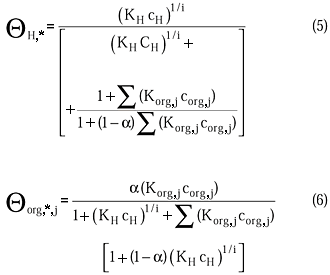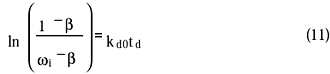Abstract
The kinetics of hydrogenation of xylose to xylitol on a sponge nickel catalyst (commonly referred to as Raney Ni catalyst) and of catalyst deactivation were studied. Plausible explanations for the decrease in catalytic activity by means of surface studies, nitrogen adsorption and thermogravimetric analyses of the fresh and spent catalysts are presented. The kinetic parameters of the process were estimated by the use of a semi-competitive model, which allows full competition between the organic species and the hydrogen atoms for the adsorption sites on the catalyst surface (competitive case). In the model, a competitiveness factor (alpha) is introduced to take into account that even after complete coverage of the surface by the organic species, interstitial sites remain for the adsorption of the hydrogen atoms.
xylitol; xylose; hydrogenation; catalyst deactivation; kinetics; sponge nickel catalyst
Hydrogenation of xylose to xylitol on sponge nickel catalyst a study of the process and catalyst deactivation kinetics
J.-P. MikkolaI* * To whom correspondence should be addressed ; T.SalmiI; A.VillelaI; H.VainioI; P.Mäki-ArvelaI; A.KalantarI; T.OllonqvistII; J.VäyrynenII; R.SjöholmIII
ILaboratory of Industrial Chemistry, Process Chemistry Group, Åbo Akademi FIN-20500 Åbo, Finland, E-mail: jpmikkol@abo.fi
IILaboratory of Surface Science, Department of Applied Physics, University of Turku FIN-20014 Åbo, Finland
IIILaboratory of Organic Chemistry, Åbo Akademi FIN-20500 Åbo, Finland
ABSTRACT
The kinetics of hydrogenation of xylose to xylitol on a sponge nickel catalyst (commonly referred to as Raney Ni catalyst) and of catalyst deactivation were studied. Plausible explanations for the decrease in catalytic activity by means of surface studies, nitrogen adsorption and thermogravimetric analyses of the fresh and spent catalysts are presented. The kinetic parameters of the process were estimated by the use of a semi-competitive model, which allows full competition between the organic species and the hydrogen atoms for the adsorption sites on the catalyst surface (competitive case). In the model, a competitiveness factor (a) is introduced to take into account that even after complete coverage of the surface by the organic species, interstitial sites remain for the adsorption of the hydrogen atoms.
Keywords: xylitol, xylose, hydrogenation, catalyst deactivation, kinetics, sponge nickel catalyst.
INTRODUCTION
Xylitol
Xylitol is a sweetening agent with interesting properties and very diverse applications. It is very soluble in water (approximately 60 wt-% at 25oC) and its crystals resemble those of plain sugar. Xylitol remains stable in storage, it does not caramelize at elevated temperatures and it has a sweetening capacity exceeding that of sugar (saccarose) by 20-25 % (Wisniak et al., 1974). However, it has become a popular sweetening agent, particularly because of its anti-caries properties (Mikkola et al., 1999a.) Its uses are widespread in the pharmaceutical, cosmetic, synthetic resin and alimentary industries (Mikkola et al., 1999b)
The Principles of Xylitol Production
Xylitol can be prepared from xylose in a batchwise, three-phase hydrogenation process. High hydrogen pressures and relatively high temperatures are used. High pressure is needed in order to improve the solubility of hydrogen in bulk liquid, whereas the need for high temperatures derives from the fact that the reaction is very temperature-dependent: the hydrogenation velocity is considerably enhanced by increased temperature. A Rushton turbine impeller, coupled to a propeller mixer, was used to provide efficient gas dispersion and mixing of the reactor contents (Mikkola et al.>, 1998). Vigorous shaking is needed for elimination of the outer liquid-solid mass transfer (Mikkola et al., 1999a). The catalyst used in the process was a sponge Ni one (commonly referred to as a Raney Nickel catalyst), and the most common solvent used was deionized water.
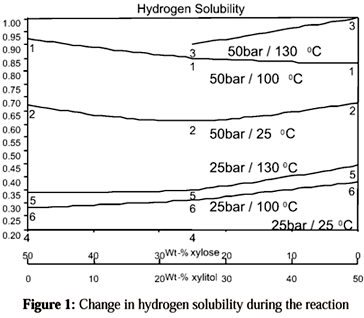
Earlier studies had shown that mixtures of solvents, such as 2-propanol-water, 2-pentanol-water and ethanol-octane-water, improve the velocity of hydrogenation. Nevertheless, their use can cause some practical difficulties due to the lower solubility of the xylose in these reaction media. Also, the mass transfer of the hydrogen from the gas to the liquid phase is faster when using the aqueous alcohol mixtures, since the solubility of hydrogen is much higher in alcohols than in water (Mikkola et al., 1998). Earlier studies had shown that the solubility of hydrogen is lower for the reaction mixture than for pure water. An increase in the solubility of hydrogen, although very moderate, was observed during the course of the reaction (Fig. 1), (Mikkola et al., 1999c).
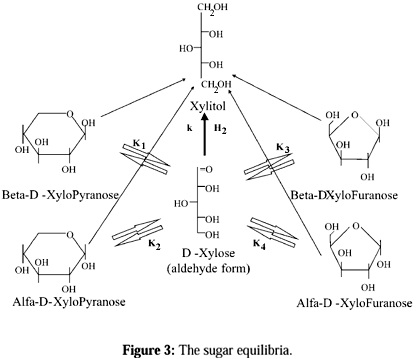
The main by-products obtained are D-arabinitol, D-xylulose, furfural and D-xylonic acid. Their formation is primarily influenced by the factors temperature, pH of the reaction media and hydrogen mass transfer. The higher the temperature, the more prominent the formation of by-products. Alkaline conditions facilitate the formation of D-xylonic acid via the Cannizzaro reaction. It has been observed that xylulose was formed at the beginning of the experiments and, thereafter, hydrogenated to xylitol and D-arabinitol. In the experiments carried out below 90oC neither xylulose nor D-arabinitol was formed. Generally, the furfural formation was moderate. At higher temperatures, some furfural formation took place (Mikkola et al., 1998). The (Fig. 2) shows the structures of the starting material, main product and above-mentioned main by-products.
Sugar Equilibria
An increase in the relative amount of the furanosidic forms was observed with rising temperature, and no peak could be assigned to be the carbon atom of an aldehyde group. The study, addressing the relative distributions of the pyranosidic forms, revealed that equilibrium shifts towards the a-pyranosidic form with increasing temperature (Fig.3), (Mikkola et al., 1999b).
This article intends to present some of the studies realised at the Laboratory of Industrial Chemistry of Åbo Akademi during the past few years. Thus, the results of some studies already published elsewhere are presented. In addition, however, some results obtained during the course of Alexandre Villela's Master's degree work (1999) are also shown.
OBJECTIVES
A study of the kinetics of the hydrogenation of xylose to xylitol and the catalyst deactivation was conducted. Plausible explanations for the deactivation of the catalyst, based on surface and structural analyses, are also presented.
EXPERIMENTAL SECTION
Hydrogenation Experiments
A laboratory-scale, pressurised, three-phase batch reactor with total capacity of 600 mL was used (Parr Instruments, model 4560). The reactor was equipped with a heating jacket, a cooling coil, a filtering system, a coupled Rushton turbine-propeller mixer and a preheater. The temperature and stirrer controller models were a Parr 4843 and a Watlow Control Series 982. The pressure controller was a Brooks Microprocessor Control & Read Out Unit (0152). A microcomputer was used to register the temperature and pressure profiles. Aqueous solutions of xylose; hydrogen with a purity of 99.999% (AGA Oy) and a commercial, Mo-promoted, sponge nickel catalyst, delivered in slurry form, were used. The catalyst dry content was approximately 50 wt-%. The same batch of the catalyst was used in consecutive hydrogenation cycles. To recycle it, the catalyst was washed with deionised water while being filtered and care was taken to avoid oxidation of the catalyst during washing.
The experiments were performed as follows: the catalyst was placed in the reactor, which was filled with hydrogen and preheated to the hydrogenation temperature. The aqueous xylose solution was heated in a hydrogen atmosphere prior to its insertion into the reactor. Immediately after the addition of the sugar solution, the hydrogen pressure was applied and the stirring rate was set. Samples for the HPLC analyses were withdrawn by quickly cracking a sampling valve and removing small amounts of the reaction medium. A 7 mm filter was placed in the sampling line in order to avoid catalyst loss.
HPLC Analyses
The HPLC analyses were performed off-line in a HP 1100 LC Series equipped with a HP RI-detector model 1047A, a degasser, a double-channel binary pump and an autosampler. The column used was a carbohydrate Bio-Rad Aminex HPX-87C (300 x 7.8 mm) coupled to a pre-column (Micro-Guard from Bio-Rad). The working temperature in the column was set at 60oC. The data generated were recorded with HP Chemstation software. The eluent used was an aqueous solution of approximately 0.002 M of Ca(NO3)2. Analysis time was 50 minutes.
Kinetic Modelling
This section describes the models that were used in order to study the kinetics of the process and the temperature dependence of the catalyst deactivation.
a) Reaction Mechanisms and Rate Equations
A kinetic model for xylose hydrogenation, based on plausible catalytic surface reaction mechanisms, is described: a semi-competitive model, in which the classical competitive and non-competitive models are obtained as special cases, was used in the quantitative treatment of the kinetic data. Principles: The semi-competitive model is based on the hypothesis that organic molecules occupy several primary sites, i.e., sites for the adsorption of a hydrogen atom. It is assumed that the organic species are not able to completely cover the catalyst surface. Thus, interstitial sites for hydrogen adsorption always remain, even after complete adsorption of the organic species. A competitiveness factor (a) for the organic molecules and the hydrogen atoms represents the maximal fraction of active sites covered by the organic species. The competitive model is obtained as a special case of the semi-competitive one when a =1, whereas the non-competitive case is obtained when a =0. The adsorption of the hydrogen molecules is assumed to be dissociative, although it is assumed that the hydrogen atoms are added to the organic molecules in pairs. Further assumptions made are that the reaction between the adsorbed species and the hydrogen atoms was the rate-determining step and that the ring and acyclic forms of the sugar rapidly reach equilibrium in the reaction media. The Table 1 represents the reaction steps:
The rate equations can be defined as
where
According to the adsorption equilibria the term, S(Korg,jCorg,j), is defined as
b) Modelling Catalyst Deactivation
The catalyst deactivation kinetics was modelled in a semi-empirical way. In the model used, based on the concept of relative activity, the plausible reasons for catalyst deactivation are lumped together. A description of the model can be given as follows: the relative activity (w) of the catalyst is proportional to the number of accessible active sites and for the disappearance of those sites, a first-order kinetic model is applied. Since the catalyst decay is not complete, i.e., even after successive experiments some activity always remains, the conventional deactivation model was extended with a term accounting for the reversibility of the adsorption of the organic species. The change in the relative activity of the catalyst (w) is described by
where b is the asymptotic activity and kd0 is the activity decay the rate constant. The values of w were estimated with an integral method based on the disappearance of xylose, and thus a factor, f, can be given as
where CS is the concentration of xylose, with C0S being the initial one, and  is the duration of the experiment. Activity factors are then obtained from
is the duration of the experiment. Activity factors are then obtained from
Where f1 denotes the factor obtained from the first experiment of the series. A rearrangement of the solution of differential equation 10 gives
where td is the total time for which the catalyst has been exposed to the reaction conditions. A plot of the left-hand side of this equation vs. time gives a straight line.
Catalyst Characterisation
a) Nitrogen Adsorption Analyses
The fresh molybdenum-promoted sponge nickel catalyst and the catalyst used in four hydrogenation experiments at 110oC and 70 bar were analysed. Prior to analysis, the catalysts were washed with deionised water. The B.E.T. surface area and the pore size distribution were determined using a Sorptomatic 1900 Series, Carlo Erba Instruments. High-purity nitrogen and nitrogen temperature were used. The samples were heated for three and four hours, respectively, at 100oC at reduced pressure.
b) X-Ray Photoelectron Spectroscopy
As in the nitrogen adsorption analyses, the fresh molybdenum-promoted sponge nickel catalyst and the catalyst used in four hydrogenation experiments at 110oC and 70 bar were analysed. Prior to the analyses, the catalysts were washed with deionised water. Then they were placed in the sample holder in a nitrogen atmosphere to prevent oxidation (glove box). The analyses were performed in a Perkin Elmer PHI 5400. The spectra were recorded by using an exitation of monochromatic MgKa radiation (1253.6 eV) with a total instrumentation resolution of about 1 eV. The samples were degassed for several hours in the preparation chamber before being transferred to the analyser chamber. The base pressure in the analyser chamber during measurement was less than 5.10-9 torr.
c) X-Ray Diffraction
The X-ray diffraction analysis of the fresh Molybdenum-promoted sponge nickel catalyst was carried out with a Philips PW 1820 diffractometer. Ni-filtered Cu-Ka radiation was used (voltage 50 kV, current 40 mA) with Ni powder (99.995 wt-% Ni, Koch Light Laboratories Ltd.) as a standard. A reflection peak was observed for the sponge Ni catalyst at 44.46º
d) Thermogravimetric Analyses
The fresh molybdenum-promoted sponge nickel catalyst and the catalyst used in seven hydrogenation experiments were analysed in a microbalance (Cahn D 200, precison 5.10-5 g). The catalysts were reduced with hydrogen (AGA 99.999% purity) and nitrogen (AGA 99.999% purity) was used as a shielding gas for the balance. The gas flows were regulated with mass flow controllers (Brooks 5850E, Bronkhorst EL-FLOW for nitrogen). The gas flow was 75 mL.min-1 (25oC, 1 atm). The reducing gas was fed to the gravimetric apparatus from the bottom, whereas the shielding gas was introduced into the system at the top.
RESULTS AND DISCUSSION
Reaction Kinetics
The kinetic parameters were estimated based on a fitting of multiple data sets, i.e., the experiments were performed at different temperatures and pressures. They were obtained from the model with non-linear regression analysis. The following table shows the parameters obtained for both, the competitive and the semi-competitive models.
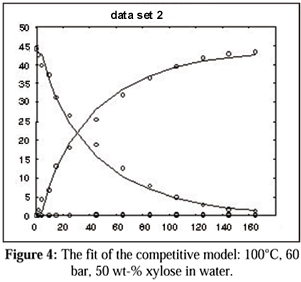
Since the number of parameters to be fit in the models was quite large, obtaining reliable estimations of the adsorption coefficients was rather cumbersome. Although the order of magnitude of the fit obtained was the same for both models, it is suggested that the approach presented here introduces a more realistic view of the phenomena that take place on the catalyst surface. Samples of model fits are shown on the following figures Figs. 4 and 5).
Catalyst Deactivation
Since the experiments for this study were carried out at three different temperatures, a rather good understanding of the temperature dependence of the deactivation constant (kd0) on the reaction temperature was achieved, as shown in Fig. 6.
The result, which is in agreement with those results obtained earlier (Vainio, 1998), shows that deactivation of the catalyst is more prominent at higher hydrogenation temperatures.
Catalyst Characterization Results
In agreement with previous results (Vainio, 1998), the nitrogen adsorption analyses revealed a decrease (from 112 to 80 m2.g-1) in the specific surface area from the fresh to the catalyst used in four hydrogenation cycles. In the same way, the pore size distribution shifted towards larger pores. The XPS analyses revealed compositional changes at the catalyst surface, again in agreement with Vainio (1998), as shown in Table 3 below.
By means of XRD analysis, the sizes of the nickel crystals was determined to be 49 nm. The thermogravimetric curves obtained for both fresh and catalyst used in seven hydrogenation cycles were virtually the same. Nevertheless, a slightly higher decrease in catalyst mass was observed in the case of the spent one.
The results of these analyses of the catalyst surface indicate that the reasons for the observed decay of catalyst activity with use are the collapse of the pore structure, the leaching of the active (Ni) and promoter (Mo) metals and the accumulation of organic species.
CONCLUSIONS
The semi-competitive model of the kinetics of hydrogenation explained the experimental concentrations of the main species involved and determined the kinetic parameters quite well. Although similar degrees of explanation were obtained with the classical competitive model, the use of the semi-competitive one is preferred since it presents a more realistic view of the phenomena that are taking place on the catalyst surface. From the modelling of catalyst deactivation, the profile of the temperature dependence of the deactivation constant (kd0) is now known. It was also discovered, from the analyses performed with the catalysts, that with use the pore structure of the catalyst collapses, there is a leaching of the active and promoter metals from the surface and there is probably some accumulation of organic species. These factors would then be responsible for the observed decrease in catalyst activity.
ACKNOWLEDGEMENTS
The project was financially supported by the Technology Development Centre of Finland (TEKES) and the Xyrofin Division of Danisco (formerly Cultor).
This work is part of the activities of the Åbo Akademi Process Chemistry Group within the Finnish Centre of Excellence Programme (2000-2005) supported by the Academy of Finland.
NOMENCLATURE
Received: March 26, 2002
Accepted: February 27, 2003
- Mikkola, J.-P., Salmi, T. and Sjöholm, R., The Effect of Solvent Polarity on the Hydrogenation of Xylose. 13th International Congress of Chemical and Process Engineering (CHISA `98). Prague, Czech Republic (1998).
- Mikkola, J.-P., Salmi, T. and Sjöholm, R., Kinetics and Mass Transfer Effects in the Hydrogenation of Xylose to Xylitol. Reaction Kinetics and the Development of Catalytic Processes (Intl. Symp.). Bruges, Belgium (1999a).
- Mikkola, J.-P., Sjöholm, R., Salmi, T. and Mäki-Arvela, P., Xylose Hydrogenation: Kinetic and NMR Studies of the Reaction Mechanisms, Catalysis Today, 48, 73-81 (1999b).
- Mikkola, J.-P., Salmi, T. and Sjöholm, R., Modelling of Kinetics and Mass Transfer in the Hydrogenation of Xylose over Raney Nickel Catalyst,J. Chem. Technol. Biotechnol., 74, 655-662 (1999c).
- Vainio, H., Deactivation Kinetics of Mo-Supported Raney Nickel Catalyst in the Hydrogenation of Xylose to Xylitol. MSc. Thesis, Åbo Akademi University (1998).
- Wisniak, J., Hershkowitz, M. and Stein, S., Hydrogenation of Xylose to Xylitol, Ind. Eng Chem., Prod. Res. Develop., 13, 75 (1974).
Publication Dates
-
Publication in this collection
05 Sept 2003 -
Date of issue
Sept 2003
History
-
Received
26 Mar 2002 -
Accepted
27 Feb 2003