Abstract
The simulated moving-bed (SMB) technology has been successfully used in separations in petrochemical, food and fine chemical industries. This work is intended to show a potencial economic alternative for the industrial processing of the cashew apple juice. The cashew tree is a native tropical plant abundant in Northeastern Brazil, whose commercial value relies mainly on the processing of its nut. The penduncle of the fruit is called the cashew apple. Despite its high nutrition value, around 90% of the crop spoils on the soil. Simulation and experimental results are presented for SMB separation of fructose from glucose, both present (<FONT FACE="Symbol">~</FONT>40 kg/m³) in the aqueous phase of comercial cashew apple juice. Kinetic and equilibrium data for fructose and glucose on packed columns using cation-exchange resins are reported. Experimental results for SMB operation indicate close to 90% purity in each product (fructose-rich extract and glucose-rich raffinate). Simulated unit performance and internal profiles agree well with experimental values. To increase the added-value and versatility of the products, either a step of isomerization of the raffinate or diverse SMB fluid-solid flowrate ratios may be applied. By this way, a wide range of products may be obtained, from nearly pure fructose to 42%, 55% and 90% solutions, which are the standard high fructose syrup concentrations. If solids content is conveniently raised to the usual HFCS (high fructose corn syrup) comercial standards, these products may be used as food additives, thus confirming a potentially attractive use of cashew apple juice.
SMB; fructose; cashew
SMB CHROMATOGRAPHY APPLIED TO THE SEPARATION/PURIFICATION OF FRUCTOSE FROM CASHEW APPLE JUICE
D.C.S. Azevedo1 and A.Rodrigues2* * To whom correspondence should be addressed
1GPSA, Departamento de Engenharia Química, Universidade Federal do Ceará,
Campus do Pici, 60455-760 Fortaleza, CE, Brazil
2Laboratory of Separation and Reaction Engineering (LSRE), University of Porto
FEUP, Rua dos Bragas, 4050-123, Phone: +(351) (22) 2041672,
Fax: +(351) (22) 2041674, Porto, Portugal
E-mail: arodrig@fe.up.pt
(Received: August 29, 1999 ; Accepted: April 6, 2000 )
Abstract - The simulated moving-bed (SMB) technology has been successfully used in separations in petrochemical, food and fine chemical industries. This work is intended to show a potencial economic alternative for the industrial processing of the cashew apple juice. The cashew tree is a native tropical plant abundant in Northeastern Brazil, whose commercial value relies mainly on the processing of its nut. The penduncle of the fruit is called the cashew apple. Despite its high nutrition value, around 90% of the crop spoils on the soil. Simulation and experimental results are presented for SMB separation of fructose from glucose, both present (~40 kg/m3) in the aqueous phase of comercial cashew apple juice. Kinetic and equilibrium data for fructose and glucose on packed columns using cation-exchange resins are reported. Experimental results for SMB operation indicate close to 90% purity in each product (fructose-rich extract and glucose-rich raffinate). Simulated unit performance and internal profiles agree well with experimental values. To increase the added-value and versatility of the products, either a step of isomerization of the raffinate or diverse SMB fluid-solid flowrate ratios may be applied. By this way, a wide range of products may be obtained, from nearly pure fructose to 42%, 55% and 90% solutions, which are the standard high fructose syrup concentrations. If solids content is conveniently raised to the usual HFCS (high fructose corn syrup) comercial standards, these products may be used as food additives, thus confirming a potentially attractive use of cashew apple juice.
Keywords: SMB, fructose, cashew
INTRODUCTION
The cashew tree (Anacardium Occidentale) is a Brazilian tropical native plant which is cultivated in fields on the north coast of the country, the state of Ceará being its major producer [Lima, 1988]. The other two greatest world producers are Moçambique and Índia. The cashew agroindustry has an outstanding role in the economic and social context of these places since it is a highly labor intensive activity which offers one of the few, if not only, job opportunities in such under-developed areas. At present, according to Leite (1994), the cashew nut is the most largely exported agro-product in the state of Ceará (Brazil). Botanically speaking, the nut is the fruit of the tree, which is topped by a fleshy and juicy penduncle (see Figure 1), whose color varies from yellow to intense red. This penduncle, also called the pseudo-fruit, apple or pear, may be consumed "in natura" or it may be processed industrially to produce a wide range of products from concentrated juice to desserts. These industrially processed products are basically consumed by the local market and such industry does not play an important role to the economy of the state. Furthermore, sadly enough, the majority of the cashew apple production spoils in the soil. In the state of Ceará, for instance, according to Price (1974), less than 10% of the annual production of cashew apples in 1974 was estimated to have been explored industrially. This scenery seems not to have changed since then. A most recent record is given by Lopes Neto (1981), who reports that the industrial exploration of the cashew apple ranges from 2 to 6%. In such context, the use of the cashew apple as a source of high added-value sugars may represent an attractive economic alternative to the Northeast region.
This work investigates the technical feasibility for obtaining a high-fructose solutions from cashew apple juice by using the simulated moving bed technology, in a similar way to the industrial high fructose corn syrup (HFCS) widely used in beverage and food industry.
The simulated moving bed technology has been successfully used for the last decades in separations that are impossible or highly energy consuming by conventional methods (e.g. distilation, crystalization, etc.). It is a chromatography-based technique which consists of simulating the countercurrent motion of a solid adsorbent relative to a mobile phase. This is accomplished by having a set of fixed beds looped in a closed circuit with two inlet streams (feed and eluent/desorbent) and two outlet streams (extract and raffinate), which shift their positions one bed ahead in the direction of fluid flow at regular time intervals (see figure 2). The simulated moving bed technology is already industrially applied to the separation of fructose from glucose in a process known as SAREX based on the SORBEX technology as proposed by UOP [Johnson, 1989]. The feed usually comes from the hydrolization of corn starch and subsequent isomerization of glucose. In the case of the cashew apple juice, glucose and fructose are already present in equal amounts, which makes it an attractive raw material to be fed into a SMB plant. Some aspects yet to be considered are: (a) the concentration of the feed, which may be as low as 40 g/l for each of the isomers for the juice "in natura"; and (b) the effects of other substances present (organic acids, mineral salts and vitamins) on SMB operation. This work shows chemical characterizarion data for the cashew apple juice and SMB experiments for synthetic glucose-fructose solutions. The results point out to an attractive use for this presently under-estimated natural resource.
MATERIALS AND METHOD
Sugar Content Determination in Cashew Apple Juice
The cashew apple juice has been reported in the literature [Lopes, 1972; Lima, 1988] to be rich in carbohydrates, minerals and vitamin C. The extracted juice is usually obtained through squeezing and sieving processes. It is composed of two phases: a solid phase, which is usually yellow and contains insoluble pigments and tannins, and an aqueous colorless phase, which is rich in carbohydrates and may be somewhat turbid due to tannins in suspension.
The sugar content of the aqueous phase of the juice was determined by HPLC analysis. Due to the difficulty in obtaining fresh fruit, two well-known brands of comercial cashew apple juice were analysed and they will be referred to as A and B. Samples of the supernatant liquid which tops the solid phase inside the bottles were collected and centrifuged until a clear supernatant was obtained. These clarified samples were injected in a chromatographic ion-exchange column with a refractive index detector. The analysis parameters were:
a) Mobile phase: 0.005 N H2SO4 solution
b) Mobile phase flowrate: 0.3 ml/min
c) Pressure: 90 bar
d) Column: Organic acids column Interaction ION-300, 300x7.8 mm
e) Sample volume: 20 ml
The obtained chromatograms were compared with other chromatograms of the following standards: ascorbic acid, fructose, glucose and sucrose. These substances are reported as the main constituents of the aqueous phase of cashew apple juice.
Column Packing and Characterization
Eleven Superformance® glass columns SP 300x26 (by Götec Labortechnik), to be used in the simulated moving bed pilot plant, were packed with cationic resin Dowex Monosphere 99/Ca of gel type (Supelco) by the slurry method. These columns have a thermostatic jacket for temperature control, 2.6 cm internal diameter and an adjustable length of 25-30 cm. They were packed 29 cm long each and submitted to a flowrate of 30 ml/min under 30 bar back pressure for 30 min in order to ensure compact packing. The following determinations were carried out in order to estimate required parameters for the SMB model:
a) Tracer pulse experiments using a blue dextran solution (5 g/l). Samples of 0.2 ml were injected under different flowrates and the column response was monitored using a UV detector. The bed porosity was calculated from the experimental stoichiometric time. By calculating the second moment of the experimental curve, an average Peclet number was obtained for the range of flowrates to be employed in the SMB (25-40 ml/min)
b) Equilibrium isotherm determination for fructose and glucose onto the cationic resin by the column saturation method, which consists of the following steps:
-
A solution of fructose-glucose of known composition is pumped through the column for enough time to ensure complete saturation of the adsorbent. This may be done by monitoring the outlet concentration with a RI detector;
-
Then, fresh eluent (deionized water) is pumped through the previously saturated column for as much time as in the saturation step (linear isotherms assumed). The eluate must be collected in a previously weighed reservoir.
-
The concentration of the eluate must be measured for both fructose and glucose
-
Knowing the initial solution concentration (
Co), the volume of eluate (
Vel), the bed void fraction (m ), the bed volume (
Vcol), any extra-column volume (
Vex) and the concentration of the eluate (
Cel), the adsorbed phase concentration (
qads) in equilibrium with
Co is given by the following equation:

(1)
-
Repeat procedure described in items (I) through (IV) for another fluid phase concentration
Co
c) Pulse, breakthrough and elution experiments with fructose and glucose individual solutions. By comparison with the corresponding simulated curves and through a "best-fit" procedure, equilibrium and kinetic data for both fructose and glucose were calculated.
d) Pulse experiment with a glucose-fructose binary solution using the whole set of SMB columns placed in series.
SMB Experiments
The eleven SP 300X26 columns were connected to the SMB pilot plant LICOSEP® 12-26, by Novasep. The jackets were connected to one another by silicone hoses and to a thermostat bath (Lauda). Between every two columns there was a four-port valve actuated by the control system. When required, the valves allowed either pumping of feed/eluent into the system or withdrawal of extract/raffinate streams so as to execute the switching scheme described in the Introduction section. Each of the inlet (feed and eluent) and outlet (extract and raffinate) streams were pumped by means of Merck-Hitachi HPLC pumps.
Simulated moving bed experiments were conducted for glucose-fructose separation by pumping the binary solution as feed and using distilled deionized water as eluent. The operating conditions are those shown in Table 1. The dispersion, kinetic and equilibrium parameters shown in the first column were those measured experimentally from the experiments described in the previous section. The operating conditions were chosen from a design procedure as described by Azevedo and Rodrigues (1999a).
MATHEMATICAL MODEL FOR SMB
The theoretical model chosen to represent a simulated moving bed was that of a true countercurrent or true moving bed (TMB). Figure 2 shows the difference between the concepts of a simulated moving bed (SMB) and a true moving bed (TMB).
In the TMB representation, the solid moves countercurrently in relation to the fluid phase and the problem is reduced to writing the pertinent mass balance equations to each of the species involved in each of the four countercurrent sections, together with the global mass balances in the eluent, feed, raffinate and extract nodes. The mathematical model proposed to represent the steady state of species i in a section j of a true countercurrent unit is presented in equations 2 to 17. Axially dispersed flow is assumed for the fluid phase and plug flow for the solid phase. Diffusion through the external film is also considered and intraparticle mass transfer is described by means of a bi-linear driving force described elsewhere (Azevedo and Rodrigues, 1999b).
Mass balance in outer fluid phase:

(2)
Mass balance in intraparticle fluid phase (pores):

(3)
Mass balance in intraparticle "solid" phase (microparticles)

(4)
Boundary conditions for section j:

(5)

(6)

(7)

(8)
The space dimensionless variable is
j, where Ljis the length of jth section. The dimensionless numbers present in the model equations are:Fluid-solid flowrate ratio

(9)
Number of macropore mass transfer units

(10)
Number of microparticle mass transfer units

(11)
Mass Biot number (film diffusion/effective pore diffusion)

(12)
Peclet number (convection/dispersion)

(13)
Cin present in the boundary condition at the section inlet can be found from the node balances.
Eluent node:

(14)
Extract node:

(15)
Feed node:

(16)
Raffinate node:

(17)
In this approach, the steady-state concentrations are constant both for the products (extract and raffinate) as well as throughout the column axial direction. On the other hand, a real simulated moving bed reaches the steady-state in a periodic fashion. The internal concentration profiles also move along the axial direction in a periodic way. The profile measured at a half-period matches that calculated from a TMB model at steady state. Moreover, the product concentrations also vary within a period, even though the cycle average is the same as that calculated from the TMB model. This equivalence between models has been shown before [Pais and Rodrigues, 1998] for down to 2 columns per section. Since we used 3 columns per section (except in section 4) and the TMB model requires a much less computational effort, this approach has been chosen to predict SMB performance.
The key to a correct design of a SMB unit relies on the correct choice of flowrate ratios gj. There are constraints on these flowrates which must be observed, especially on sections II and III, where adsorption occurs. In the simplest case, if dispersion and mass transfer effects are neglected, Ruthven and Ching (1989) have shown the effect of the relative values of gII and gIII on the quality of separation. They are summarized as follows:
If nKB <gII <gIII < nKA
Pure extract and raffinate are obtained
If gII < nKB and nKB <gIII < nKA
Only pure raffinate is obtained
If gIII > nKA and nKB <gII < nKA
Only pure extract is obtained
If gII < nKB and gIII > nKA
No separation is obtained
Where nis equal to (1-e )/e and A, the preferentially adsorbed component.
RESULTS
Cashew Apple Juice Characterization
Samples of cashew apple juice from two comercial brands were analysed by HPLC for sugar content assesment. The aqueous colorless phase was collected and centrifuged. The supernatant was diluted in equal volume of pure water and injected into a chromatographic column connected to a refractive index detector.
Figure 3 shows chromatograms of samples of two comercial brands of cashew apple juice A and B. Good reproducibility between the two comercial brands was observed, sample B being richer in tannins. A considerable amount of ascorbic acid was verified confirming long term literature records [Campos, 1946] about the nutritious value of the fruit.
Chromatograms from standards of sugars and vitamin C (not shown) were confronted with possible cashew apple juice composition as given by various authors. They allowed us to propose the identification of the peaks shown in Figure 3. The relative percentual composition as given by the chromatograms may be summarized in Table 2. The average sugar content found in both samples of juice is 40 g/l for each (glucose and fructose). This figure is in accordance with literature records [Lima, 1988; Lopes, 1972]; they mention a content of reducing sugars of 80-100 g/l present in the aqueous phase of cashew apple juice.
Model Parameters Estimation
From pulse experiments in each of the eleven SMB columns using blue dextran as a tracer, it was possible to determine the bed porosity and Peclet number from the obtained response curves. The calculated average values were 0.4 for the bed porosity and 500 for the Peclet number.
The isotherms were measured in the range of 0 to 30 g/l at 30oC according to the column saturation method as described previously. The isotherms showed to be linear in this concentration range with equilibrium constants equal to 0.29 and 0.55 for glucose and fructose, respectively, assuming the adsorbent to be a homogeneous solid.
The pulse curves obtained for pure fructose and glucose solutions (not shown) were well correlated by simulation with KFR=0.58 and KGL=0.24 and kp=2 min-1 and km=0.8 min-1. As for
the breakthrough and elution experiments, figure 4 shows the breakthrough curves obtained for pure fructose and glucose solutions (elution curves not shown). In each graph, the equilibrium constant calculated from the experimental stoichiometric time is shown.
These calculated values were used in the simulation of the curves. For all simulations, kp=2 min-1 and km=0.8 min-1, for both isomers, yielded the best fit of experimental data.
Table 3 summarizes the model parameters obtained for the various experiments described. The average values shown in the last row were the ones used in the simulation of the SMB performance.They are included in Table 1 as the experimental conditions in which the SMB pilot plant was operated.
In order to have a final confirmation of the dispersion, equilibrium and kinetic parameters obtained through the previous described experiments, a pulse experiment was conducted in the eleven SMB columns placed in series using a binary glucose and fructose solution. A sample of 300 ml and 20 g/l was injected into the set of 11 columns and then eluted under a flowrate of 30 ml/min. Figure 5 shows the exit concentration obtained experimentally as compared with the simulated curves. Very good agreement is obtained for KFR=0.6 and KGL=0.28 and kp=2 min-1 and km =0.8 min-1.
SMB Experiments
The LICOSEP 12-26 unit was operated under the conditions shown in Table 1. Steady-state attainment was monitored by collecting samples of extract and raffinate for a whole cycle at every 2 cycles and measuring the corresponding concentrations. When these concentrations remained unchanged and mass balances for both fructose and glucose were checked (amount that enters=amount that leaves), the internal concentration profile was sampled and measured. This happened in the 15th cycle. Figure 6 shows the experimental steady-state concentration profile as compared with that obtained by simulation. Very good agreement between the predicted and the experimental profiles may be observed.
However, both extract and raffinate purities obtained experimentally were lower than those predicted from simulation. This may be due to the use of the TMB model instead of the real SMB or isotherm non-linearities in the high concentration range. Since our target product is fructose, one may obtain a higher fructose-enriched extract by increasing gII and/or gIII values at the expense of decreasing the raffinate purity. Again, this may be desirable in order to obtain products at 42 and 55% fructose, which are standards for high-fructose syrups [Cen and Tsao, 1993]. Alternatively, the raffinate stream may isomerized with the immobilized enzime glucose-isomerase. For instance, if the 89% raffinate stream shown in Figure 6 is fed to a fixed bed isomerization reactor, it may be converted to a 42% fructose stream.
CONCLUSION
This work proposes a potential economic alternative for the cashew apple juice as a fructose source. HPLC analysis of the juice aqueous phase indicated significant presence of both fructose and glucose in equal amounts. A binary mixture of fructose and glucose was separated by SMB chromatography and the experimental pilot-plant performance was well predicted with a proposed mathematical model. Choice of different operating conditions and/or isomerization of the glucose-rich product may lead to a wide range of fructose syrups if solids content (Brix) is conveniently elevated to industrial standards. Further investigation on the effects of cashew juice "impurities" and economic evaluation of complementary unit operations are necessary to confirm the feasibility of the cashew apple juice as a fructose resource.
NOMECLATURE
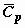
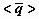
ACKNOWLEDGEMENT
Financial support from Capes (process 1140/96-5), Brazil, and project PRAXIS XXI/3/3.1/CEG/2644/95, Portugal, are gratefully acknowledged.
REFERENCES
- Azevedo, D.C.S. and Rodrigues, A.E. (1999a), "Design of a Simulated Moving Bed in the Presence of Mass Transfer Resistances", AIChE Journal, 45, 956-966.
- Azevedo, D.C.S. and Rodrigues, A.E. (1999b), Bilinear Driving Force Approximation in the Modeling of Simulated Moving bed using Bidisperse Adsorbents", Ind. Eng. Chem. Res., 38, 3519-3529.
- Campos, F.A.M. (1946), "Virtudes Nutritivas do Cajú", Anais Fac. Med. Univ. S. Paulo, vol. XXII, 79-94.
- Cen, P. and Tsao, G.T. (1993), "Recent Advances in the Simultaneous Bioreaction and Product Separation Processes", Sep. Technol., 3, 58-75.
- Johnson, J. (1989), "Sorbex: Continuing Inovation in Liquid-Phase Adsorption", in Adsorption Science and Technology; A.E. Rodrigues, D. Tondeur and M.D. LeVan.(eds.), Kluwer, Dordrecht.
- Leite, L.A.S. (1994), "A Agroindústria do Caju no Brasil", 1st ed., edited by Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA), Fortaleza.
- Lima, V.P.M.S. (1988), "A Cultura do Cajueiro no Nordeste do Brasil," 1st ed., edited by Banco do Nordeste do Brasil (BNB), Fortaleza.
- Lopes Neto, A. (1981), "A Agroindústria do Caju no Nordeste do Brasil e em outros Países Grandes Produtores", 1st ed., edited by Banco do Nordeste do Brasil (BNB), Fortaleza.
- Lopes, M.H.C. (1972), "Composição Química e Aproveitamento da pêra de Caju de Moçambique", Agron. Moçamb., 6(2), 119-131.
- Pais, L.S., Loureiro, J.M. and Rodrigues, A.E. (1998), "Modeling strategies for enantiomers separation by SMB chromatography", AIChE Journal, 44, 561-569.
- Price, R.L. (1974), "Industrialization of cashew in the state of Ceará, Brazil", Progressive Agriculture in Arizona, 26, 13-16.
- Ruthven, D.M. and Ching, C.B. (1989), "Counter-Current and Simulated Counter-Current Adsorption Separation Processes", Chem. Eng. Sci., 44, 1011-1038.
Publication Dates
-
Publication in this collection
16 Mar 2001 -
Date of issue
Dec 2000
History
-
Accepted
06 Apr 2000 -
Received
29 Aug 1999












