Abstract
The ability of nano-silversol-coated activated carbon (NSSCAC) to adsorb Pb2+ from aqueous solution has been investigated through batch experiments. The adsorption of lead onto NSSCAC has been found to depend on adsorbent dose, initial concentration and contact time. The experiments were carried out at natural solution pH. Equilibrium data fitted well with the Langmuir model and Freundlich model with a maximum adsorption capacity of 23.81 mg of Pb/g of NSSCAC. The experiments showed that the highest removal rate was 92.42% for Pb2+ under optimal conditions. The sorption of Pb2+ on NSSCAC was rapid during the first 30 min and the equilibrium attained within 60 min. The kinetic processes of Pb2+ adsorption on NSSCAC were described by applying pseudo-first-order and pseudo-second-order kinetic models. The kinetic data for the adsorption process obeyed a pseudo-second-order kinetic model, suggesting that the adsorption process is chemisorption. The NSSCAC investigated in this study showed good potential for the removal of Pb2+ from aqueous solution.
Lead (Pb2+); Adsorbent; Adsorption isotherms; Batch adsorption; Removal
Kinetics and equilibrium studies of Pb2+ in removal from aqueous solutions by use of nano-silversol-coated activated carbon
P. Senthil Kumar* * To whom correspondence should be addressed , C. Vincent, K. Kirthika, and K. Sathish Kumar
Department of Chemical Engineering, SSN College of Engineering, Phone: +91 44 32909855, Fax: +91 44 27475063, Mobile: +91 9884823425, Chennai, 603 110, India. E-mail: senthilkumarp@ssn.edu.in ; senthilchem8582@gmail.com
Abstract
The ability of nano-silversol-coated activated carbon (NSSCAC) to adsorb Pb2+ from aqueous solution has been investigated through batch experiments. The adsorption of lead onto NSSCAC has been found to depend on adsorbent dose, initial concentration and contact time. The experiments were carried out at natural solution pH. Equilibrium data fitted well with the Langmuir model and Freundlich model with a maximum adsorption capacity of 23.81 mg of Pb/g of NSSCAC. The experiments showed that the highest removal rate was 92.42% for Pb2+ under optimal conditions. The sorption of Pb2+ on NSSCAC was rapid during the first 30 min and the equilibrium attained within 60 min. The kinetic processes of Pb2+ adsorption on NSSCAC were described by applying pseudo-first-order and pseudo-second-order kinetic models. The kinetic data for the adsorption process obeyed a pseudo-second-order kinetic model, suggesting that the adsorption process is chemisorption. The NSSCAC investigated in this study showed good potential for the removal of Pb2+ from aqueous solution.
Keywords: Lead (Pb2+); Adsorbent; Adsorption isotherms; Batch adsorption; Removal.
INTRODUCTION
Lead is one of the potentially toxic heavy metals adsorbed into the body (Friberg et al., 1979). The pollution of water resources due to indiscriminate disposal of lead metal has been causing worldwide concern for the last few decades. The presence of lead in drinking water even at low concentration may cause diseases such as anemia, encephalopathy, hepatitis and nephritic syndrome (Lo et al., 1999). Lead is non-biodegradable and can accumulate in living tissues, thus becoming concentrated throughout the food chain, and can be readily absorbed into the human body (Wong et al., 2003). Even a very small amount can cause severe physiological or neurological damage to the human body. It is a general metabolic poison and enzyme inhibitor, also causing mental retardation and semipermanent brain damage in young children.
Lead, an element which has been used by man for centuries, can be regarded as a longstanding environmental contaminant. It is released into the environment in a number of ways: by process industries engaged in lead acid batteries, pulp and paper, petrochemicals, refineries, printing, pigments, photographic materials and explosive manufacturing, ceramics, glass, paint, oil, metal, phosphate fertilizer, electronics, wood production and also combustion of fossil fuel, forest fires, mining activity, automobile emissions, sewage wastewater, and sea spray, to mention just a few examples (Jalali et al., 2002; Gupta et al., 2001; Conrad et al., 2007). Industrial wastewaters are considered to be the main source of lead impurities. Its removal from wastewater prior to discharge into the environment is, therefore, necessary. The current EPA drinking water standard for lead is 0.05 mg/l, but a level of 0.02 mg/l has been proposed and is under review (Groffman et al., 1992). According to the Indian Standard Institution, the tolerance limit for discharge of lead into drinking water is 0.05 mg/l and in land surface waters is 0.1 mg/l (I.S.I., 1982). Increasingly stringent legislation on the purity of drinking water has created a growing interest in the development of conventional treatment processes. Various chemical and physical-chemical methods for the treatment of wastewaters containing lead wastes are known, such as chemical precipitation, electrochemical reduction, ion exchange, biosorption and adsorption (Husein et al., 1998; Lin and Navarro, 1999; Petruzzelli et al., 1999; Saeed et al., 2005; Doyurum and Celik, 2006). The choice of treatment depends on effluent characteristics such as concentration of lead, pH, temperature, flow volume, biological oxygen demand, the economics involved and social factors like standards set by government agencies. Various methods of wastewater treatment were examined and adsorption emerged as one of the most promising techniques (Pollard et al., 1992). It is generally preferred for the removal of lead due to its high efficiency, easy handling, availability of different adsorbents and cost effectiveness.
The use of activated carbon (AC) is still very popular and different grades are available. Activated carbon has been chosen as an adsorptive media for removal of lead by many researchers (Goel et al., 2005; Issabayeva et al., 2006; Singh et al., 2008; Mohanty 2005). It is widely used as adsorbent in wastewater and gas treatments as well as in catalysis. The inherent drawback of AC, however, is that it has excellent biocompatibility with bacteria. Bacteria breed on carbon during the purification process when left overnight, thus making the carbon materials themselves pollutants. To address this problem, some researchers have explored depositing colloidal-sized silver, a wide-spectrum antibacterial agent, onto the carbon as bactericide. This is done by coating with SilverSol, an antibacterial coating agent produced from pure silver (Ag 99.99%) by using the latest nanotechnology. In the present paper, NSSCAC was examined for its sorption properties towards Pb2+ ion at 25oC. The parameters that influence adsorption are: adsorbent dose, effect of contact time, and effect of initial Pb2+ concentration. The adsorption process is studied via isotherms and kinetics.
EXPERIMENTAL
Adsorbent
A commercial Nano-SilverSol-Coated Activated Carbon from Nano Silver Manufacturing Sdn Bhd. (644873-M), Malaysia, was used as the adsorbent material in this study. It has an average particle size of 3-5 mm.
Adsorbate
A stock solution of Pb2+ was prepared (1000 mg/l) by dissolving the required amount of, Pb(NO3)2 in double distilled water. The stock solution was diluted with double distilled water to obtain desired concentration, ranging from 100 to 1000 mg/l.
Adsorption Experiments
Batch adsorption experiments were performed by contacting 2.5 g of the selected NSSCAC with 100 mL of aqueous solution of different initial concentrations (100 to 1000 mg/l) at natural solution pH (5.5). The experiments were performed in a wrist action shaker for a period of 1 hour at 120 rpm using 250 mL Erlenmeyer flasks containing 100 mL of different Pb2+ concentrations at room temperature. Continuous mixing was provided during the experimental period with a constant agitation speed of 120 rpm for better mass transfer with high interfacial area of contact. The kinetic study was carried out by agitating 250 mL flasks containing 2.5 g of NSSCAC and 100 mL of lead solution in a wrist action shaker. The mixture was agitated at 120 rpm at 25oC. The contact time was varied from 0 to 60 minutes. At predetermined times, the flasks were withdrawn from the shaker and the reaction mixtures were filtered through Whatman No. 40 filter paper. The isotherm study was performed using various concentrations of lead solutions; 2.5 g of NSSCAC with 100 mL of lead solution of various initial concentrations was shaken at 120 rpm for 60 min at 25oC. All the experiments were performed in duplicate. The remaining concentration of Pb2+ in each sample after adsorption at different time intervals was determined by atomic-absorption spectroscopy after filtering the adsorbent with Whatman No. 40 filter paper to make it carbon free. The batch process was used so that there is no need for volume correction. The percentage removal of lead from the aqueous solution was calculated according to the following equation:
where Ci and Cf are the initial and final lead concentration (in mg/l), respectively.
Analysis
The concentrations of Pb2+ in the solutions before and after equilibrium were determined with a Perkin-Elmer 3100 Atomic absorption spectrometer. The pH of the solution was measured with a Hanna pH meter using a combined glass electrode.
RESULTS AND DISCUSSION
Effect of Adsorbent Dose
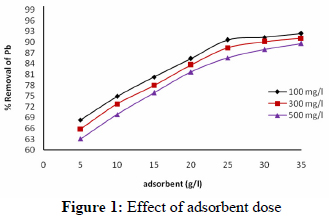
The effect of adsorbent dosage on the percentage removal of Pb2+ is shown in Fig. 1. It can be seen from the figure that the percentage removal initially increases very sharply with the increase in adsorbent dosage, but beyond a value of 25 g/L, the percentage removal reaches an almost constant value. This may be due to an overlapping of adsorption sites as a result of over-crowding of adsorbent particles (Namasivayam et al., 1998). The percentage removal of Pb2+ increased from 68.4 to 92.4%, 65.8 to 91.1% and 63.1 to 89.6% for the three different initial concentrations of 100, 300 and 500 mg/L, respectively, with an increase in the adsorbent doses from 5 g/l to 35 g/l at room temperature (25oC) and pH 5.5. A maximum removal of 92.42% was observed at an adsorbent dosage of 35 g/L at pH 5.5 for an initial Pb2+ concentration of 100 mg/l. Therefore, the use of a 25 g/L adsorbent dose is justified for economical purposes.
Effect of Contact Time
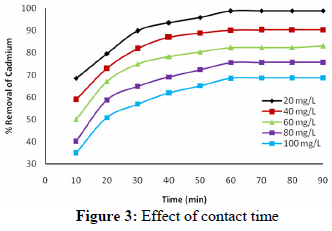
The relationship between contact time and lead sorption onto NSSCAC at different initial lead concentrations is shown in Fig. 2. The adsorption was very fast from the beginning to 30 min and the percentage removal decreased from 90 to 80% over the lead concentration range of 100 to 500 mg/l at a contact time of 30 min. With further increase of time, the sorption kinetics decreased progressively and, finally, the adsorption approached equilibrium within 60 min in all the cases. The percentage removal corresponding to equilibrium adsorption decreased from 93 to 89% with the increase in lead concentration from 100 to 500 mg/l. The fast adsorption at the initial stage is probably due to the increased concentration gradient between the adsorbate in solution and adsorbate in the adsorbent as there must be an increased number of vacant sites available at the beginning. The progressive increase in adsorption and, consequently, the attainment of equilibrium adsorption may be due to limited mass transfer of the adsorbate molecules from the bulk liquid to the external surface of NSSCAC, initially and subsequently by slower internal mass transfer within the NSSCAC particles.
Effect of Initial Concentration
The effect of initial Pb2+ concentration in the range of 100 to 1000 mg/l on adsorption was investigated and is shown in Fig. 3. It is evident from the figure that the percentage Pb2+ removal decreased with the increase in initial concentration of Pb2+ due to the fixed quantity of adsorbents used in this study. The initial Pb2+ concentration provides the necessary driving force to overcome the resistance to the mass transfer of Pb2+ between the aqueous phase and the solid phase. The increase in initial Pb2+ concentration also enhances the interaction between Pb2+ and NSSCAC. Therefore, an increase in initial concentration of Pb2+ enhances the adsorption uptake of Pb2+. This is due to the increase in the driving force of the concentration gradient with the increase in the initial Pb2+ concentration. While the percentage lead removal was found to be 90.08% for 100 mg/l of initial concentration, this value was 70.35% for that of 1000 mg/l.
Adsorption Isotherms
Adsorption isotherms are mathematical models that describe the distribution of the adsorbate species between liquid and adsorbent, based on a set of assumptions that are mainly related to the heterogeneity/homogeneity of the adsorbent, the type of coverage and possibility of interaction between the adsorbate species. Adsorption data are usually described by adsorption isotherms such as the Langmuir and Freundlich isotherms. These isotherms relate metal uptake per unit weight of adsorbent (qe) to the equilibrium adsorbate concentration in the bulk fluid phase Ce. The results of the adsorption experiment conducted in this study were fitted with the well known Langmuir and Freundlich adsorption models.
The Langmuir model is based on the assumption that maximum adsorption occurs when a saturated monolayer of solute molecules is present on the adsorbent surface, the energy of adsorption is constant and there is no migration of adsorbate molecules in the surface plane. The Langmuir isotherm is given by:
The constants in the Langmuir isotherm can be determined by plotting 1/(qe) versus (1/ Ce) and making use of above equation rewritten as:
where qm and KL are the Langmuir constants representing the maximum adsorption capacity for the solid phase loading and the energy constant related to the heat of adsorption, respectively. It can be seen from Fig. 4 that the isotherm data fits the Langmuir equation well (R2=0.997). The values of qm and KL were determined from the figure and were found to be 23.81 mg/g and 0.0178 l/mg, respectively.
The Freundlich isotherm model is an empirical relationship describing the adsorption of solutes from a liquid to a solid surface and assumes that different sites with several adsorption energies are involved. The Freundlich adsorption isotherm is the relationship between the amounts of lead adsorbed per unit mass of adsorbent (qe) and the concentration of the lead at equilibrium (Ce).
The logarithmic form of the equation becomes,
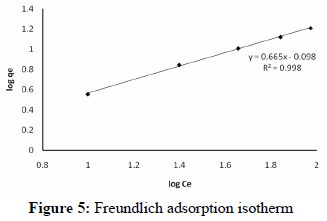
where Kf and n are the Freundlich constants characteristic of the system. Kf and n are the indicator of the adsorption capacity and adsorption intensity, respectively. The ability of the Freundlich model to fit the experimental data was examined. For this case, the plot of log Ce vs log qe was employed to obtain from the intercept the value of Kf and from the slope that of n. From Fig. 5, the Freundlich constants Kf and n were found to be 0.798 mg/g and 1.504 respectively. The isotherm data fit the Freundlich model well (R2=0.998). The magnitudes of Kf and n show easy separation of Pb2+ ions from the wastewater and indicate favorable adsorption. The intercept Kf value is an indication of the adsorption capacity of the adsorbent; the slope 1/n indicates the effect of concentration on the adsorption capacity and represents adsorption intensity. As seen from the table, the n value was high enough for separation.
The Langmuir and Freundlich adsorption constants with the correlation coefficients are presented in Table 1. The correlation coefficients show that the adsorption process could be described by both Langmuir and Freundlich models. The comparison of maximum monolayer adsorption capacity of Pb2+ onto various adsorbents is presented in Table 2. It shows that the NSSCAC studied in this work has a very large adsorption capacity.
Adsorption Kinetics
In order to investigate the controlling mechanism of adsorption processes such as mass transfer and chemical reaction, the pseudo-first-order and pseudo-second-order kinetic models were applied to model the kinetics of lead adsorption onto NSSCAC. The equilibrium adsorption capacity of NSSCAC was found to increase with increasing initial lead concentration. This indicates that there are plenty adsorption sites in NSSCAC available for the adsorption of lead. The enhanced adsorption of lead ion with the increase in agitation time may be due to the decrease in boundary layer resistance to mass transfer in the bulk solution and an increase in the kinetic energy of hydrated ions. By increasing the agitation time, the boundary layer resistance is reduced and there is an increase in the mobility of ions in the solution. The pseudo-first-order rate equation, given as (Lagergren 1898):
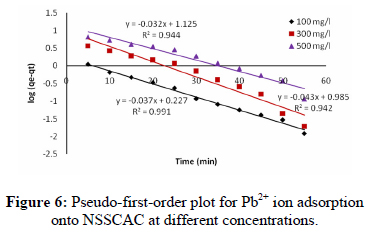
where qt is the adsorption capacity at time t (mg/g) and kad (min−1) is the pseudo-first order rate constant of the adsorption, was applied to the present study of Pb2+ ions adsorption. The rate constant, kad and correlation coefficients for different concentrations of the Pb2+ ions were calculated from the linear plots of log(qe − qt) versus t (Fig. 6) and are listed in Table 3. The correlation coefficients for the pseudo-first-order kinetic model are low. Moreover, a large difference of equilibrium adsorption capacity (qe) between the experiment and calculation was observed, indicating a poor pseudo first-order fit to the experimental data.
The pseudo-second-order equation is expressed as (Ho and McKay 1999):
where h = kqe2 (mg g-1min-1) can be regarded as the initial adsorption rate when  and k is the pseudo-second-order rate constant of adsorption (g mg-1min-1). A plot of t/qt versus t should give a straight line if pseudo-second-order kinetics are applicable and qe, k and h can be determined from the slope and intercept of the plot, respectively. The plots of the linearized form of the pseudo-second-order reaction at different Pb2+ concentrations are shown in Fig. 7. The pseudo-first-order and pseudo-second-order rate constants determined from Figs. 6 and 7 are presented in Table 3, along with the corresponding correlation coefficients.
and k is the pseudo-second-order rate constant of adsorption (g mg-1min-1). A plot of t/qt versus t should give a straight line if pseudo-second-order kinetics are applicable and qe, k and h can be determined from the slope and intercept of the plot, respectively. The plots of the linearized form of the pseudo-second-order reaction at different Pb2+ concentrations are shown in Fig. 7. The pseudo-first-order and pseudo-second-order rate constants determined from Figs. 6 and 7 are presented in Table 3, along with the corresponding correlation coefficients.
The plot of t/qt versus t for the pseudo-second-order model (Fig. 7) yields very good straight lines (correlation coefficient, R2>0.99) as compared to the pseudo-first order plot. The pseudo-second-order rate constants were in the range of 0.0088 to 0.0963 g/mg-min. The theoretical values of qe also agree very well with the experimental ones. Both facts suggest that the adsorption of Pb2+ ions by NSSCAC follows the pseudo-second-order kinetic model, which relies on the assumption that chemisorption may be the rate-limiting step. In chemisorption, the metal ions stick to the adsorbent surface by forming a chemical (usually covalent) bond and tend to find sites that maximize their coordination number with the surface (Atkins, 1995). The pseudo-second-order kinetic analysis reveals that the value of the initial adsorption rates (h) increases with increase in the initial Pb2+ ion concentration. The lower the concentration of metal ions in the solution, the lower the probability of collisions between these species and, hence, the faster Pb2+ ions can bond to the active sites on the surface of the adsorbent (Wong et al., 2003).
CONCLUSION
The present investigation shows that NSSCAC is an effective adsorbent for the removal of Pb2+ from aqueous solutions. From the kinetics studies it is observed that the adsorption of Pb2+ is very rapid in the initial stage and decreases upon approaching equilibrium. A maximum adsorption efficiency of 92.42% has been obtained for the initial Pb2+ concentration of 100 mg/L. The percentage removal of Pb2+ increases with the increase in adsorbent dosage due to the availability of more active sites and the percentage removal of Pb2+ decrease with an increase in initial Pb2+ concentration due to the presence of a fixed number of active sites. Experimental results are in good agreement with both the Langmuir and Freundlich adsorption isotherm models .The kinetics of Pb2+ ions adsorption obey the pseudo-second-order model, which suggests chemisorption as the rate-limiting step in the adsorption process.
NOMENCLATURE
List of Symbols and Units
a
Langmuir constant representing the maximum adsorption capacity for the solid phase loading
mg/g
b
Langmuir constant representing the energy constant related to the heat of adsorption
L/mg
Ci
Initial concentration of Pb2+ solution
mg/L
Ce
Equilibrium concentration of Pb2+ solution
mg/L
h
initial adsorption rate
mg g-1min-1
k
pseudo-second-order rate constant for adsorption
g mg-1min-1
Kad
pseudo-first-order rate constant for the adsorption process
min-1
kf
Freundlich constant representing the adsorption capacity
(mg/g)
(L/mg)(1/n)
n
Freundlich constant representing the adsorption intensity
qe
amount adsorbed at equilibrium
mg/g
qt
amount adsorbed at time t
mg/g
t
Time
min
V
Volume
L
W
Weight of the adsorbent
g
(Submitted: August 4, 2009 ; Revised: January 7, 2010 ; Accepted: March 29, 2010)
- Atkins, P. W., Physical Chemistry. 5th Edition, Oxford. Oxford University Press (1995).
- Calero, M., Hernainz, F., Blazquez, G., Martín-Lara, M. A. and Tenorio, G., Biosorption kinetics of Cd (II), Cr (III) and Pb (II) in aqueous solutions by olive stone. Braz. J. Chem. Eng., 26, 265-273 (2009).
- Conrad, K. and Hansen, H. C. B., Sorption of zinc and lead on coir. Bioresour. Technol., 98, 89-97 (2007).
- Doyurum, S., and Celik, A., Pb(II) and Cd(II) removal from aqueous solutions by olive cake. J. Hazard. Mater., 138, 22-28 (2006).
- Dwivedi, C. P., Sahu, J. N., Mohanty, C. R., Mohana, B. R. and Meikap B. C., Column performance of granular activated carbon packed bed for Pb(II) removal. J. Hazard. Mater., 156, 596-603 (2008).
- Friberg, L., Nordberg, G. F. and Vouk, B., Handbook on the Toxicology of Metals. Elsevier, North-Holland, Biomedical Press, Amsterdam (1979).
- Giraldo, L. and Moreno-Pirajan, J. C., Pb2+ adsorption from aqueous solutions on activated carbons obtained from lignocellulosic residues, Braz. J. Chem. Eng., 25, 143-151 (2008).
- Goel, J., Kadirvelu, K., Rajagopal, C. and Garg, V. K., Removal of lead(II) by adsorption using treated granular activated carbon: batch and column studies. J. Hazard. Mater. 125, 211-220 (2005).
- Groffman, A., Peterson, S. and Brookins, D., Removing lead from wastewater using zeolites. Water Environ. Technol., 5, 54-59 (1992).
- Gueu, S., Yao, B., Adouby, K. and Ado, G., Kinetics and thermodynamics study of lead adsorption on to activated carbons from coconut and seed hull of the palm tree. Int. J. Environ. Sci. Tech., 4, 11-17 (2007).
- Gupta, V. K., Gupta, M. and Sharma, S., Process development for the removal of lead and lead from aqueous solutions using red mud-an aluminium industry waste. Water Res. 35, 1125-1134 (2001).
- Ho, Y. S. and McKay, G., The sorption of lead(II) ions on peat, Water Res., 33, 578-584 (1999).
- Husein, M. M., Vera, J. H. and Weber, M. E., Removal of lead from aqueous solutions with sodium caprate. Sep. Sci. Technol. 33, 1889-1904 (1998).
- I.S.I., Tolerance limits for industrial effluents prescribed by Indian Standards Institution. IS: 2490 (Part II), New Delhi, India (1982).
- Issabayeva, G., Aroua, K. M. and Sulaiman, N. M. N., Removal of lead from aqueous solutions on palm shell activated carbon. Bioresour. Technol., 97, 2350-2355 (2006).
- Jalali, R., Ghafourian, H., Asef, Y., Davarpanah, S. J. and Sepehr, S., Removal and recovery of lead using nonliving biomass of marine algae. J. Hazard. Mater., 253-262 (2002).
- Lagergren, S., About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakad. Handlingar Band, 24, 1-39 (1898).
- Lin, S. W. and Navarro, R. M. F., An innovative method for removing Hg2+ and Pb2+ in ppm concentrations from aqueous media. Chemosphere, 39, 1809-1817 (1999).
- Lo, W., Chua, H., Lam, K.H. and Bi, S. H., A comparative investigation on the biosorption of lead by filamentous fungal biomass, Chemosphere 39, 2723-2736 (1999).
- Mohanty. K., Jha, M., Meikap, B. C. and Biswas, M. N., Preparation, Characterization of activated carbons from terminalia arjuna nut with zinc chloride activation for the removal of phenol from waste water. Ind. Eng. Chem. Res., 44, 4128-4138 (2005).
- Namasivayam, C., Prabha, D., Kumutha, M., Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresour. Technol. 64, 77-79 (1998).
- Petruzzelli, D., Pagano, M., Tiravanti, G. and Passino, R., Lead removal and recovery from battery wastewaters by natural zeolite clinoptilolite. Solvent Extr. Ion Exch. 17, 677-694 (1999).
- Pollard, T. J. S., Fowler, D. G., Sollars, J. C. and Perry, R., Low-cost adsorbents for waste and wastewater treatment: a review. Sci. Total Environ., 116, 31-52 (1992).
- Saeed, A. Iqbal, M. and Akhtar, M. W., Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J. Hazard. Mater., 117, 65-73 (2005).
- Singh, C. K., Sahu, J. N., Mahalik, K. K., Mohanty, C. R., Raj Mohan, B. and Meikap, B. C., Studies on the removal of Pb(II) from wastewater by activated carbon developed from Tamarind wood activated with sulphuric acid. J. Hazard. Mater., 153, 221-228 (2008).
- Wong, K. K., Lee, C. K., Low, K. S. and Haron, M. J., Removal of Cu(II) and Pb(II) by tartaric acid modified rice husk from aqueous solutions. Chemosphere, 50, 23-28 (2003).
Publication Dates
-
Publication in this collection
04 Aug 2010 -
Date of issue
June 2010
History
-
Reviewed
07 Jan 2010 -
Received
04 Aug 2009 -
Accepted
29 Mar 2010