Abstract
The activated carbon produced from olive stones was chemically activated using sulfuric acid, (OS-S), and utilized as an adsorbent for the removal of Cr(VI) from aqueous solution in the concentration range 4-50 mg/L. Adsorption experiments were carried out in a batch process and various experimental parameters such as effect of contact time, initial chromium ion concentration, carbon dosage, and pH on percentage removal have been studied. Adsorption results obtained for activated carbon (OS-S) were compared with the acid-treated commercial activated carbon (CAC-S). The optimum efficiency shows that the Cr(VI) uptake being attained at pH 1.5. The equilibrium adsorption data was better fitted to the Langmuir adsorption model. The results of kinetic models showed that the pseudo-first-order kinetic model was found to correlate the experimental data well. It was concluded that activated carbon produced from olive stones (OS-S) has an efficient adsorption capacity compared to (CAC-S) sample.
Activated carbon; Adsorption; Kinetics of Cr(VI) removal
THERMODYNAMICS AND SEPARATION PROCESSES
Adsorption of chromium ion (VI) by acid activated carbon
A. A. Attia* * To whom correspondence shoul be addressed ; S. A. Khedr; S. A. Elkholy
Surface Chemistry and Catalysis Laboratory, National Research Center, 12622, Dokki, Giza, Egypt. E-mail: mollyattia@hotmail.com
ABSTRACT
The activated carbon produced from olive stones was chemically activated using sulfuric acid, (OS-S), and utilized as an adsorbent for the removal of Cr(VI) from aqueous solution in the concentration range 4-50 mg/L. Adsorption experiments were carried out in a batch process and various experimental parameters such as effect of contact time, initial chromium ion concentration, carbon dosage, and pH on percentage removal have been studied. Adsorption results obtained for activated carbon (OS-S) were compared with the acid-treated commercial activated carbon (CAC-S). The optimum efficiency shows that the Cr(VI) uptake being attained at pH 1.5. The equilibrium adsorption data was better fitted to the Langmuir adsorption model. The results of kinetic models showed that the pseudo-first-order kinetic model was found to correlate the experimental data well. It was concluded that activated carbon produced from olive stones (OS-S) has an efficient adsorption capacity compared to (CAC-S) sample.
Keywords: Activated carbon; Adsorption; Kinetics of Cr(VI) removal.
INTRODUCTION
Chromium and its compounds are toxic metals introduced into natural water from a variety of industrial wastes. The major sources are from leather tanning, textile dyeing, electroplating, and metal finishing industries, which cause severe environmental and public health problems. The hexavalent form of chromium is considered to be a group "A" human carcinogen because of its mutagenic and carcinogenic properties (Cieslak, 1996). It leads to liver damage, pulmonary congestion, edema, and causes skin irritation, resulting in ulcer formation (Raji and Amiridhan, 1998). Its concentration in industrial waste water ranges from 0.5 mg/L to 270,000 mg/L (Patterson, 1985). The tolerance limit for the discharge of Cr(VI) into inland surface water is 0.1 mg/L and in potable water is 0.05 mg/L (EPA, 1990). A wide range of physical and chemical processes are available for the removal of Cr(VI) from waste water such as electro-chemical precipitation, ultra-filtration, ion exchange, electro-dialysis, reverse osmosis, chemical precipitation, and adsorption (Jung and Shiau, 2000, Yan and Viraraghavan, 2001, Balel and Kumiawan, 2004, Donati et al., 2003). The major drawbacks with these processes are high cost, toxic sludge generation or incomplete metal removal.
Several commercial activated carbons have been used as received and also after chemical modifications for Cr(VI) adsorption (Balel and Kumiawan, 2004, Barros et al., 2006). Many reports have appeared on the development of low-cost activated carbon from renewable resources and also how to decontaminate water in an environmentally friendly manner. Agricultural and industrial waste materials have been utilized as activated carbon precursors, by a large number of researchers for the removal of chromium. Feedstocks such as sawdust (Sumathi and Naidu, 2005), nut shells (Agarwal et al., 2006), cactus, olive stone/cake, wood charcoal, oil palm fibre (Dakiky et al., 2002), fruit gum dust (Samantaroy et al., 1997), wheat bran (Dupont and Guillon, 2003), and sugar beet pulp (Atlundogan, 2005) have all been reported in the literature. The removal of Cr(III), by olive stone (Lyubchik et al., 2004) and commercial active carbons was also reported (Hu et al., 2003). Some basic information about the adsorptive properties of activated carbon prepared from olive stone was presented by Pereira et al., (2006). The results made clear that olive stones, a very abundant agricultural by-product in Mediterranean countries, could be a very adequate feedstock to obtain active carbons with good adsorptive properties and hardness, which could be of interest in future environmental protection programs. The adsorption of Cr(VI) from aqueous solutions under different kinetic and equilibrium conditions has been investigated in detail in the present study.
MATERIALS AND METHODS
Adsorbent Preparation
Olive stones freed from their fruit were obtained from the olive oil industry. They were crushed in a 10% solution of sulfuric acid and refluxed in distilled water to complete acid removal. A commercially activated carbon (decolorized powder ADWIC, Alnasr Drug-Chemical) was packed according to Prolabo. A known amount (100 g) of the dried (OS) and (CAC) was impregnated with concentrated H2SO4 in the weight ratio 1:1 (w/v), followed by heating in a hot air oven at 437K for 24 h to produce materials designated (OS-S) for olive stones and (CAC-S) for commercial active carbon. Pyrolysis for 3 hours in a furnace at 873K completed the carbonization and activation. The resulting carbons were washed with distilled water to remove any free acid and the activated carbons soaked in 1% NaHCO3 solution to remove any remaining acid and designated as (OS-S) and (CAC-S). Then it was washed with distilled water until the pH of the activated carbons attained a value of 4.5, following which it was dried at 373 K. The analyses of sulfuric acid treated olive stones and commercial active carbon are shown in Table (1).
Batch Adsorption Studies
All reagents used were A.R. grade (Aldrich). The chromium sample was prepared by dissolving calculated amount of potassium dichromate (K2Cr2O7) in doubly distilled water. This was then used as a stock solution. Adsorption studies were carried out using the batch technique to obtain both the rate and equilibrium data. The batch adsorption experiments were performed an a rotary shaker using 100-cm3 screw-cap conical flasks under constant conditions (stirring rate, 200 rpm, temperature, 303 K). The Cr(VI) solution concentration of 50 mg/L was prepared at different initial pH values before addition of 0.25 g of either OS-S or CAC-S. The effect of initial pH on adsorption kinetics was investigated in the range pH 1.5-10 employing an initial Cr(VI) ion concentration of 50 mg/L, while the effect of initial Cr(VI) concentrations was studied over the range 4, 20, 30 and 50 mg/L at different pH values. The pH adjustments were carried out either by the addition of 0.1N NaOH or 0.1N H2SO4 before addition of activated carbons and were maintained throughout the experiment.
The batch adsorption equilibrium isotherm tests were also carried out on a rotary shaker for 72 h using the same amount of (OS-S) and (CAC-S) (0.25 g) was thoroughly mixed with 100 ml of chromium solution. The isotherm studies were performed by varying the initial chromium concentrations from 5 to 50 mg/L at pH 1.5.
Analytical Procedure
After all kinetic and equilibrium studies, the resulting mixture was filtered through a 0.45 µm membrane filter and the filtrate analyzed. The concentrations of Cr(VI) in the solutions derived from all runs were measured spectrophotometrically (Perkin-Elmer model 550S UV-Vis Spectrophotometer) using 1,5-diphenyl carbazide in an acid medium. Absorbance was measured at the wavelength λ = 540 nm (APHA, 1985). The amount of adsorption at equilibrium (qe mg/g) and sorption efficiency (%) were calculated according to the expressions:
Where Co and Ce are the initial and equilibrium concentrations (mg/L), V the volume of solution (L), m is the mass of the carbon material (g) (Oliveria et al., 2005).
RESULTS AND DISCUSSIONS
Effect of initial pH
Solution pH is one of the most important parameters while assessing the adsorption capacity of an adsorbent for metal ion sequestration from aqueous solution (Kapoor et al., 1999, Aksu, 2001). The pH of the system controls the adsorption capacity due to its influence on the surface properties of the adsorbent and ionic forms of the chromium solutions. Adsorption experiments were carried out in the pH range of 1.5-10, keeping all other parameters constant, (stirring speed = 250 rpm, contact time = 60 min, adsorbent dose = 0.25 g, and room temperature) while initial chromium concentrations are 4, 20, 30, and 50 mg/L. The pH of the chromium solution was adjusted after adding the adsorbent. As depicted in Fig. (1), the maximum removal of chromium was 90% for (CAC-S) and for (OS-S) was 82% from a solution 4 mg/L at pH 1.5. There was a sharp decline in percent adsorption with an increase in either pH or concentration of Cr(VI) of the aqueous solution due to the fact that the covalent coordination bonds between Cr (III) ions and the surface functional groups of both activated carbons, such as carboxylic and hydroxyl are very weak in basic conditions (Sharma and Foster, 1994). Maximum adsorption was observed at pH 1.5. Our results are consistent with other workers, that the removal of Cr(VI) decreases with the increase of pH > 5.0. The pH dependence of metal adsorption can largely be related to the type and ionic state of the functional groups present on the adsorbent and the metal chemistry in solution (Babel and Kurniauan, 2004). In the pH range of 1.0-6.0, chromium ions co-exists in different forms such as Cr2O72 , HCrO4, Cr3O102 , Cr4O132 of which HCrO4 predominates. As the pH of the solution increases, the predominant species are then CrO42 and Cr2O72. More adsorption at acidic pH indicates that the lower pH results in an increase in H+ ions on the adsorbent surface that results in significantly strong electrostatic attraction between positively charged adsorbent surface and chromate ions. Lesser adsorption of Cr(VI) at pH values greater than 6.0 may be due to the dual competition of both the anions (CrO42 and OH) to be adsorbed on the surface of the adsorbent, of which OH predominates. This is in accordance with earlier studies that reported the removal of Cr(VI) by different adsorbents (Mohanty et al., 2006). It has also been postulated that, under acidic conditions, Cr(VI) could be reduced to Cr(III) in the presence of an adsorbent.
Dichromate ions (Cr2O72) under acidic conditions get reduced to Cr3+:
Cr2O72+14H++6 e→ 2 Cr32++7 H2O ;
Eº = 1.33 V (Mohanty et al., 2006)
However, in basic solutions it is much less oxidizing and exists as Cr(OH)3:
Cr2O72+4 H2O+3 e→ Cr (OH)3 +5 OH ;
Eº = -0.13 V
The favorable effect at low pH can be attributed to the neutralization of negative changes on the surface of the adsorbents by excess hydrogen ions, thereby facilitating the diffusion of the hydrogen chromate ion (HCrO4) and its subsequent adsorption, because HCrO4 is the dominant anionic form of Cr(VI) between pH 1.0 and 4.0. This ionic form was found to be preferentially adsorbed on the surface of carbon. The negative charges could result from oxygenated functional groups of basic character such as lactone or hydroxyl groups, chemisorbed at the surface of the pores. The possible explanation for higher adsorption in the acidic region is that the Cr2O72 ion is oxidized to Cr3+. Being small in size, it can be easily replaced by the positively charged species (Karthiheyan et al., 2005).
Fourier Transform Infrared Analysis (FTIR)
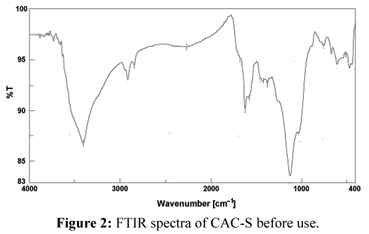
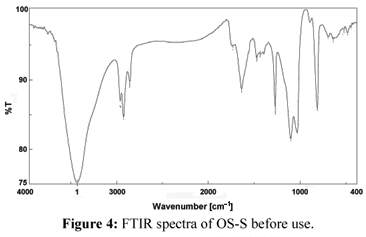
The FTIR spectra of chemically activated (OS-S) and (CAC-S) before and after sorption of chromium were used to determine the vibrational frequency changes of the functional groups in the adsorbents. The spectra of adsorbents were measured within the range of 400-4000 cm-1 wave numbers. The spectra were plotted using the same scale on the transmittance axis for all the adsorbents before and after adsorption. The FTIR spectra of the adsorbents display number of adsorption peaks, indicating the complex nature of the studied adsorbents. Table (2) presents the fundamental peaks of the adsorbents before and after use.
In the treated-commercial active carbon (CAC-S), the absorption peak around 3406 cm-1 indicates the existence of free and intermolecular bonded hydroxyl groups. The peaks observed at 2923 cm-1 can be assigned to the C-H stretching vibration of the (-CH2-) group. The peaks around 1627 cm-1 correspond to the carbonyl group in a quinone as well as representing a γ-pyrone structure with strong vibrations from a combination of C=O and C=C (Hamadi et al., 2001). The strong CO band at 1126 cm-1 is due to the OCH3 group and ether type structure as shown in Figure (2). The additional peaks at 725 and 602 cm-1 can be assigned to bending modes of aromatic compounds. The OH and CO band absorption peaks are observed to shift to 3399 and 1516 cm-1 when (CAC-S) is loaded with chromium (Fig. 3). It seems that this functional group participates in metal binding.
In (OS-S) the absorption peak around 3430 cm-1 is indicative of the existence of a bonded hydroxyl group. The other prominent peaks are due to the carbonyl group C=O and OCH3 groups. However, in the case of (OS-S)-Cr(VI), there is a shift in position and shape of OH, OCH3, and C=O groups. Similarly, the bending modes of aromatics have also shifted, indicative of association with the aromatic ring in Figs. 4 and 5.
Kinetic Studies
The timedependent behavior of Cr(VI) uptake adsorption was measured by varying the equilibrium contact time in the range of 15140 min. The Cr(VI) concentration was kept at 50 mg/L, while the amount of added carbon dose was 0.25 g/L. The adsorption efficiency of Cr(VI) removal by CAC at pH 1.5 increased from 64 to 87%, while at pH 5.0 increased from 56 to 77% with an increase in contact time. Equilibrium was attained within 100 min and, thereafter, it become constant. It was also observed that the equilibrium time for (OS) to attain equilibrium to sequester the anion from the solution was 100 min. The Cr(VI) removal was 62% at pH 1.5 and 51% at pH 5.0 as shown from Fig. (6). This indicates that the process is uniform with time and with decrease in pH. The positive charge density on the sorbent surface increases and hence the electrostatic force of attraction between the oxyanions of the chromate and the sorbent surface increases. In addition to that, there is the possibility of prevalent intraparticle diffusion of sorbate ions from the surface into the pores of sorbent (Tsai et al., 2001). At pH 1.5, the dominant anionic chromium species is HCrO4, which has a relatively smaller size in comparison to Cr2O42. The combined effect of increase in electrostatic force of attraction and faster intraparticle diffusion may account for the slight decrease in equilibrium time at pH 1.5 as compared to the pH 5.0.
If the movement of the solute from the bulk liquid film surrounding the adsorbent is ignored, the adsorption process for porous solids can be separated into three stages, viz.: (1) mass transfer (boundary layer diffusion); (2) sorption of ions onto sites; and (3) intraparticle diffusion inside the pore system. In many cases, there is a possibility that intraparticle diffusion will be the rate limiting step and this is normally determined by using the equation described by Weber and Morris:
where qt (mg/g) is the amount adsorbed at time t and Kid is the intraparticle rate constant (mg/g min0.5). The various values of Kid are shown in Table (3), whereas the larger Kid values illustrate a better adsorption mechanism which is related to an improved bonding between Cr(VI) ions and the adsorbent particles. It appears from Table (3) that the rate of the adsorption reaction increased as long as lower values of pHadj are maintained (Khezami and Capart, 2005). In many cases, the kinetics of adsorption by any biological material have been described by first-order kinetics to explain the mechanism of the adsorption process, as given by the Lagergren equation (Khezami and Capart, 2005). However, it has also been shown that a pseudo-second order approach can sometimes provide a better description of the adsorption kinetics. The first Lagergren equation is:
The pseudo-second order equation is:
where qe is the mass of metal adsorbed at equilibrium (mg/g), qt is the mass of metal at time t (min), K1 the firstorder reaction rate constant of adsorption (min-1), and K2 the pseudo-second order rate constant of adsorption (mg/g min). The values of K1 and qe were calculated from the intercept and slope of the plot of ln (qeqt) vs. t, while the values of K2 and qe were evaluated from the intercept and slope of a plot t /qt vs. t. Table (3) summarizes the values of the corresponding model parameters. The correlation coefficient for the pseudo-first order kinetic model are greater than 0.990. These indicate that the adsorption system is probably best described by the pseudo-first order kinetic model. On the other hand, Crag et al., (2004) have shown a better performance of the pseudo-first order kinetics model in the case of adsorption of chromium from aqueous solutions on treated sawdust. Kobya (2004) reached similar conclusions in the case of adsorption of Cr(VI) onto H2SO4activated carbons produced from hazelnut shell. In conclusion, it appears from the literature that the kinetics of adsorption is strongly dependent on the type of adsorbent material.
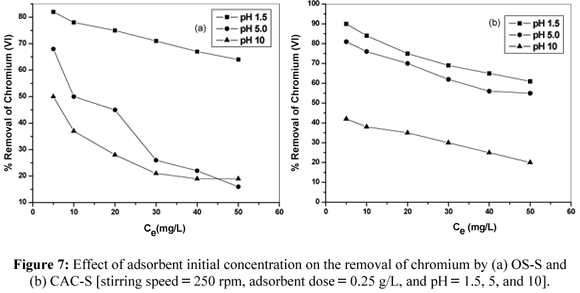
Effect of Initial Chromium Concentration
The present uptake of Cr(VI) with the two adsorbents was studied by varying pH (1.5, 5, 10) and chromium concentration (5, 20, 30, and 50 mg/L), keeping constant the adsorbent dose (0.25 g), stirring speed (250 rpm), and contact time (60 min). The percentage sorption is given in Fig. (7), which showed that the percentage of Cr(VI) sorption increases with decreasing sorbate concentration and pH in both CAC and OS (Devaperasath et al., 2007). This is because at lower concentration there are sufficient active sites that the sorbate can easily occupy. However, at higher concentrations, active sorption sites are not sufficiently available for the sorbate to occupy. Hence, Cr(VI) ions not completely adsorbed in solution due to the saturation of binding sites. In addition, the electrostatic repulsion between negative charges of adsorbate ions results in the decrease of the adsorption percentage (Hamadi et al., 2001).
Sorption as a Function of Dosage
The effect of sorbent dosages on the percent removal of chromium has been shown in Fig. (8). It followed the predicted pattern of increasing percentage sorption as the dosage was increased and reaches a saturation level at high doses for both CAC-S and OS-S. This is probably because of the resistance to mass transfer of Cr(VI) from bulk liquid to the surface of the solid, which becomes important at high adsorbent loading in the conical flask in which the experiment was conducted. Increase in adsorption with dose can be attributed also to increase in surface area and the availability of more adsorption sites.
Adsorption Isotherms
The isotherm of adsorption indicates how the quantities of target species are distributed between the liquid and solid phases when the adsorption process reach balance. It is employed to establish the maximum capacity of adsorption of metals on adsorbents, which is expressed in term of quantity of metal adsorbed per unit of mass of adsorbent used (mg/g or mmol/g). In the present investigation, the data have been correlated with a suitable isotherm. The Langmuir and Freundlich equations are commonly used for describing the adsorption equilibrium for Cr(VI) removal. The linear forms of the Langmuir and Freundlich isotherms are represented by the equations as follows:
Langmuir
where
KL = Langmuir equilibrium constant for adsorption (L/mg)
Qo = maximum adsorption capacity (mg/g)
qe = amount adsorbed at equilibrium (mg/g)
Ce = equilibrium concentration (mg/L)
Freundlich
where KF and n are the isotherm constants of the Freundlich equation.
The isotherm constants of Freundlich and Langmuir were calculated from the plots of Ce / qe vs. Ce as depicted from Fig. (9) and log qe vs. log Ce, respectively, and the results are listed in Table (4). The values of the coefficient of determination (R2) are (0.989 and 0.991) for CAC-S and (0.972 and 0.985) for OS-S. It is clear that the correlation coefficients for the Langmuir isotherm are somewhat higher than for the Freundlich isotherm, which indicates that the uptake occurs on a homogenous surface by monolayer adsorption and can be described in terms of chemisorption as the formation of an ionic or covalent bonds between adsorbent and adsorbate (Dhar Das et al., 2000). The essential characteristics of the Langmuir isotherm may also be expressed in terms of a dimensionless separation factor of equilibrium (RL) which may be calculated from equation (Badu and Gupta, 2008):
The parameter (RL) is related to the shape of the isotherm according to the following characteristics: RL > 1 represents unfavorable adsorption; RL = 1 corresponds to a linear relationship; 0 < RL < 1 is favorable adsorption and RL = 0 is irreversible. In the present study, RL remained between 0.018 and 0.063 (0 < RL < 1), which indicates that both (OS-S) and (CAC-S) are good adsorbents for Cr(VI) ion removal.
Comparison with Other Adsorbents
The adsorptive capacities of (CAC-S) and (OS-S) have been compared with other adsorbents reported in the literature as having been examined for the removal of Cr(VI) under similar conditions to those employed in the present work. These have been tabulated in Table (5). It is clearly seen that both (OS) and (CAC) exhibited considerably higher adsorption capacity when the removal of Cr(VI) was undertaken at very low pHadj values.
CONCLUSIONS
New alternative adsorbents for Cr(VI) removal have been explored by making simple chemical modifications of olive stones and commercial active carbon by sulfuric acid. The results indicate that the biomass (OS-S)-activated carbon is a good sorbent as compared to the treated commercial active carbon (CAC-S). The adsorption of Cr(VI) was found to be highly dependent on the pH value of the system, with the best results being obtained at pH 1.5. A high percentage of Cr(VI) may be reduced to the Cr(III) form at low pH values. Chromium (VI) was rapidly adsorbed when lower concentrations were used. The kinetic data were well fitted by a pseudo-first order model. The Langmuir isotherm provided the best correlation for adsorption of Cr(VI) onto the activated carbons. The adsorption capacity as calculated from the Langmuir isotherm was 71 mg/g at initial pH 1.5 for a 50 mg/L Cr(VI) solution. The FTIR spectra showed that the hydroxyl group was the chromium binding site within the pH range (pH 1-4) where chromium does not precipitate.
NOMENCLATURE
(Submitted: August 16, 2009 ; Revised: October 30, 2009 ; Accepted: November 29, 2009)
- Abul Hossain, M., Kumita, M., Michigami, Y. and Mori, S., Optimisation of parameters for Cr(VI) adsorption on used black tea leaves. Adsorption, 11, (5-6), 561 (2005).
- Agarwal, G. S., Bhuptawat, K. H. and Chaudhari, S., Bisorption of aqueous chromium (VI) by Tamarindus indica seeds. Bioresour. Technol. 97, 7, 949 (2006).
- Aksu, Z., Equilibruim and kinetic modeling of cadmium bisorption by C.vulgaris in a batch system effect of temperature. Sep. Purf. Technol. 21, 285 (2001).
- APHA, Standard Methods for Examination of Water and waste water, 16th Edn. American Public Health Association, Washington, DC, USA (1985).
- Atlundogan, H. S., Cr(VI) removal from aqueous solution by iron (III) hydroxideloaded sugar feet pulp. Process. Biochem. 40 (3/4), 1443 (2005).
- Babel, S. and Kumiawan, T. A., Cr(VI) removal from synthetic waste water using coconut shell charcoal and commercial activated carbon modified with oxidizing a gents and/or chitosan. Chemosphere 54, 951 (2004).
- Badu, B. V. and Gupta, S., Adsorption of Cr(VI) using activated neem leaves, kinetic studies. Adsorption 14, 85 (2008).
- Barros, A. J. M., Prasad, S., Leite, V. D. and Souza, A. G., The process of biosorption of heavy metals in bioreactors loaded with sanitary sewage sludge. Braz. J. Chem. Eng. 23(2), 153 (2006).
- Cieslak, G. M., Toxis and magnetic effects of chromium (VI) Polyhedron Aug, 21, 3665 (1996).
- Devaparasath, P. M., Soloman, J. S. and Thomas, B. V., Removal of Cr(VI) from aqueous solution using natural material J. Appl. Environ. Sanit., 2 (3), 77 (2007).
- Dhar Das, D., Pradhan. J., Nath Das, S. and Sing Thakur, R., Removal of Cr(VI) from Aqueous solution using activated Cow Dung Carbon. J. of colloid 2 Int. Sci. 232, 235 (2000).
- Donati, E., Oliver, C. and Curutchet, G.. Reduction of chromium (VI) by the indirect action of Thiobacillus thioparus. Braz. J. Chem. Eng. 20(1), 69 (2003).
- Dupont, L. and Guillon, E., Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran. Environ. Sci. Technol. 37, 18, 4235 (2003).
- EPA, Environmental Pollution Control Alternatives EPA/625/5-90/25, EPA/625/4-89/023. Envirommental Protection Agency Cincinaati OH, USA (1990).
- Grag, V. K., Gupta, P., Kumar, R. and Gupta, R. K., Adsorption of chromium from aqueous solution treated saw dust. Bioresor. Technol. 92, 79 (2004).
- Hamadi, N. K., Chen, X. D., Farid, M. M. and Lu, M. G. Q., Adsorption kinetics for the removal of chromium (VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chem. Eng. J. 84, 95 (2001).
- Hu, Z., Lei, L., Li, Y. and Ni, Y., Chromium adsorption on high performance activated carbons from aqueous solution, Sep. Purif. Technol. 31(1), 13 (2003).
- Isa, M. H., Ibrahim, N., Abdel Aziz, H., Adlan, N. N., Sabiani, N. H. M. D., Zinatizadeh, A. A. L. and Kutty, S. R. M., Removal of chromium (VI) from aqueous solution using treated oil palm fiber. J. Hazardous Materials 152, 662 (2008).
- Jung, R. S. and Shiau, R. C., Metal removal from aqueous solution using chitosan enhanced membrane filtration. J. Membrane Science, 165(2), 159 (2000).
- Kapoor, A., Viraraghavan T. and Cullimore D. R., Removal of heavy metals using the fungus Asperigililus niger Biores. Technol 70, 95 (1999).
- Karthiheyan, T., Rajgopal, S., and Miranola, L. R., Chromium (VI) adsorption from aqueous solution by Hevea Brasiliensis sawdust activated carbon. J. Hazard. Mater. 124, 192 (2005).
- Khezami, L. and Capart, R., Removal of chromium (VI) from aqueous solution by activated carbons. Kinetic and equilibrium studies. J. Haz. Materials B 123, 223 (2005).
- Kobya, M., Adsorption, Kinetic and Equilibrium studies of Cr(VI) by Hazelnut Shell activated Carbon, Ads. Sci. Technol. 22, 1, (2004).
- Lyubchik, S.I., Lyubchik, A.I., Galushko, O. L., Tikhonova, L. P., Vital, J., Onseca, I. M. and Lybchik, S. B., Kinetic and thermodymamics of the Cr(III) adsorption on the activated carbon from co-mingled wastes. Colloids Surf A: Physiochem. Eng. Aspects 242 (1-3), 151, (2004).
- Mohanty, K., Jha, M., Meikap, B. C. and Biswas, M. N., Bisorption of Cr(VI) from aqueous solutions by Eichhornia crassipes. Chem. Eng. J. 117, 71 (2006).
- Oliveria, E. A., Montanher, S. F., Andrader, A. D., Nobrega, J. A. and Rollemberg, M. C., Equilibrium studies for the sorption of chromium and nickel from aqueous solution using rice bran. Process Biochemistry, 40, 3485 (2005).
- Patterson, J. W., Industrial waste water treatment technology, 25d edn. Butter worth. Heineman London (1985).
- Pereira, M. R., Arroya, P. A., Dornellas de Barros, M. A., Sanches, V. M., da Silva E. A., Fonseca, I. M. and Lovera, R. G., Chromium adsorption in olive stone activated carbon. Adsorption 12(2), 155 (2006).
- Raji, C. and Anirudhan, T. S., Batch Cr(VI) removal by poly acrylamide-grafted sawdust: Kinetics and thermodynamics, Water Research 32, 3772 (1998)
- Samantaroy, S., Mohanty, A. K. and Misra, M., Removal of hexavalent chromuim by Kendu fruit gum dust. J. Appl. Polym. Sci. 66(8), 1443 (1997).
- Sharma, D. C. and Foster, C. F., The treatment of chromium waste waters using the sorptive potential on leaf mould. Bioresour. Tech. 49, 31 (1994).
- Sumathi, K. M. S., Mahimairaja S. and Naidu, R., Use of low-cost biological wastes and vermiculite for removal of chromium from tannery effluent. Biores. Technol 96(3), 309 (2005).
- Tazrouti, N. and Amrani, M., Chromium (VI) adsorption onto activated Kraft lignin produced from Alfa grass (Sipta tenacissima). BioResources 4(3) 740 (2009).
- Tsai, W. T., Chang, C. Y., Wang, S. Y., Chang, C. F., Chien, S. F. and Sun, H. F., Preparation of activated carbon from corn cob catalyzed by potassium salts and subsequent gasification with CO2 Biores. Technol. 78, 203 (2001).
- Wang, X. S., Tang, Y. P. and Tao, S. R., Removal of Cr(VI) from aqueous solutions by the nonliving biomass of alligator weed Kinetics and equilibrium. Adsorption 14(6), 823 (2008).
- Yang, G. Y. and Viraraghavan T., Heavy metal removal in biosorption column by immobilized Mrouxii biomass. Bioresource Tech. 78(3), 243 (2001).
Publication Dates
-
Publication in this collection
14 Apr 2010 -
Date of issue
Mar 2010
History
-
Received
16 Aug 2009 -
Reviewed
30 Oct 2009 -
Accepted
29 Nov 2009