Abstract
Bioactivation, a procedure to obtain anaerobic sulphidogenic sludge, was developed in order to increase sulphate reduction and, consequently, sulphide production to remove metals from effluents. This procedure, in which the source of carbon/energy (lactate) is gradually replaced, consisted of three operational conditions. It was observed that bioactivation took six months so there was a 100-fold increase in the population of sulphate-reducing bacteria estimated by the most-probable-number (MPN) when molasses was employed as a new source.
Bioactivation; Sulphidogenic sludge; UASB reactor
BIOTECHNOLOGY
The bioactivation procedure for increasing the sulphate-reducing bacteria in a UASB reactor
M. M. M. GonçalvesI, II, * * To whom correspondence should be addressed ; S. G. F. LeiteIII; G. L. Sant'Anna JrI
ICOPPE, Programa de Engenharia Química/UFRJ, Centro de Tecnologia, Bloco G, Ilha do Fundão, 21941-900, Rio de Janeiro - RJ, Brazil. E-mail: lippel@peq.coppe.ufrj.br
IIInstituto de Química/UERJ, Phone/Fax (55) (21) 2587-7322, Extension 49 Rua São Francisco Xavier 524, Maracanã, 20550-013, Rio de Janeiro, RJ - Brazil. E-mail: marciamg@uerj.br
IIIEscola de Química/UFRJ, Centro de Tecnologia - Bloco E, Ilha do Fundão, 21941-900, Rio de Janeiro - RJ, Brazil. E-mail: selma@eq.ufrj.br
ABSTRACT
Bioactivation, a procedure to obtain anaerobic sulphidogenic sludge, was developed in order to increase sulphate reduction and, consequently, sulphide production to remove metals from effluents. This procedure, in which the source of carbon/energy (lactate) is gradually replaced, consisted of three operational conditions. It was observed that bioactivation took six months so there was a 100-fold increase in the population of sulphate-reducing bacteria estimated by the most-probable-number (MPN) when molasses was employed as a new source.
Keywords: Bioactivation; Sulphidogenic sludge; UASB reactor.
INTRODUCTION
In most of the methods available to obtain predominantly sulphidogenic anaerobic sludge, the main focus is inhibition of methanogenic organisms, since these organisms compete with sulphate-reducing bacteria (SRB) for several substrates. Hulshoff Pol et al. (1998) show that the outcome of this competition may be favourable to SRB under the following conditions: sludge exposure to oxygen (Omil et al., 1997b), alteration of pH (Omil et al., 1996), shock treatment with an increase in temperature to 65ºC (Visser et al., 1993) or the use of a staged sludge bed reactor (Lens et al., 1998). However, there are some problems with these alternatives that should be considered.
Some authors (Harada et al., 1994; Omil et al., 1998) say that the predominance of SRB over methanogenic bacteria in sulphate-rich medium is only achieved after long-term operation (more than 100 days) of UASB reactors. A quick way to obtain sulphidogenic sludge is by bioaugmentation, which is the addition of SRB pure cultures (Omil et al., 1997b) or sulphidogenic sludge (O'Flaherty & Colleran, 1999) to the inoculum. The use of pure cultures has not been successful, apparently due to problems with the colonization of granular sludge (Omil et al., 1997b), whereas the use of sulphidogenic sludge has been able to improve bioreactor performance, increasing COD removal from 65% to 95% in 48 hours (O'Flaherty & Colleran, 1999). According to O'Flaherty and Colleran (1999) the development of suitably adapted seed sludge is of paramount importance, since there are no successful inoculation strategies.
The main of this work was to establish a procedure to obtain anaerobic sulphidogenic sludge, in order to increase sulphate reduction and, consequently, sulphide production for use in metals removal from several types of effluents. The aim of bioactivation, the method used, was to adapt this sludge to complex substrate usage as a source of carbon/energy. In this study, the organic waste chosen was molasses, since it contains a high concentration of organic matter and is widely available in Brazil.
MATERIALS AND METHODS
Experimental Set-up
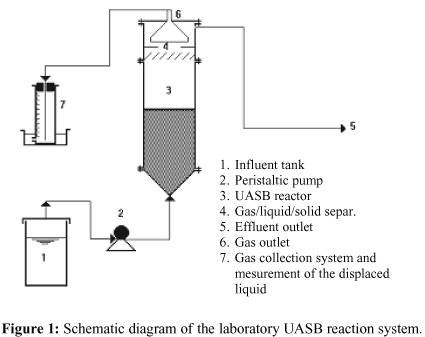
The experiments were carried out in a continuous bench-scale reactor (13 l) during 224 days. A schematic view of the experimental set-up is shown in Figure 1. It is comprised of a storage tank (200 l), from which the solution is fed through a peristaltic pump into the upflow anaerobic sludge blanket reactor (UASB), and a gas collection system.
The upflow anaerobic sludge blanket process is characterised by a reactor containing no packing or any other type of biomass support material. The UASB reactor is basically made up of a vertical tank in the shape of a cone with a round cross section and a gas/solids separator located directly on top of the reactor. The influent is fed in at the bottom of the reactor in an upstream flow. Its even distribution throughout the sludge blanket is ensured by a perforated plate. The anaerobic bacteria in the sludge provide the organic matter to stabilise. An important feature of this design is the gas/solids separator (GSS), which provides a quiescent zone in the upper part of the reactor, where suspended solids will settle and return to the sludge blanket. Any gas being produced will flow through the GSS, to be trapped by a conical gas collector whose edges are immersed in the liquid above the GSS. The solution flowing through the GSS leaves the reactor through an outlet pipe at the top.
Experimental Procedure
The bioreactor was inoculated with 7 liters of anaerobic sludge from the effluent treatment plant of a yeast factory. An enrichment procedure for increasing the number of SRB (called bioactivation) was then started. This bioactivation was conducted under three different operational conditions, during which time the sludge was adapted to a different composition of feed solution. The source of carbon/energy used was a mixture of molasses and lactate, which was added in the form of lactic acid. Lactate was chosen because it has been used by most of the sulphate reducers already identified and therefore it is able to supported better growth of SRB in mixed culture (White & Gadd, 1996) and because it is one of the anaerobic degradation intermediaries. According to Madigan et al. (2000), H2, lactate and pyruvate are used by a wide variety of SRB. Moreover, the methanogenic ones do not use lactate as a substrate.
During the bioactivation process, the bioreactor was fed with a mixture of synthetic solution containing sulphate only (400 mg SO4=/l), which was added in the form of sodium sulphate, and a source of carbon/energy. At first, the source of carbon/energy, expressed as chemical oxygen demand (COD), was 200 mg/l. Therefore, the COD/sulphate ratio was 0.5.
Table 1 summarizes the main operational characteristics of the experimental runs carried out. It may be observed that the average values of pH in the effluent were always higher than those in the influent, due to neutralization of acidity by the bicarbonate formed during sulphate reduction. As for Eh, it is important to observe that the average values were measured in the reactor effluent; therefore, it is assumed to be higher than those found in the sludge bed.
Analytical Methods
The amount of sulphate and COD were determined according to the Standard Methods for the Examination of Water and Wastewater (APHA, 1998). The SRB were enumerated by the most probable number (MPN) technique (n=3) utilising Postgate B semi-solid medium (Postgate, 1984). This medium was supplemented with resazurine (0.0025%), its pH was adjusted to 7.6 and it was purged with nitrogen before sterilisation. Inoculated tubes were incubated at 35°C for 28 days. The growth of SRB was indicated by the formation of a black FeS precipitate.
RESULTS AND DISCUSSION
The bioactivation procedure was assessed by consumption of organic matter, sulphate reduction and quantification of SRB in the sludge bed.
Removal of Organic Matter
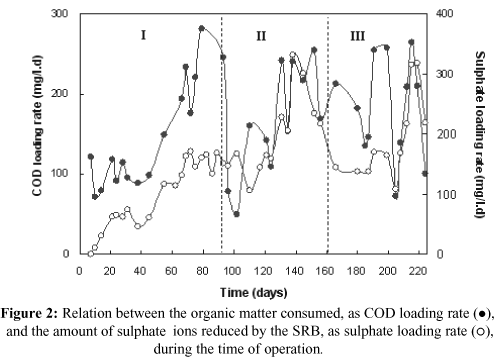
In Figure 2 the correlation of the organic matter consumed ([COD loading rate]infl. - [COD loading rate]effl.) to the amount of sulphate ions reduced by the SRB is shown. This amount of sulphate ions was calculated as the difference between the sulphate loading rate in the influent and that in the effluent of the UASB reactor ([Sulphate loading rate]infl. - [Sulphate loading rate]effl.).
The results presented in Figure 2 show that up to the 49th day of operation, the amount of organic matter consumed was not enough (stoichiometrically) for sulphate removal to reach the level aimed. It was estimated that it would be necessary to reduce the sulphate by around 200mg/l.d to produce enough sulphide for removing metals in the wastewater from drainage from a metallurgical industry dam containing around 80 mg/l zinc, e 2 mg/l cadmium and 400 mg/l sulphate. According to Isa et al. (1986) the amount of organic matter, expressed as COD, necessary for SRB is the number of moles of SO4= multiplied by 64g (1 mol of sulphate reduced º1 mol of sulphide produced º1 mol of COD º64g of COD). However, it was not the intention to reduce all of the sulphate to sulphide, but rather just the amount necessary for removing the metals as sulphide. Therefore, the amount of organic matter added was only that needed to produce sulphide for metal precipitation, i. e., the study was carried out under conditions of organic substrate limitation.
Molasses is a quite complex source of carbon, but it is approximately 30 to 50% (w/w) sucrose, which is easily converted by fermentative bacteria into carbon dioxide, hydrogen and, mainly, low-chain fatty acids. These acids can be used by SRB as the source of carbon. The other components of molasses, approximately 50 to 70% (w/w), are not easily biodegradable; thus carbon availability to SRB may be reduced (Annachhatre & Suktrakoolvait, 2001). In this case, the composition of the source of carbon/energy added to the synthetic effluent was 50% lactate and 50% molasses, expressed as COD. So, probably only lactate was used, since COD consumption was around 42% of the COD fed to the reactor. Based on these observations and the need to increase sulphate reduction achieved so far, it was decided to raise the concentration of organic matter in the bioreactor feeding to about 400mg/l, expressed as COD.
Table 2 shows that for run I the COD removal efficiency remained lower, although the organic matter concentration increased (55th to 90th day). In other words, microbial consortia were not able to metabolize complex organic molecules present in the molasses (Annachhatre & Suktrakoolvait, 2001). Nevertheless, due to lactate reduction in the composition of the source of carbon/energy from 50% to 25% (run II), an increase in COD removal efficiency was observed. In this case, it seems that the reduction in lactate availability forced an association between fermentative bacteria and SRB.
Bacterial Reduction of Sulphate
It can be seen from the results shown in Figure 2 that sulphate reduction obtained at first was very small and this reduction increased only after the 50th day, when the concentration of organic matter in the influent increased to about 400mg/l, expressed as COD. In fact, during the entire operation, immediately after the organic load consumed increased, an increase of sulphate reduction was observed and vice-versa, as for example, between the 124th and 145th days. Therefore, sulphate reduction and consequent sulphide generation may be controlled through consumption of organic matter, since the dissimilatory sulphate reduction is a consequence of the metabolization of simple organic compounds by SRB (Postgate, 1984).
SRB Quantification
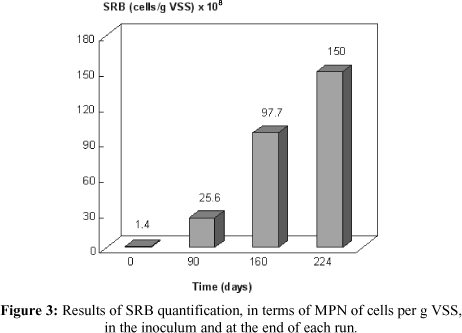
The SRB population was quantified in the inoculum and at the end of each run. The results are shown in Figure 3.
It should be pointed out that the medium growth used, semi-solid Postgate B, had just lactate as the source of carbon/energy and that this compound is not used by some SRB, including several species of Desulfobacter, Desulfotomaculum acetoxidans and some species of Desulfobacterium (Holt et al., 1994). Therefore, the number of SRB in the sludge bed may have been underestimated; however, lactate was used in bioactivation, so the number of SRB may not have been much higher. According to Vester and Ingvorsen (1998), the best solution to this problem is the use of natural medium like sludge or sediments.
In Figure 3 it is shown that the procedure to increase the number of SRB in anaerobic sludge succeeded. There was a 100-fold increase in the number of SRB at the end of the run, although the period of time was so long (224 days). It would have been possible to reduce this time by at least 50 days if the concentration of organic matter, expressed as COD, in the bioreactor loading had been 400 mg/l from the beginning of the procedure. Therefore, for a 100-fold increase in concentration of SRB, this bioactivation procedure must last for approximately 6 months when molasses is employed as a new source.
Although many factors interfere with competition between SRB and methanogenic Archaea, the predominance of SRB depends mainly on the sulphate concentration in the influent (>200 mg/l) and/or the COD/sulphate ratio (<0.67) (Omil et al., 1997a). In this work, during the bioactivation procedure sulphate concentration in the influent was always higher than 200 mg/L, but the COD/sulphate ratio was slightly higher than recommended, as shown in Table 3. However, due to the complexity of the substrate used (molasses), it may be assumed that not all the organic matter was available. So, in fact, the COD/sulphate ratio may have been lower than the one shown in Table 3, what would make it easier to obtain sulphidogenic anaerobic sludge (Annachhatre & Suktrakoolvait, 2001).
Also, it should be pointed out that the bioactivation procedure adopted in this study not only involved an increase in SRB in the inoculated anaerobic sludge, but also the adaptation of these microorganisms to a complex source of carbon/energy. A slightly similar procedure was used to stimulate SRB growth and increase their density using granular peat moss as support and source of carbon/energy (Beaulieu et al., 2000). The bioactivation procedure followed by these authors used small cubes of moss inoculated with sediment from an inactive mine. Lactate, sulphate and nutrients were periodically added to the reactor. No SRB quantification was done; however, it was assumed that the cubes of moss increased the number of these bacteria because, during a period of about 50 days, the sulphate reduction rate increased from 69 to 167 mg/l.d.
CONCLUSIONS
The following conclusions can be drawn from the experiments:
-
The bioactivation procedure proposed to increase the SRB in the anaerobic sludge was carried out successfully;
-
The time of operation for a 100 fold increase in SRB population was six months when molasses was employed as a new source;
-
The bioactivation procedure adopted in this study also involved adaptation of the inoculated sludge to a complex source of carbon/energy.
ACKNOWLEDGEMENTS
We thank the National Council of Scientific and Technological Development - Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) - for its financial support of this study and the Center of Mineral Technology - Centro de Tecnologia Mineral (CETEM) - for the use of its facilities and the analytical support received.
Received: October 20, 2004
Accepted: June 17, 2005
- Annachhatre, A.P. and Suktrakoolvait, S., Biological Sulfate Reduction Using Molasses as a Carbon Source, Water Environment Research, vol. 73, p. 118-126 (2001).
- APHA, Standard Methods for the Examination of Water and Wastewater, 20th edition. APHA-AWWA-WEF, Washington, D.C. (1998).
- Beaulieu S., Zagury G.J., Deschênes L.and Samson, R. Bioactivation and Bioaugmentation of a Passive Reactor for Acid Mine Drainage Treatment. In: Singhal & Mehrotra. Environmental Issues and Management of Waste in Energy and Mineral Production. Balkema, Rotterdam, pp. 533-537 (2000).
- Harada H., Uemura S. and Momonoi, K., Interaction Between Sulfate-reducing Bacteria and Methane-producing Bacteria in UASB Reactors Fed with Low Strength Wastes Containing Different Levels of Sulfate, Water Research, vol. 28, pp. 355-367 (1994).
- Holt J.G., Krieg N.R., Sneath P.H.A., Staley J.T. and Williams S.T., Bergey's Manual of Determinative Bacteriology, 9th edition. Williams & Wilkins, Baltimore, Maryland (1994).
- Hulshoff Pol L.W., Lens P.N.L., Stams A.J.M. and Lettinga G., Anaerobic Treatment of Sulphate-rich Wastewaters, Biodegradation, vol. 9, pp. 213-224 (1998).
- Isa Z., Grusenmeyer S. and Verstraete W., Sulfate Reduction Relative to Methane Production in High-rate Anaerobic Digestion: Microbiological Aspects, Applied and Environmental Microbiology, vol. 51, pp. 580-587 (1986).
- Lens P.N.L., Van Den Bosch M.C., Hulshoff Pol L.W. and Lettinga G., Effect of Staging on Volatile Fatty Acid Degradation in Sulfidogenic Granular Sludge Reactor, Water Research, vol. 32, pp. 1178-1192 (1998).
- Lima A.C.F., Gonçalves M.M.M., Granato M. and Leite S.G.F., Anaerobic Sulphate-reducing Microbial Process Using UASB Reactor for Heavy Metals Decontamination, Environmental Technology, vol. 22, pp. 261-270 (2001).
- Madigan M.T., Martinko J.M. and Parker J., Brock Biology of Microorganisms, 9th edition. Prentice Hall, New Jersey (2000).
- O'Flaherty, V. and Colleran, E., Effects of Sulphate Addition on Volatile Fatty Acid and Ethanol Degradation in an Anaerobic Hybrid Reactor. I: Process Disturbance and Remediation, Bioresouce Technology, vol. 68, pp. 101-107 (1999).
- Omil F., Bakker C.D., Hulshoff Pol L.W. and Lettinga G., Effect of pH and Low Temperature Shocks on the Competition Between sulphate Reducing Bacteria and Methane Producing Bacteria in UASB Reactors, Environmental Technology, vol. 18, pp. 255-264 (1997a).
- Omil F., Lens P., Hulshoff Pol L.W. and Lettinga G., Effect of Upward Velocity and Sulphide Concentration on Volatile Fatty Acid Degradation in a Sulphidogenic Granular Sludge Reactor, Process Biochemistry, vol. 31, pp. 699-710 (1996).
- Omil F., Lens P., Visser A., Hulshoff Pol L.W. and Lettinga G., Long-term Competition Between Sulfate Reducing and Methanogenic Bacteria in UASB Reactors Treating Volatile Fatty Acids, Biotechnology and Bioengineering, vol. 57, pp. 676-685 (1998).
- Omil F., Oude Elferink S.J.W.H., Lens P., Hulshoff Pol L.W. and Lettinga G., Effect of Inoculation with Desulforhabdus amnigenus and pH or O2 Shocks on the Competition Between Sulphate Reducing and Methane Bacteria in an Acetate Fed UASB Reactor, Bioresource Technology, vol. 60, pp. 113-122 (1997b).
- Postgate, J R., The Sulphate-reducing Bacteria. Cambridge University Press, Cambridge (1984).
- Vester, F. and Ingvorsen, K., Improved Most-probable-number Method to Detect Sulfate-reducing Bacteria with Natural Media and Radiotracer, Applied and Environmental Microbiology, vol. 64, pp. 1700-1707 (1998).
- Visser A., Gao Y. and Lettinga G., Effects of Short-term Temperature Increases on the Mesophilic Anaerobic Breakdown of Sulfate Containing Synthetic Wastewater, Water Research, vol. 27, pp. 541-550 (1993).
- White, C. and Gadd, G.M., A Comparison of Carbon/energy and Complex Nitrogen Sources for Bacterial Sulphate-reduction: Potential Applications to Bioprecipitation of Toxic Metals as Sulphides, Journal of Industrial Microbiology, vol. 17, pp. 116-123 (1996).
Publication Dates
-
Publication in this collection
02 Jan 2006 -
Date of issue
Dec 2005
History
-
Accepted
17 June 2005 -
Received
20 Oct 2004