Abstract
In this work, we present the calculation of critical coordinates for the esterification of acetic acid with ethanol in compressed carbon dioxide. Determination of the critical pressure for this system is useful, since the conversion of this reaction increases with pressure in the two-phase region, reaching a maximum at the critical point. We used a calculation framework based on a coordinate transformation for molar fractions, producing a new compositional domain. For a system with five components (acetic acid + ethanol + ethyl acetate + water + carbon dioxide) and one equilibrium reaction, the compositional domain is entirely described by three independent transformed coordinates. The results obtained were compared with experimental observations presented in the literature. The results illustrate the capability of the framework used to determine critical coordinates for reactive systems, and thus its usefulness as a tool for pressure tuning for this esterification reaction in compressed carbon dioxide.
Esterification reaction; Critical point; Ethyl-acetate production; Reactive vapor + liquid equilibria
THERMODYNAMICS
Determination of critical conditions for the esterification of acetic acid with ethanol in the presence of carbon dioxide
G. M. PlattI, * * To whom correspondence should be addressed ; N. HendersonI; J. L. de MedeirosII
IInstituto Politécnico, Universidade do Estado do Rio de Janeiro, CEP 28601-970, Nova Friburgo - Rio de Janeiro, RJ, Brazil. E-mail: gmplatt@iprj.uerj.br
IIDepartamento de Engenharia Química, Escola de Química, Universidade Federal do Rio de Janeiro, CEP 21949-900, Rio de Janeiro - RJ, Brazil
ABSTRACT
In this work, we present the calculation of critical coordinates for the esterification of acetic acid with ethanol in compressed carbon dioxide. Determination of the critical pressure for this system is useful, since the conversion of this reaction increases with pressure in the two-phase region, reaching a maximum at the critical point. We used a calculation framework based on a coordinate transformation for molar fractions, producing a new compositional domain. For a system with five components (acetic acid + ethanol + ethyl acetate + water + carbon dioxide) and one equilibrium reaction, the compositional domain is entirely described by three independent transformed coordinates. The results obtained were compared with experimental observations presented in the literature. The results illustrate the capability of the framework used to determine critical coordinates for reactive systems, and thus its usefulness as a tool for pressure tuning for this esterification reaction in compressed carbon dioxide.
Keywords: Esterification reaction; Critical point; Ethyl-acetate production; Reactive vapor + liquid equilibria.
INTRODUCTION
Critical point calculations have received considerable attention in the chemical engineering literature over the past decades (Peng and Robinson, 1977; Heidemann and Khalil, 1980; Kohse, 1989; Henderson et al., 2004), but the effect of equilibrium chemical reactions on critical phenomena has not been considered. On the other hand, there are several advantages of conducting chemical reactions in supercritical fluids; for instance, reaction yields can be increased by adjusting pressure of the system. In this context, Platt and de Medeiros (1999) presented a suitable formalism and an algorithm for exploration of multireactive critical loci in systems with equilibrium chemical reactions. This formalism is based on the coordinate transformation proposed by Ung and Doherty (1995), widely used in reactive distillation problems with equilibrium chemical reactions (Taylor and Krishna, 2000). Several recent studies approach the problem of critical calculations in reactive systems, but only with nonequilibrium reactions (Ke et al., 2001).
In this work, the algorithm proposed by Platt and de Medeiros (1999) was applied in the well-known reaction of esterification of acetic acid with ethanol (conducted in the presence of carbon dioxide).
The esterification of acetic acid with ethanol in compressed carbon dioxide at pressures up to 16 MPa was studied by Hou et al. (2001), who demonstrated that this reaction has an increasing conversion with pressure in the two-phase region, reaching a maximum at the critical point. We present a simulation approach for the critical point calculation of the (acetic acid + ethanol + ethyl acetate + water + carbon dioxide) reactive system. The reactive critical locus was generated in the Ung-Doherty coordinate transformation (1995).
THEORY
The Ung-Doherty Transformation
Ung and Doherty (1995) proposed a transformation variable framework (henceforth referred to simply as UD coordinates), based on the vectors of molar fractions, as follows: assuming a system with c components and r chemical reactions, r reference components (whose subset is denoted by the designator k) are chosen; thus, the UD coordinates for the liquid and vapor phases are given by
In this equation, x and y stand for liquid and vapor molar fraction vectors, respectively, and v refers to the stoichiometric coefficient matrix, with the following partition:
In Eq. (1), 1T indicates a vector for which all components are equal to one and the superscript T indicates the transpose operation.
Accordingly, vectors of molar fractions are partitioned as
With the partition presented in Eqs. (2) and (3), it is clear that Xk=Yk=0. All characters typed in bold represent vectors. As previously reported by Ung and Doherty (1995) and Platt and de Medeiros (1999), the choice of reference components must guarantee the existence of the inverse of matrix vk. Inert components must be avoided because they will lower the rank of matrix vk.
In the (acetic acid + ethanol + ethyl acetate + water + carbon dioxide) system, the following equilibrium reaction occurs in the presence of inert carbon dioxide:
Applying Eq. (1) to this system, and taking ethyl acetate as the reference component, one can obtain the vector of transformed coordinates:
The Shape of the Transformed Compositional Domain
The compositional domain (in the Ung-Doherty sense) is formed of the following hemi-spaces (which produce a polyhedron):
Thus, the transformed compositional domain can be described by:
Using Eq. (5), one can construct the following pyramid (using components 1, 2 and 5 as independent coordinates).
An analysis of Figure 1 shows that each vertex of the pyramid corresponds to a pure component of the system.
Critical Point Calculations in Reactive Systems
In this work, we used the approach to critical point calculation in reactive systems presented by Platt and de Medeiros (1999). These authors developed a suitable algorithm for this purpose, based on the Heidemann and Khalil approach (1980), but using molar densities instead of molar volumes.
For a system with c components and one chemical reaction, we have the following critical equations (see Platt and de Medeiros, 1999):
where AV refers to the Helmholtz free energy per unit of volume (J/m3), is the chemical equilibrium constant (evaluated as proposed by Platt and de Medeiros, 1999), 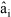 is the activity of component i and ri refers to the molar density of the ith component. The operator ÑÑT represents the Hessian matrix (the differential operator Ñ applied to a scalar function is the gradient of the function and the superscript T again refers to the transpose operation).
is the activity of component i and ri refers to the molar density of the ith component. The operator ÑÑT represents the Hessian matrix (the differential operator Ñ applied to a scalar function is the gradient of the function and the superscript T again refers to the transpose operation).
Equations (6) and (7) are simultaneously solved by a Newton-Raphson procedure, generating a new estimate for (T, r). The number of degrees of freedom is satisfied introducing a constraint on the molar density  and specifying the Ung-Doherty coordinates (c r 1 coordinates) from the molar density vectors. Then Eq. (9) (a homogeneous system) is solved for u. Eq. (8) the cubic form is then evaluated and the molar density is re-estimated in an external loop (by the secant method in our algorithm). A more detailed explanation of this algorithm and the expression for AV are presented by Platt and de Medeiros (1999).
and specifying the Ung-Doherty coordinates (c r 1 coordinates) from the molar density vectors. Then Eq. (9) (a homogeneous system) is solved for u. Eq. (8) the cubic form is then evaluated and the molar density is re-estimated in an external loop (by the secant method in our algorithm). A more detailed explanation of this algorithm and the expression for AV are presented by Platt and de Medeiros (1999).
In this work we used the Soave equation (1972) with classical mixing rules (all binary interaction parameters were set equal to zero).
RESULTS AND DISCUSSION
yIn this section, we present calculations for the reactive system described previously. Hou et al. (2001) presented critical conditions (verified by strong critical opalescence) for an original molar ratio of CO2:CH3COOH:C2H6O = 90:5:5. The reaction time required to reach equilibrium was measured at approximately 4 hours, with an ethanol conversion of 0.68 (see Hou et al., 2001). Performing a stoichiometric calculation, one can obtain the number of mols of ethanol under equilibrium conditions, nC2H6O. Thus, the molar fractions under equilibrium conditions are
With this result, we can calculate the Ung-Doherty coordinates for acetic acid, ethanol and carbon dioxide using Eq. (3) in order to satisfy the number of degrees of freedom for the system of equations (6)-(7). The Ung-Doherty coordinates remain constant during the reaction (see Ung and Doherty, 1995) and can also be calculated using initial molar fractions (before the reaction). Thus, it is not necessary to know the equilibrium composition of the system. For example X1=x1+x3=0.05+0=0.016+0.034=0.05.
In the following table the calculated results on critical temperature and critical pressure for the conditions measured by Hou et al. (2001) (using the methodology developed by Platt and de Medeiros, 1999) and the experimental results of Hou et al. (2001) are presented. An excellent agreement between experimental and calculated data can be verified.
In Figures 2 and 3, we also present the critical temperature and pressure loci (plotted in a gray scale scheme) for the entire Ung-Doherty compositional domain. The bar in these figures shows the correspondence between colors and temperature/ pressure values.
CONCLUSIONS
In this work we presented a scheme for calculation of critical coordinates for the esterification of acetic acid with ethanol in compressed carbon dioxide a reactive system. The formulation used here was entirely detailed by Platt and de Medeiros (1999) and is based on a formalism for the exploration of multireactive critical loci, using the Ung-Doherty (1995) coordinate transformation.
We presented the shape of the transformed compositional domain for the reactive system under study and calculated the critical coordinates. A gray scale scheme was used to plot the critical temperature and pressure loci.
ACKNOWLEDGMENTS
Gustavo M. Platt is grateful to FAPERJ for its financial support of this research (Grant Nº E-26/170.108/03).
NOMENCLATURE
Latin Letters
Helmholtz free energy per unit of volume
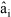
activity of component i
cubic form {Eq. (8)}
number of components
determinant
chemical equilibrium constant
absolute pressure
universal gas constant
number of equilibrium chemical reactions
absolute temperature
unitary vector {Eq. (9)}
vector of Ung-Doherty coordinates for liquid phase
vector of Ung-Doherty coordinates for vapor phase
vector of mole fractions for liquid phase
vector of mole fractions for vapor phase
Greek Letters
Superscripts
transpose operator
inverse matrix
Subscripts
types of molecules
reference components
Received: October 20, 2004
Accepted: March 7, 2006
- Heidemann, R.A. and Khalil, A.M., The Calculation of Critical Points, AIChE Journal, 26, No. 5, 769 (1980).
- Henderson, N., Freitas, L. and Platt, G.M., Prediction of Critical Points: A New Methodology Using Global Optimization, AIChE Journal, 50, No. 6, 1300 (2004).
- Hou, Z., Han, B., Zhang, X., Zhang, H. and Liu, Z., Pressure Tuning of Reaction Equilibrium of Esterification of Acetic Acid with Ethanol in Compressed CO2, J. Phys. Chem. B, 105, 4510 (2001).
- Ke, J., Han, B., George, M.W., Haike, Y. and Poliakoff, M., How Does the Critical Point Change during a Chemical Reaction in Supercritical Fluids? A Study of Hydroformilation of Propene in Supercritical CO2, Journal of American Chemical Society, 123, 3661 (2001).
- Kohse, B.F., The Calculation of Tricritical Points, M.Sc. thesis, The University of Calgary (1989).
- Peng, D. and Robinson, D.B., A Rigorous Method for Predicting the Critical Properties of Multicomponent Systems from an Equation of State, AIChE Journal, 23, No. 2, 137 (1977).
- Platt, G.M. and de Medeiros, J.L., Phase Rule Calculations and the Thermodynamics of Reactive Systems under Chemical Equilibrium, Brazilian Journal of Chemical Engineering, 16, No. 3, 247 (1999).
- Soave, G.S., Equilibrium Constants from a Modified Redlich-Kwong Equation of State, Chemical Engineering Science, 27, 1197 (1972).
- Taylor, R. and Krishna, R., Modeling Reactive Distillation, Chemical Engineering Science, 55, 5183 (2000).
- Ung, S. and Doherty, M.F., Vapor-liquid Equilibrium in Systems with Multiple Chemical Reactions, Chemical Engineering Science, 50, 23 (1995).
Publication Dates
-
Publication in this collection
13 Dec 2006 -
Date of issue
Sept 2006
History
-
Received
20 Oct 2004 -
Accepted
07 Mar 2006














