passed through the extractor during that period (V90, in liters). cps and V90 were evaluated according to equations 5 and 6:
where

(6)
and · represents the density of SCO2 at experimental temperature and pressure. Values of · were evaluated by double-square interpolation in tables for the compressibility factor of CO2 as a function of T-1 and p (Pickering, 1928), as proposed by del Valle and Aguilera (1988). Values of c reported in Table 4 for 50 °C and 300 bar, 40 °C and 400 bar, 60 °C and 400 bar, and 50 °C and 500 bar correspond to averages (standard deviations) of estimates for experiments of 90 min duration, and the first 90 min of extraction in experiments lasting 270 min. All other combinations reported in Table 4 correspond to results derived from data on oil recovered during the first 90 min of extraction in experiments lasting 180 min. As expected, cps increased consistently with p and less pronouncedly with T and values ranged from 1.94 g/l at 40 °C and 300 bar to 2.69 g/l at 60 °C and 500 bar. However, pseudosolubility values for equivalent experimental conditions decreased when they were evaluated based on oil recoveries and volumes of CO2 used up in longer extractions (data not shown). The reduction in pseudosolubility was between 31.3 and 40.4% (average of 35.3%) after 180 min of extraction and between 42.4 and 54.5% (average of 48.7%) after 270 min of extraction. This indicates a reduction in extraction rate after 90 min, probably due to depletion of surface oil on flaked rosehip seeds. However, since no data was collected for very short extraction times, this depletion of surface oil may have occurred in less than 1.5 h, specially when using SCO2 with high solvent power (high density, high temperature). This hypothesis may be confirmed by comparing our pseudosolubility values with true triglyceride solubility values for oilseeds, in general (del Valle & Aguilera, 1988), or rosehip seed, in particular (Illés et al., 1997). It is relevant to point out that del Valles and Aguileras formula appears to be valid in the 20-80 °C and 150-680 bar region, whereas that of Illés and coauthors was generated using solubility data measured at 35 and 55 °C and several pressures between 100 and 400 bar.
Eggers (1996) reviewed the effect of several variables on extraction rates. He claimed that extraction rates are determined by equilibrium restraints only during the initial stages of the extraction process. In the case of rapeseed extracted with SCO2 at 40 °C and 300 bar, Eggers (1996) reported that for mechanically deoiled seed containing 24.8% residual oil, the amount of SCO2 required to extract 55% of the initial oil content (the percentage that was removed in our case under equivalent extraction conditions) was between 136 and 152% of that required if conditions of loading to saturation were achieved. The actual SCO2 requirement depended on whether the press cake was extracted as is or following additional flaking treatment (which caused pseudosolubility values to be 26.5-34.2% smaller than true solubility values). It is conceivable that this percentage would increase for seeds containing less oil initially, or oil that is more tightly held (less intensive microstructural destruction as a result of seed pretreatment). The solvent flow rate also has some effect on solvent requirements for extraction. In the case of milled corn germ (·23.4% oil; Christianson et al., 1984) treated at 50 °C and 500 bar, the SCO2 requirement for extracting up to a residual content of 2.57% oil (the residual content in flaked rosehip seeds following 90 min extraction under equivalent conditions) was 114, 125 and 181% of the theoretical value (solvent loaded to saturation) when using 21.7, 33.3 and 66.7 g CO2/100 g substrate/min, respectively (Eggers, 1996). This would cause a decrease in pseudosolubility of between 12.3 and 44.8% of the true solubility values. It is interesting to point out that in our case we utilized 21.0 g CO2/100 g substrate/min, whereas Illés et al. (1997) presumably utilized 4.8 g CO2/100 g substrate/min in their solubility determinations (this value was estimated based on the assumption that their 114 mL extraction vessel was loaded with 0.5 g/mL of hiprose seed, the packing utilized in our studies). Eggers (1996) recommends using 16.6-83.3 g CO2/100 g substrate/min, which corresponds to superficial solvent velocities of between 1 and 5 mm/s. Illés et al. (1997) claimed that they could obtain solubility values at the outlet of their extractor if they used ·12.9 g CO2/100 g substrate/min.
Considering the variations in true solubility values reported by del Valle and Aguilera (1988), under ideal solvent loading conditions, oil depletion from the rosehip seed should have taken between 12.9 (60 °C and 500 bar) and 44.3 min (40 °C and 300 bar). Thus, it is clear that either rosehip extraction was kinetically controlled in our experiments or oil recovery in our cyclone separators was controlled to a great extent by the transport of precipitated oil in connecting lines by expanded CO2 flowing through them.
Effects of Extraction Temperature, Extraction Pressure and Treatment Time on Oil Color
We proceeded in a similar fashion to analyze the response surface for photometric color as a function of extraction temperature, extraction pressure and processing time. In this case, we had a single measurement of color (of recovered oil) and we were also unable to consider the color of oil obtained in Replicate 2. This was probably due to cleaning and handling considerations that may have negatively affected the delicate color of the rosehip oil. Thus, the replicate had a considerable effect on color, but no statistical test was performed to verify this.
On the other hand, we were able to demonstrate that processing time did not have a significant effect (p > 10%) of on oil color, which was significantly affected (p · 5%) by both extraction temperature and pressure (data not shown). Furthermore, we demonstrated that second-order terms had not a significant effect (p > 10%), suggesting that the response for color as a funtion of extraction temperature and pressure was a fairly flat surface (data not shown). When we attempted to eliminate individual terms from the response surface equation in order to simplify it, we demonstrated that besides all second-order terms, all first-order that included  as variable were not significant (p > 10%) (data not shown). Statistical analysis notwithstanding, we selected the response surface reported below (eqn. 7) because SD decreased from an initial value of 0.411 to a minimal of 0.367 (after eliminating the terms with
as variable were not significant (p > 10%) (data not shown). Statistical analysis notwithstanding, we selected the response surface reported below (eqn. 7) because SD decreased from an initial value of 0.411 to a minimal of 0.367 (after eliminating the terms with  ,
,  *
*  ,
,  *
*  ,
,  *
*  ,
,  2 and
2 and  2) before increasing to 0.409 (after eliminating all of the above and
2) before increasing to 0.409 (after eliminating all of the above and  2).
2).
The selected response surface equation

(7)
suggests a photometric color of 6.88 for oil extracted during 3 h using SCO2 at 50 °C and 400 bar, and that color becomes worse (photometric color increases) as a result of increases in either temperature (increase of 0.99 in PhC with an increase of 10 °C) or, to a lesser extent, pressure (increase of 0.69 in PhC with an increase of 100 bar). However, the second-order term in equation 7 indicates that the negative effect of increasing pressure is more pronounced at extremely high pressures (500 bar). Increases in temperature and pressure usually increase solubility and coextraction of undesirable pigments (Friedrich & List, 1982). In addition, thermal degradation may be responsible for additional darkening of oil samples.
The response surface for photometric color is plotted in Figure 3. It is clear that photometric color decreases (with an associated improvement in oil color quality) as a result of a decrease in extraction pressure or, more effectively, a decrease in extraction temperature. In agreement with this plot, experimental values of PhC ranged from 5.29 (3 h extraction with SCO2 at 40 °C and 300 bar) to 8.97 (3 h extraction with SCO2 at 60 °C and 500 bar) (Table 1).
It is thus clear that the objectives of increased yield and improved color may not be achieved simultaneously. In fact, increases in temperature and pressure that result in faster extraction also result in an improved coextraction of undesirable compounds such as reddish pigments and a reduction in the color quality of oil samples. For this reason, we decided to compare SCO2-extracted with commercial rosehip oil samples, using the most benign conditions in our original experimental design of low temperature (40 °C) and low pressure (300 bar). We also decided to increase extraction time to the maximal value under analysis (4.5 h) to compensate for the reduced extraction rates.
Comparison of Oil Obtained Using Different Extraction Techniques
Table 5 shows the results of the comparisons between rosehip oil samples obtained using different technologies. It can be seen that the yield of sco2 (4.5 h at 40 °c and 300 bar) extraction was slightly lower than that of hexane extraction, but considerably higher than that of cold-pressed oil from the seed. To improve the yield of the sco2 process, extraction time could have been increased. An increase in either extraction temperature or extraction pressure could have also increased yield, but a decrease in oil quality would have been expected under those conditions. On the other hand, the yield of the cold-pressing process has already been optimized.
The SCO2-extracted oil had the best color, followed by the cold-pressed and the hexane-extracted oil (table 5). On the other hand, there were only small differences between the three oil samples, as assessed using simple chemical tests (iodine value, saponification value) (table 5). The iodine value gives an indication of the degree of unsaturation of constituent fatty acids. On the other hand, the saponification value is related to the average molecular mass of the fatty acids. Thus, the results of these two tests are related to the fatty acid composition of triglyceride samples.
Only very small differences in fatty acid composition were detected between the oil samples (table 5). This has already been reported by many others, including stahl et al. (1980). Friedrich and list (1982) also reported that SCO2- and hexane-extracted oils have a similar content of unsaponifiable matter. The only chemical differences that have been observed are that smaller contents of polar lipids (phospho- and glycolipids) (mangold, 1982) and liposoluble pigments (chlorophyll, carotenoids) are present in sco2- than in conventional solvent-extracted oils (friedrich & list, 1982). However some of the latter differences depend largely on extraction temperature and pressure conditions. As a consequence of these differences, the naoh requirement and refining losses may be smaller when sco2 rather than an organic solvent is used to extract the oils. On the other hand, sco2-extracted oils manifest lower oxidative stability than conventional oils because of reduced coextraction of native antioxidants in the oilseed (list et al., 1984; list & friedrich, 1985).
ACKNOWLEDGEMENTS
This work was carried out under the auspices of the Chilean science and technology funding agencies, Fondef (Research and Development project D97I2026) and Fondecyt (project 100-0382). The donation of carbon dioxide by AGA S.A. is greatly appreciated.
REFERENCES
Aguilera, J.M., Stanley, D.W., Microstructural Principles of Food Processing and Engineering, 2nd. Ed. Aspen Publ., Inc., Frederick, MD (1999).
Alexander, W.S., Brusewitz, G.H., Maness, N.O., Pecan Oil Recovery and Composition as Affected by Temperature, Pressure, and Supercritical CO2 Flow Rate, Journal of Food Science, 62, 762 (1997).
AOCS, Official Methods and Recommended Practices of the American Oil Chemists Society. American Oil Chemists Society, Champaign, IL (1989).
Calame, J.P., Steiner, R., CO2 Extraction in the Flavor and Perfumery Industries, Chemistry and Industry (London), 399 (1982).
Christianson, D.D., Friedrich, J.P., List, G.R., Warner, K., Bagley, E.B., Stringfellow, A.C., Inglett, G.E., Supercritical Fluid Extraction of Dry-Milled Corn Germ with Carbon Dioxide, Journal of Food Science, 49, 229 (1984).
del Valle, J.M., Aguilera, J.M., An Improved Equation for Predicting the Solubility of Vegetable Oils in Supercritical CO2, Industrial Engineering and Chemistry Research, 27, 1551 (1988).
del Valle, J.M., Aguilera, J.M., Extracción con CO2 a Alta Presión. Fundamentos y Aplicaciones en la Industria de Alimentos, Food Science and Technology International, 5, 1 (1999).
Eggers, R., Supercritical Fluid Extraction (SFE) of Oilseeds/Lipids in Natural Products, In King, J.W., List, G.R. (Eds.): Supercritical Fluid Technology in Oil and Lipid Chemistry, p. 35. AOCS Press, Champaign, IL (1996).
Fattori, M., Bulley, N.R., Meisen, A., Fatty Acid and Phosphorus Contents of Canola Seed Extracts Obtained with Supercritical Carbon Dioxide, Journal of the Agricultural and Food Chemistry, 35, 739 (1987).
Fattori, M., Bulley, N.R., Meisen, A., Carbon Dioxide Extraction of Canola Seed: Oil Solubility and Effect of Seed Treatment, Journal of the American Oil Chemists Society, 65, 968 (1988).
Favati, F., King, J.W., Manzanti, M., Supercritical Carbon Dioxide Extraction of Evening Primrose, Journal of the American Oil Chemists Society, 68, 422 (1991).
Friedrich, J.P., List, G.R., Characterization of Soybean Oil Extraction by Supercritical Carbon Dioxide and Hexane, Journal of Agricultural and Food Chemistry, 30, 192 (1982).
Friedrich, J.P., List, G.R., Heakin, A.J., Petroleum-Free Extraction of Oil from Soybean with Supercritical CO2, Journal of the American Oil Chemists Society, 59, 288 (1982).
Friedrich, J.P., Pryde, E.H., Supercritical CO2 Extraction of Lipid Bearing Materials and Characterization of the Products, Journal of the American Oil Chemists Society, 61, 223 (1984).
Goodrum, J.W, Kilgo, M.B., Peanut Oil Extraction with SC-CO2: Solubility and Kinetic Functions, Transactions of the American Society of Agricultural Engineers, 30, 1865 (1987).
Illés, V., Szalai, O., Then, M., Daood, H., Perneczki, S., Extraction of Hiprose Fruit by Supercritical CO2 and Propane, Journal of Supercritical Fluids, 10, 209 (1997).
King, J.W., Critical Fluids for Oil Extraction, In Wan, P.J., Wakelyn, P.J. (Eds.): Technology and Solvents for Extracting Oilseeds and Nonpetroleum Oils, p. 283. AOCS Press, Champaign, IL (1997).
King, J.W., List, G.R., Supercritical Fluid Technology in Oil and Lipid Chemistry. AOCS Press, Champaign, IL (1996).
Kuk, M.S., Hron, R.J. Sr., Supercritical Carbon Dioxide Extraction of Cottonseed with Co-Solvents, Journal of the American Oil Chemists Society, 71, 1353 (1994).
List, G.R., Friedrich, J.P., Processing Characteristics and Oxidative Stability of Soybean Oil Extracted with Supercritical Carbon Dioxide at 50 °C and 8000 psi, Journal of the American Oil Chemists Society, 62, 82 (1985).
List, G.R., Friedrich, J.P., Christianson, D.D., Properties and Processing of Corn Oils Obtained by Extraction with Supercritical Carbon Dioxide, Journal of the American Oil Chemists Society, 61, 1849 (1984).
Mangold, H.K., Extraction with Supercritical Fluids: A Progress Report from Germany, Journal of the American Oil Chemists Society, 59, 673A (1982).
Mehlenbacher, V.C., Análisis de Grasas y Aceites. Ediciones Urmo, Bilbao, España (1979).
Molero Gómez, A., Pereyra López, C., Martínez de la Ossa, E., Recovery of Grape Seed Oil by Liquid and Supercritical Carbon Dioxide Extraction: A Comparison with Conventional Solvent Extraction, The Chemical Engineering Journal, 61, 227 (1996).
Moyler, D.A., Commercial Extraction of Flavours and Perfumes, Journal of Chemical Technology and Biotechnology, 65, 296 (1996).
Muñoz, A., Perspectivas comerciales del fruto de la Rosa aff. rubiginosa en la VIII region. B.S. thesis, Universidad de Chile (1982).
Muñoz, M., Barrera, E., Meza, I., El Uso Medicinal y Alimenticio de las Plantas Nativas y Naturalizadas en Chile. Publicación ocasional N°33, Museo Nacional de Historia Natural, Santiago, Chile (1981).
Omarova, M.A., Artamonova, N.A., Proskurin, B.M., Chemical Composition of a CO2 Extract of Rose Fruit, Chemistry of Natural Compounds, 33, 689 (1997).
Pareja, B., Kehl, H., Contribución a la Identificación de los Principios Activos en el Aceite de Rosa aff. rubigonosa, Anales de la Real Academia de Farmacia, 56, 283 (1990).
Peña, J.E., Utilización de la Pepa de Mosqueta (Rosa aff. rubigonosa) en la Alimentación de Aves de Postura Durante el Período de Recria. B.S. thesis, Universidad de Concepción (1978).
Pickering, S.F., P-V-T Relations in the Gaseous State for Substances which are Gases at 0 °C and 1 atm, In Washburn, E.W. (Ed.): International Critical Tables of Numerical Data, Physics, Chemistry and Technology, 1st. Ed., Vol. 3, p. 3. McGraw-Hill, New York, NY (1928).
Roy, B.P., Goto, M., Hirose, T., Temperature and Pressure Effects on Supercritical CO2 Extraction of Tomato Seed Oil, International Journal of Food Science and Technology, 31, 137 (1996).
Sankar, K.U., Supercritical Fluid Carbon Dioxide Technology for Extraction of Spices and Other High Value Bio-active Compounds, In Rizvi, S.S.H. (Ed.): Supercritical Fluid Processing of Food and Biomaterials, p. 155. Blackie Academic & Professional, London (1994).
Snyder, J.M., Friedrich, J.P., Christianson, D.D., Effect of Moisture and Particle Size on the Extractability of Oils from Seeds with Supercritical CO2, Journal of the American Oil Chemists Society, 61, 1851 (1984).
Stahl, E., Schutz, E., Mangold, H.K., Extraction of Seed Oils with Liquid and Supercritical Carbon Dioxide, Journal Agricultural and Food Chemistry, 28, 1153 (1980).
Temelli, F., Extraction of Tryglycerides and Phospholipids from Canola with Supercritical Carbon Dioxide and Ethanol, Journal of Food Science, 57, 440 (1992).
Valladares, J., Palma, M., Sandoval, C., Crema de Aceite de Mosqueta (Rosa aff. rubigonosa) I Parte: Formulación, Preparación y Aplicación Sistemática en Clínica, Anales de la Real Academia de Farmaceútica, 51, 327 (1985).
Wan, P.J., Wakelyn, P.J., Technology and Solvents for Extracting Oilseeds and Nonpetroleum Oils. AOCS Press, Champaign, IL (1997).
- Aguilera, J.M., Stanley, D.W., Microstructural Principles of Food Processing and Engineering, 2nd. Ed. Aspen Publ., Inc., Frederick, MD (1999).
- Calame, J.P., Steiner, R., CO2 Extraction in the Flavor and Perfumery Industries, Chemistry and Industry (London), 399 (1982).
- del Valle, J.M., Aguilera, J.M., An Improved Equation for Predicting the Solubility of Vegetable Oils in Supercritical CO2, Industrial Engineering and Chemistry Research, 27, 1551 (1988).
- del Valle, J.M., Aguilera, J.M., Extracción con CO2 a Alta Presión. Fundamentos y Aplicaciones en la Industria de Alimentos, Food Science and Technology International, 5, 1 (1999).
- Eggers, R., Supercritical Fluid Extraction (SFE) of Oilseeds/Lipids in Natural Products, In King, J.W., List, G.R. (Eds.): Supercritical Fluid Technology in Oil and Lipid Chemistry, p. 35. AOCS Press, Champaign, IL (1996).
- Fattori, M., Bulley, N.R., Meisen, A., Fatty Acid and Phosphorus Contents of Canola Seed Extracts Obtained with Supercritical Carbon Dioxide, Journal of the Agricultural and Food Chemistry, 35, 739 (1987).
- Fattori, M., Bulley, N.R., Meisen, A., Carbon Dioxide Extraction of Canola Seed: Oil Solubility and Effect of Seed Treatment, Journal of the American Oil Chemists Society, 65, 968 (1988).
- Favati, F., King, J.W., Manzanti, M., Supercritical Carbon Dioxide Extraction of Evening Primrose, Journal of the American Oil Chemists Society, 68, 422 (1991).
- Friedrich, J.P., List, G.R., Characterization of Soybean Oil Extraction by Supercritical Carbon Dioxide and Hexane, Journal of Agricultural and Food Chemistry, 30, 192 (1982).
- Friedrich, J.P., List, G.R., Heakin, A.J., Petroleum-Free Extraction of Oil from Soybean with Supercritical CO2, Journal of the American Oil Chemists Society, 59, 288 (1982).
- Friedrich, J.P., Pryde, E.H., Supercritical CO2 Extraction of Lipid Bearing Materials and Characterization of the Products, Journal of the American Oil Chemists Society, 61, 223 (1984).
- Goodrum, J.W, Kilgo, M.B., Peanut Oil Extraction with SC-CO2: Solubility and Kinetic Functions, Transactions of the American Society of Agricultural Engineers, 30, 1865 (1987).
- Illés, V., Szalai, O., Then, M., Daood, H., Perneczki, S., Extraction of Hiprose Fruit by Supercritical CO2 and Propane, Journal of Supercritical Fluids, 10, 209 (1997).
- King, J.W., Critical Fluids for Oil Extraction, In Wan, P.J., Wakelyn, P.J. (Eds.): Technology and Solvents for Extracting Oilseeds and Nonpetroleum Oils, p. 283. AOCS Press, Champaign, IL (1997).
- King, J.W., List, G.R., Supercritical Fluid Technology in Oil and Lipid Chemistry. AOCS Press, Champaign, IL (1996).
- Kuk, M.S., Hron, R.J. Sr., Supercritical Carbon Dioxide Extraction of Cottonseed with Co-Solvents, Journal of the American Oil Chemists Society, 71, 1353 (1994).
- List, G.R., Friedrich, J.P., Processing Characteristics and Oxidative Stability of Soybean Oil Extracted with Supercritical Carbon Dioxide at 50 °C and 8000 psi, Journal of the American Oil Chemists Society, 62, 82 (1985).
- List, G.R., Friedrich, J.P., Christianson, D.D., Properties and Processing of Corn Oils Obtained by Extraction with Supercritical Carbon Dioxide, Journal of the American Oil Chemists Society, 61, 1849 (1984).
- Mangold, H.K., Extraction with Supercritical Fluids: A Progress Report from Germany, Journal of the American Oil Chemists Society, 59, 673A (1982).
- Mehlenbacher, V.C., Análisis de Grasas y Aceites. Ediciones Urmo, Bilbao, Espańa (1979).
- Molero Gómez, A., Pereyra López, C., Martínez de la Ossa, E., Recovery of Grape Seed Oil by Liquid and Supercritical Carbon Dioxide Extraction: A Comparison with Conventional Solvent Extraction, The Chemical Engineering Journal, 61, 227 (1996).
- Moyler, D.A., Commercial Extraction of Flavours and Perfumes, Journal of Chemical Technology and Biotechnology, 65, 296 (1996).
- Muńoz, A., Perspectivas comerciales del fruto de la Rosa aff. rubiginosa en la VIII region. B.S. thesis, Universidad de Chile (1982).
- Muńoz, M., Barrera, E., Meza, I., El Uso Medicinal y Alimenticio de las Plantas Nativas y Naturalizadas en Chile. Publicación ocasional N°33, Museo Nacional de Historia Natural, Santiago, Chile (1981).
- Omarova, M.A., Artamonova, N.A., Proskurin, B.M., Chemical Composition of a CO2 Extract of Rose Fruit, Chemistry of Natural Compounds, 33, 689 (1997).
- Pareja, B., Kehl, H., Contribución a la Identificación de los Principios Activos en el Aceite de Rosa aff. rubigonosa, Anales de la Real Academia de Farmacia, 56, 283 (1990).
- Peńa, J.E., Utilización de la Pepa de Mosqueta (Rosa aff. rubigonosa) en la Alimentación de Aves de Postura Durante el Período de Recria. B.S. thesis, Universidad de Concepción (1978).
- Pickering, S.F., P-V-T Relations in the Gaseous State for Substances which are Gases at 0 °C and 1 atm, In Washburn, E.W. (Ed.): International Critical Tables of Numerical Data, Physics, Chemistry and Technology, 1st. Ed., Vol. 3, p. 3. McGraw-Hill, New York, NY (1928).
- Roy, B.P., Goto, M., Hirose, T., Temperature and Pressure Effects on Supercritical CO2 Extraction of Tomato Seed Oil, International Journal of Food Science and Technology, 31, 137 (1996).
- Sankar, K.U., Supercritical Fluid Carbon Dioxide Technology for Extraction of Spices and Other High Value Bio-active Compounds, In Rizvi, S.S.H. (Ed.): Supercritical Fluid Processing of Food and Biomaterials, p. 155. Blackie Academic & Professional, London (1994).
- Snyder, J.M., Friedrich, J.P., Christianson, D.D., Effect of Moisture and Particle Size on the Extractability of Oils from Seeds with Supercritical CO2, Journal of the American Oil Chemists Society, 61, 1851 (1984).
- Stahl, E., Schutz, E., Mangold, H.K., Extraction of Seed Oils with Liquid and Supercritical Carbon Dioxide, Journal Agricultural and Food Chemistry, 28, 1153 (1980).
- Temelli, F., Extraction of Tryglycerides and Phospholipids from Canola with Supercritical Carbon Dioxide and Ethanol, Journal of Food Science, 57, 440 (1992).
- Valladares, J., Palma, M., Sandoval, C., Crema de Aceite de Mosqueta (Rosa aff. rubigonosa) I Parte: Formulación, Preparación y Aplicación Sistemática en Clínica, Anales de la Real Academia de Farmaceútica, 51, 327 (1985).
- Wan, P.J., Wakelyn, P.J., Technology and Solvents for Extracting Oilseeds and Nonpetroleum Oils. AOCS Press, Champaign, IL (1997).
Publication Dates
-
Publication in this collection
18 Oct 2000 -
Date of issue
Sept 2000
History
-
Accepted
05 June 2000 -
Received
09 Mar 2000