Abstract
The thermal stability of the extracellular fructosyltransferase (FTase) from Rhodotorula sp., recovered from cultivation medium by ethanol precipitation and immobilized onto niobium ore, was studied by Arrhenius plot, half - life profile, half - inactivation temperature (T50) and thermodynamic parameters. The Arrhenius plot showed two different behaviors with different deactivation energies (Ead) only after immobilization, the transition occurring in the temperature interval between 51 and 52ºC. T50 for the free enzyme was estimated to be around 62ºC and, after immobilization, 66ºC. After 15 minutes at 52ºC, it was also possible to observe enzymatic activation for both the free and immobilized forms, but greater activation was achieved at pH 4.5 with the immobilized enzyme. Between 47 - 51ºC the immobilized enzyme was more stable than the free enzyme, with pH 6.0 being the more stable condition for the immobilized enzyme. However, above 52ºC the free form was more stable.
Niobium; Adsorption; Half-life; Thermal activation; Arrhenius plot; Dimer
BIOPROCESS ENGINEERING
Thermal stability of the immobilized fructosyltransferase from Rhodotorula sp.
E. Aguiar-Oliveira* * To whom correspondence should be addressed ; F. Maugeri
Department of Food Engineering (DEA), Faculty of Food Engineering (FEA), University of Campinas (UNICAMP), Phone: + (55) (19) 3521-4052, Fax: + (55) (19) 3521-4027, R. Monteiro Lobato 80, Cidade Universitária Zeferino Vaz, Postal Code: 13083-862, Campinas - São Paulo, Brazil. E-mail: elizamaguiar@yahoo.com.br
ABSTRACT
The thermal stability of the extracellular fructosyltransferase (FTase) from Rhodotorula sp., recovered from cultivation medium by ethanol precipitation and immobilized onto niobium ore, was studied by Arrhenius plot, half-life profile, half-inactivation temperature (T50) and thermodynamic parameters. The Arrhenius plot showed two different behaviors with different deactivation energies (Ead) only after immobilization, the transition occurring in the temperature interval between 51 and 52ºC. T50 for the free enzyme was estimated to be around 62ºC and, after immobilization, 66ºC. After 15 minutes at 52ºC, it was also possible to observe enzymatic activation for both the free and immobilized forms, but greater activation was achieved at pH 4.5 with the immobilized enzyme. Between 47-51ºC the immobilized enzyme was more stable than the free enzyme, with pH 6.0 being the more stable condition for the immobilized enzyme. However, above 52ºC the free form was more stable.
Keywords: Niobium; Adsorption; Half-life; Thermal activation; Arrhenius plot; Dimer.
INTRODUCTION
Fructooligosaccharides (FOS) belong to the functional class of foods, due mainly to their prebiotic properties (Gibson et al., 2004). FOS can be naturally obtained from fruits, greens and vegetables (Tanriseven and Aslan, 2005) and also from the enzymatic hydrolysis of inulin and/or the transfructosylation of sucrose residues by certain microbial enzymes; the most well-known and most widely applied enzymes in FOS production are those obtained from filamentous fungi and certain bacteria. Nevertheless, yeasts of the genus Rhodotorula, isolated from Brazilian Atlantic Forest flowers in the authors' laboratory (Maugeri and Hernalsteens, 2007), are capable of extracellularly producing the dimeric enzyme fructosyltransferase (FTase, EC 2.4.1.9), which presents high transfructosylation potential (Hernalsteens and Maugeri, 2008).
The immobilization of this enzyme on a solidacid niobium-graphite compound has been the subject of several studies, such as: characterization of the immobilized fructosyltransferase (Aguiar-Oliveira and Maugeri, 2010), studies of the thermostability (reported in the present paper), kinetic studies (Alvarado-Huallanco and Maugeri-Filho, 2010) and the optimization of FOS synthesis in shaking flasks (unpublished data). Initial studies of the characterization of the free enzyme obtained in a synthetic medium (GYMP) showed an optimum pH value of 4.5 (Hernalsteens and Maugeri, 2008). Studies performed with the same enzyme, but obtained using an industrial medium (containing industrial by-products such as corn steep liquor and sugar cane molasses) and immobilized on the same support, showed several effects caused by the strong negative charge of the support on the enzyme, such as the existence of two distinct points with maximum activity: pH 4.5 -similar to the free enzyme - and pH 6.0 - presenting excellent thermal stability (Aguiar-Oliveira and Maugeri, 2010).
The extracellular frustosyltranferase (FTase) from Rhodotorula sp. is obtained by only one purification step (ethanol precipitation - partially purified enzyme) since the transfructosylation activity and degree of purity present in the final concentrated enzymatic solution were adequate for the proposed purpose, allied with the fact that the methodology is of low cost and easy to reproduce in an industrial environment (Aguiar-Oliveira and Maugeri, 2010; Hernalsteens and Maugeri, 2008). In addition, kinetics studies with the immobilized FTase showed that there is no significant improvement in sucrose conversion between purified and partially purified enzyme (Alvarado-Huallanco and Maugeri-Filho, 2010).
The industrial application of biocatalysts relies strongly on their stock and operational stabilities. The half life, which expresses the stock stability, provides information regarding the biocatalytic maintenance capacity between manufacturing time and usage. On the other hand, the operational stability describes the enzyme persistence during the process under the conditions of use (Papinutti et al., 2008; Ó'Fágáin, 2003). Immobilization also changes the denaturation profile in both negative and positive ways, one explanation for this being that the decrease in the solubility of the enzyme reduces, for instance, molecular and submolecular mobility and vibrations (Bromberg et al., 2008).
The mechanism of enzyme denaturation can have different stages and complexities, the most common models, described by first order kinetics, being those developed by Arrhenius and/or Lumry & Eyring (Fogler, 1999; Lumry and Eyring, 1954). However, in some cases, an apparently simple, first-order inactivation can mask a set of complex molecular events. It is known that the unfolding of the tertiary structure of proteins leads to a loss of enzymefunctionality, resulting in its denaturation (Ó'Fágáin, 1997). Enzyme thermostability studies provide information on the capacity of the enzyme to resist thermal unfolding in the absence of substrate and thermo-affinity studies assess the capacity of the enzyme to perform its function in the presence of the substrate (Nadeem et al., 2007). This work presents the results of thermal stability studies of immobilized fructosyltransferase from Rhodotorula sp., which has great potential and viability for fructooligosaccharides (FOS) production, providing valuable information necessary for bioreactor design.
MATERIALS AND METHODS
Materials
As reported in a previous work (Aguiar-Oliveira and Maugeri, 2010), the strain of Rhodotorula sp. (LEB-V10: Laboratory of Bioprocess Engineering, UNICAMP, Brazil) was isolated from the Brazilian Atlantic Forest (Maugeri and Hernalsteen, 2007). The industrial substrates for the cultivation medium (by-products) were: sugar cane molasses and corn steep liquor, kindly provided by The Usina Estér (Campinas-SP/Brazil) and Corn Products (Mogi Guaçu-SP/Brazil), respectively. The glucose (hydrolytic activity) was determined by using an enzymatic glucose-oxidase kit from LaborLab® (Guarulhos/São Paulo, Brazil). The solid-acid niobium-graphite (~95% Nb) compound, used as the support, was provided by the Brazilian Company of Metallurgy and Mining (CBMM; Brazil). All the other reagents used were acquired from reliable commercial sources.
Rhodotorula sp. Cultivation and Fructosyltransferase Partial Purification
The conditions for cultivation of the Rhodotorula sp. and partial purification of the fructosyltransferase (FTase) from the medium (by precipitation with anhydrous ethanol) have been described previously (Aguiar-Oliveira and Maugeri, 2010). The concentrated enzymatic solution with high transfructosylation activity, used in all experiments, corresponds to ethanol-precipitated enzyme re-suspended in 50 mM acetate buffer, pH 4.5.
Inorganic Support and Enzyme Immobilization
Particles of the solid-acid niobium-graphite compound between 65 and 80 mesh (180 - 212 µm) were obtained and cleaned according to methodology described in previous work (Aguiar-Oliveira and Maugeri, 2010) where it is possible to obtain more information about the mechanical and chemical properties of these particles.
The standard methodology for immobilization by adsorption onto the niobium particles applied in this work was described earlier (Aguiar-Oliveira and Maugeri, 2010; Maugeri and Aguiar-Oliveira, 2007).
The Enzymatic Activity and Related Parameters
As applied and described earlier (Aguiar-Oliveira and Maugeri, 2010; Hernalsteens and Maugeri, 2008), the enzymatic activity of fructosyltransferase (FTase) was determined based on the methods of Hidaka et al. (1998) and Chen and Liu (1996). One transfructosylation activity unit (UTF; free enzyme; Eq. (1)) was defined as 1 µmol of fructose transferred (FT) in one minute (min) and one immobilized activity unit (Ui) expressed as one transfructosylation activity unit immobilized on one gram (g) of support (Eq. (2)).
The efficiency of immobilization (εimmob) was expressed as the ratio between the enzymatic activity effectively immobilized (Ui) and the total enzymatic activity submitted to immobilization (Uo), as shown in Eq. (3); by definition, Uo corresponds to the ratio between the enzymatic activity of the concentrated solution (UTF) submitted to immobilization and the weight of the support (g). The residual activity at each time (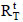 ) was determined according to Eq. (4) to monitor enzymatic activity losses with time for both the immobilized and free enzymes. For this purpose, some definitions were employed:
) was determined according to Eq. (4) to monitor enzymatic activity losses with time for both the immobilized and free enzymes. For this purpose, some definitions were employed: 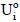 and
and 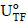 correspond to the initial enzymatic activities determined at time zero (to), and U
correspond to the initial enzymatic activities determined at time zero (to), and U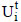 and
and  correspond to the enzymatic activities determined at time t.
correspond to the enzymatic activities determined at time t.
Effects of Temperature
The thermostabilities of the free and immobilized fructosyltransferase were followed and established from their residual activities ( ) in stocks maintained in 50 mM acetate buffer at pH values of 4.5 and 6.0 [in accordance with Hernalsteens and Maugeri (2008) for the free form and Aguiar-Oliveira and Maugeri (2010) for the immobilized form] in the absence of substrate, for various periods of time at temperatures ranging from 47 to 70ºC. All the experiments were performed in triplicate.
) in stocks maintained in 50 mM acetate buffer at pH values of 4.5 and 6.0 [in accordance with Hernalsteens and Maugeri (2008) for the free form and Aguiar-Oliveira and Maugeri (2010) for the immobilized form] in the absence of substrate, for various periods of time at temperatures ranging from 47 to 70ºC. All the experiments were performed in triplicate.
Half-inactivation temperatures (T50) and the thermal activation of the free and immobilized enzyme were determined by submitting them to a brief incubation period (in 50 mM acetate buffer without substrate) at different temperatures (ranging from 47 to 70ºC) for 15 min, followed by immersion in an ice bath for 5 min; enzymatic activities were then determined according to 2.4. The enzymatic activity obtained by this procedure corresponds to  for the immobilized enzyme, and
for the immobilized enzyme, and  for the free enzyme. As a control, the enzymatic activities of free and immobilized enzyme with no incubation were determined and correspond to the values of
for the free enzyme. As a control, the enzymatic activities of free and immobilized enzyme with no incubation were determined and correspond to the values of 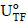 and
and 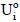 , respectively. These enzymatic activities were used in Eq. (4) to obtain the residual activity as a function of the incubation temperature [(
, respectively. These enzymatic activities were used in Eq. (4) to obtain the residual activity as a function of the incubation temperature [( ) versus T (ºC)]. For the immobilized enzyme, the assays were carried out at pH
) versus T (ºC)]. For the immobilized enzyme, the assays were carried out at pH
4.5 and 6.0 (Aguiar-Oliveira and Maugeri, 2010), while for the free enzyme, the assays were performed only at the optimum pH value of 4.5. All experiments were performed in triplicate.
General Enzyme Thermal Stability Parameters
a) kd
The enzyme denaturation constant (kd; 1/min) was obtained from a graph of ln(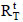 ) versus time (min) (data not presented), corresponding to the value of the slope of the regression line obtained. For this calculation, only regressions obtained with a minimum of 5 points and a value for R2 above 0.93 were accepted.
) versus time (min) (data not presented), corresponding to the value of the slope of the regression line obtained. For this calculation, only regressions obtained with a minimum of 5 points and a value for R2 above 0.93 were accepted.
b) Ead and t1/2
The denaturation activation energy (Ead; kJ/mol) and half-life (t1/2; min) of the immobilized enzyme were obtained from the kd values (Catana et al., 2007; Cornish-Bowden, 1995). According to the Arrhenius equation (Eq. (5)), the kd values were plotted as [-ln(kd)] versus [103/T; (1/K)]. The value referred to as Ead is obtained by multiplying the slope of the regression by the ideal gas constant (R = 8.314×10-3 kJ/mol.K).
Eq. (6), allows the calculation of t1/2 (min) from the kd values and combination of this equation with Equation (5) allows one to express t1/2 according to Eq. (7).
c) T50 and Thermal Activation
Based on Eq. (4), the residual free and immobilized activities for each temperature were used to construct the graph of  (residual activity after 15 min) versus T (ºC). The value of the hal-finactivation temperature (T50) was determined with the mathematical software TableCurveTM 2D (SPSS, INC., 1996) by means of an adjustment of the points to a mathematical equation that allows the calculation of the temperature required for
(residual activity after 15 min) versus T (ºC). The value of the hal-finactivation temperature (T50) was determined with the mathematical software TableCurveTM 2D (SPSS, INC., 1996) by means of an adjustment of the points to a mathematical equation that allows the calculation of the temperature required for  = 0.5. The values obtained for
= 0.5. The values obtained for  also provided information on the thermal activation of the enzyme at specific temperatures.
also provided information on the thermal activation of the enzyme at specific temperatures.
d) The Effect of Pre-Incubation at 52 and 60ºC
The temperatures of 52 and 60ºC, corresponding to the points of maximum activation observed in the study of T50, were evaluated separately with respect to the intensity of the effect of this activation on enzyme stability. The immobilized enzyme (at pH values of 4.5 and 6.0) and the free enzyme (at pH 4.5), obtained under standard conditions and with initial activities of 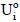 and
and 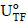 , were first submitted to a short incubation at 52 or 60ºC for 15 min and then immersed in an ice bath for 2 min before incubating at 65ºC (high temperature) for 35 min, presenting final activities of
, were first submitted to a short incubation at 52 or 60ºC for 15 min and then immersed in an ice bath for 2 min before incubating at 65ºC (high temperature) for 35 min, presenting final activities of  and
and  . The residual activities obtained in each case,
. The residual activities obtained in each case,  and
and  were compared with the residual activity obtained after incubation at only 65ºC for 35 min,
were compared with the residual activity obtained after incubation at only 65ºC for 35 min,  . The experiments were carried out in triplicate.
. The experiments were carried out in triplicate.
Thermodynamic Parameters of Enzyme Denaturation
The values of kd and Ead, obtained according to sub-items (a) and (b), were used in the calculations of: Gibbs Free Energy (ΔG; kJ/mol; Eq. (8)), Enthalpy (ΔΗ; kJ/mol; Eq. (9)) and Entropy (ΔS; J/mol.K; Eq. (10)). For these calculations, the value employed for Boltzmann constant (kB) was 1.38×10- 23 J/K and for the Planck constant (h) 11.04×10-36 J.min (Doran, 2002; Blanch and Clark, 1997).
RESULTS AND DISCUSSION
Calculations of Ead (Arrhenius Plot)
a) Free Enzyme
According to Figure 1 the linear regression of the points in the temperature range assessed (47-70ºC) was a good fit (R2 = 0.992), the value for the denaturation activation energy (Ead) being 294.35 kJ/mol. The R2 values for the residual activity (Eq. (4)) ranged from 0.93 to 0.97.
b) Immobilized Enzyme
Analyzing Figures 1, two distinct phases of the slope can be clearly noted at the two pH values evaluated. The differentiation between the phases occurred in an interval of just 1ºC, between 51 and 52ºC. The first phase corresponded to the lower temperature range, from 47 to 51ºC, and the second phase comprised the temperatures from 52 to 70ºC. In the first phase, the linear fit gave Ead values of 175.10 kJ/mol (R2 = 0.976) at pH 4.5 and 263.16 kJ/mol (R2 = 0.952) at pH 6.0; in the second phase (Ead) was calculated as 293.74 kJ/mol (R2 = 0.995) at pH 4.5 and 319.44 kJ/mol (R2 = 0.996) at pH 6.0 The R2 values for the residual activity (Eq. (4)) ranged from 0.93 to 0.96.
When compared to the free enzyme, the immobilized enzyme presented unusual behavior, since a single slope profile is more commonly observed, as in the case of the free enzyme (Bhatti et al., 2007; Santos et al., 2007). However, unusual profiles have previously been reported for Arrhenius plots, and even a "zig-zag" like profile with two negative slopes and one positive slope has been reported (Šikšnis et al., 1990).
The denaturation activation energy (Ead) values for enzymes generally vary from 170 to 400 kJ/mol (Doran, 2002) and this parameter expresses how much energy is needed to promote enzyme denaturation under the conditions assessed. The Ead values obtained shows that, at pH 4.5, the free and the immobilized fructosyltransferase (in temperature phase 2) presented very similar energy values. With respect to the immobilized enzyme, the value of Ead was higher in temperature phase 2 (52-70ºC) for both the pH values assessed. Several authors have shown that chemical modifications of the surface can drastically change enzyme behavior with respect to its thermostability and other aspects of interest, due to conformational changes (Bromberg et al., 2008; Bhatti et al., 2007; Šikšnis et al., 1990). It must be emphasized that immobilization, especially on a strongly loaded support such as niobium, can alter the enzyme behavior or highlight phenomena imperceptible when the enzyme is dispersed in the medium (Sadana, 1998).
The profiles presented here can also be analyzed considering the dimeric form presented by this enzyme (Hernalsteens and Maugeri, 2008) which, according to some authors, is the most common form presented by all fructosyltransferases (Lüscher et al., 1996). It is also possible to find references to the action of two distinct fructosyltransferases (Nemukula et al., 2009). Between 51 and 52ºC, some type of energy differentiation occurs with the immobilized enzyme and, to understand this phenomenon, one must consider the influence of the support and, principally, the form in which the enzyme is immobilized on the support. Some authors defend the idea that, in the case of dimeric enzymes, for example, immobilization can occur with just one of the sub-units, leaving the other one free, and this could alter many aspects of enzymatic functionality (Trevan, 1980). In a study carried out with a trimeric enzyme (Bolivar et al., 2009), a strong argument in favor of the partial immobilization of sub-units was that the dissociation of these sub-units could, in many cases, be the first step in the inactivation of polymeric enzymes, or the desorption of one of the non-immobilized sub-units could occur, contaminating the reactive medium. One can even conjecture that, when incubated at higher temperatures, the molecule of fructosyltransferase from Rhodotorula sp. immobilized on niobium could be in the form of two monomers and, in this case, would probably require more energy to denature these two independent units. On the other hand, at lower temperatures, the dimeric form would confer greater stability, requiring a lower value for Ead.
Calculation of t1/2
Mathematical expressions for the half life (t1/2) at different temperatures, obtained according to Eq. (7), are listed below as Eqs. (11) to (15). With the help of the software Statistica 5.0 (STATSOFT, INC., 2000), the equations were fitted to the experimental points in order to obtain the respective R2 values.
As discussed before, for the free enzyme the profile is exponential and uninterrupted and obeys only Eq. (11). However, for the immobilized enzyme at both pH values (4.5 and 6.0), the differentiation between the profiles is clear in both temperature phases (Figure 2). At 50ºC, corresponding to the optimum temperature for FOS synthesis with the free enzyme (Hernalsteens and Maugeri, 2008), the half life (t1/2) calculated for the free enzyme was 5.81 days. After immobilization, this value increased to approximately 24 days at pH 4.5 and nearly twice this value at pH 6.0. Therefore, immobilization was beneficial to the enzyme between 47 and 51ºC, increasing its stability (Figure 2). This characteristic makes the use of immobilized fructosyltransferase very promising, because if Equations (13) and (15) were followed at temperatures below 51ºC, the values estimated for the half life of the immobilized enzyme at pH 4.5 and 6.0 would be greater than 3 and 7 days, respectively. At 48ºC and pH 6.0, corresponding to the optimized temperature and pH for FOS synthesis with the immobilized fructosyltransferase (data not published yet), the half life obtained experimentally is 72 days, while that estimated according to Equation (14) is 77 days: for the free enzyme at 48ºC, this value is 14 days - from experimental data and according to Eq. (11).
Thermodynamic Denaturation Parameters
Protein unfolding is followed by the rupture of several links, generating a disorganized system. This increase in entropy (ΔS; J/mol.K) is compensated by a decrease in Gibbs free energy (ΔG; kJ/mol), making it easier for denaturation to occur, meaning that, when the susceptibility to denaturation is higher, the enzyme is in a less energetic state and in greater disorder, since its original structure has been destroyed.
The results for the Gibbs free energy (ΔG; kJ/mol) indicate that, at the temperatures of the first phase (47-51ºC), the immobilized enzyme has (Figure 3) a more stable intrinsic conformational form, since the values are higher, also indicating that this range is less favorable for protein unfolding. At temperatures above 52ºC, the Gibbs energy reached completely different levels (Figure 3). This difference between the temperature ranges can also be seen from the values of the enthalpy (ΔH; kJ/mol) and entropy (ΔS; J/mol.K), which indicate that the susceptibility of the enzyme to thermal denaturation increases with an increase in temperature (Bromberg et al., 2008).
According to some authors, one can apply the concept of ΔCp = 0 to the thermodynamic analyses of enzyme denaturation processes, leading to the conclusion that ΔH and ΔS are independent of the temperature, as observed in Figure 3 (Doran, 2002; Blanch and Clark, 1997). However, for other authors, this supposition represents a simplistic vision of the phenomenon and they defend a marked temperaturedependence (Becktel and Schellman, 1987). The present authors believe that the results and discussion presented in this paper have attended the proposed objective, which was to provide basic knowledge on the heat denaturation phenomenon of immobilized FTase.
T50 and Thermal Activation
The mean transition point for thermal protein unfolding can be described as the half-inactivation temperature or unfolding temperature (T50). This parameter corresponds to the temperature at which the residual activity (Eq. (4)) drops to 50% of its initial value after a brief period of incubation, such as 10 (Ruth et al., 2007) or 15 min (Ó'Fágáin, 1997). The basic profile for this type of analysis would be of a sigmoidal type, where, at a certain temperature, the activity decreases significantly, indicating that a brief incubation is sufficient to affect thed thermal stability of the enzyme. This analysis also makes it possible to confirm the effects of thermal activation (Papinutti et al., 2008). The values obtained for  are shown in Figure 4.
are shown in Figure 4.
According to the results, one can identify a moderate activation range for the free enzyme between 51 and 57ºC, not exceeding 8% of the initial activity. When analyzing the immobilized enzyme, one can distinguish two temperature ranges at both pH values where enzyme activation was significant, these being 57-61ºC and 51-53ºC. Comparing pH 4.5 with pH 6.0 (this latter showing greater stability than the former), the effect of activation on the immobilized enzyme was always higher at pH 4.5, which is equivalent to the optimum pH of the free enzyme. The maximum activation point was observed at a temperature of 52ºC, where the value for  reached 1.82 ± 2.2E- 2 at pH 4.5 and 1.56 ± 2.1E-2 at pH 6.0. As previously discussed in item 3.1, the temperature range between 51 and 52ºC suggests some type of energetic and/or conformational limit for the immobilized enzyme, indicating a strong effect of the support.
reached 1.82 ± 2.2E- 2 at pH 4.5 and 1.56 ± 2.1E-2 at pH 6.0. As previously discussed in item 3.1, the temperature range between 51 and 52ºC suggests some type of energetic and/or conformational limit for the immobilized enzyme, indicating a strong effect of the support.
When the free enzyme was at pH 4.5, there was a pronounced activity loss as of 61ºC, the T50 of the free enzyme being calculated as 62.06ºC. For the immobilized enzyme, the negative effect of incubation became evident at temperatures above 65ºC, the T50 being estimated as 66.57ºC at pH 4.5 and 66.21ºC at pH 6.0. However, according to the discussion presented in item 3.1, immobilization was observed to affect enzyme thermostability, at least in the temperature range from 52-70ºC. Nevertheless, Figure 4 shows that, at temperatures above 63-65ºC, the effect of a brief incubation is greater for the free enzyme. Possible explanations for this could be the existence of some type of mild activation caused by the support, even at high temperatures, or maybe some type of protection caused by immobilization.
For some enzymes, a pre-incubation step increases stability and is frequently recommended (Papinutti et al., 2008). The present study of the influence of pre-incubating the free and immobilized fructosyltranferase at the temperatures of greatest thermal activation showed that neither of the temperatures evaluated (52 and 60ºC) increased thermal stability, free or immobilized, at a higher temperature (65ºC), as can be seen from the values for the residual activity in Table 1. With preincubation at both temperatures, the free residual activity was about 70% lower than without preincubation. For the immobilized enzyme, preincubation at 52ºC gave a reduction of 17% as compared to the residual value without incubation, and of 35% at 60ºC. At pH 6.0, the immobilized enzyme showed the worst effects, with a reduction of approximately 65% at both temperatures evaluated.
CONCLUSIONS
With respect to the thermostability of the extracellular fructosyltransferase (FTase) from Rhodotorula sp. (LEB-V10: Laboratory of Bioprocess Engineering, UNICAMP, Brazil), it can be concluded that the immobilization by adsorption on niobium ore (a solid-acid compound) significantly improved the enzymatic stability, especially at temperatures lower than 51ºC and at pH 6.0, a value less acidic than the optimum condition for the free enzyme (pH 4.5). The results presented so far indicate that the support (niobium) and the immobilization technique (adsorption) can affect in Oliveira and Maugeri, 2010) the beginning of a sequence of studies related to this enzyme and its biotechnological application.
ACKNOWLEDGMENTS
The authors are grateful to FAPESP and CAPES for their financial support; to the Brazilian Company of Metallurgy and Mining (CBMM-Brazil) for their kind donation of the niobium used in all the tests and experiments and to Professors A. Converti (Genoa-Italy) and C. Ó. Fágáin (Dublin-Ireland) for their important suggestions.
NOMENCLATURE
Ead
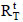
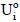 ,
, 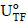
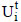 ,
, 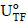
REFERENCES
Aguiar-Oliveira, E. and Maugeri, F., Characterization of the immobilized fructosyltranferase from Rhodotorula sp. International Journal of Food Engineering, v. 6, I. 3 (2010). DOI 10.2202/15563758.1894.
Alvarado-Huallanco, M. B. and Maugeri-Filho, F., Kinetics and modeling of fructooligosaccharide synthesis by immobilized fructosyltransferase from Rhodotorula sp. Journal of Chemical Technology & Biotechnology, (2010). DOI 10.1002/jctb.2477.
Becktel, W. and Schellman, J. A., Protein Stability Curves, Biopolymers. v. 26, 1859-1877 (1987).
Bhatti, H. N., Rashid, M. H., Asgher, M., Nawaz, R., Khalid, A. M., Perveen, R., Chemical modification results in hyperactivation and thermostabilization of Fusarium solani glucoamylase. Canadian Journal of Microbiology, v. 53, 177-185 (2007).
Blanch, H. W. and Clark, D. S., Biochemical Engineering. Marcel Dekker, New York (1997).
Bolivar, J. M., Mateo, C., Rocha-Martin, J., Cava, F., Berenguer, J., Fernadez-Lafuente, R. and Guisan, J. M., The adsorption of multimeric enzymes on very lowly activated supports involves more enzyme subunits: Stabilization of a glutamate dehydrogenase from Thermus Thermophilus by immobilization on heterofunctional supports. Enzyme and Microbial Technology, v. 44, 139-144 (2009).
Bromberg, A., Marx, S. and Frishman, G., Kinetic study of the thermal inactivation of cholinesterase enzymes immobilized in solid matrices. Biochimica et Biophysica Acta, v. 1784, 961-996 (2008).
Catana, R., Eloy, M., Rocha, J. R., Ferreira, B. S., Cabral, J. M. S., Fernandes, P., Stability evaluation of an immobilized enzyme system for inulin hydrolysis. Food Chemistry, v. 101, 260-266 (2007).
Chen, W. and Liu, C., Production of β-fructofuranosidase by Aspergillus japonicus. Enzyme and Microbial Technology, v. 18, I. 2, 153-160 (1996).
Cornish-Bowden, A., Fundamentals of Enzyme Kinetics. Portland Press, London (1995).
Doran, P. M., Bioprocess Engineering Principles. Academic Press, New York (2002).
Dosztányi, Z., Fiser, A. and Simon, I., Stabilization Centers in Proteins: Identification, Characterization and Predictions. Journal of Molecular Biology, v. 272, 597-612 (1997).
Fogler, H. S., Elements of chemical reaction engineering. 3rd Ed., Prentice Hall, New Jersey (1999).
Gibson, G. R., Probert, H. M., Loo, J. V., Rastall, R. A. and Roberfroid, M. B., Dietary modulation of the human colonic microbiota: updating the concept of prebiotcs. Nutrition Research Reviews, v. 17, 259-275 (2004).
Hernalsteens, S. and Maugeri, F., Purification and characterisation of a fructosyltransferase from Rhodotorula sp. Applied Microbiology Biotechnology, v. 79, 589-596 (2008).
Hidaka, H., Hirayama, M. and Sumi, N., A fructooligosaccharide-producing enzyme from Aspergillus niger ATCC20611. Agricultural and Biological Chemistry, v. 52, I. 5, 1181-1187 (1998).
Lumry, R. and Eyring, H., Conformation changes of proteins. The Journal of Physical Chemistry, v. 58, 110-120 (1954).
Lüscher, M., Erdin, C., Sprenger, N., Hochstrasser, U., Boller, T., Wiemken, A., Inulin synthesis by a combination of purified fructosyltransferases from tubers of Heliantus tuberosus. FEBS Letters, v. 385, 39-42 (1996).
Maugeri, F. and Aguiar-Oliveira, E., Método para imobilização de enzimas utilizando suporte sólido inorgânico. University of Campinas (UNICAMP), Patent: PI 070683-1, Brazil Office (2007).
Maugeri, F. and Hernalsteens, S., Screening of yeast strain for fructosylating activity. Journal of Molecular Catalysis B: Enzymatic, v. 49, I. 1-6, 43-49 (2007).
Nadeem, M., Perveen, R., Javed, M. R., Nadeem, H. and Rashid, M. H., Kinetic and thermodynamic properties of novel glucoamylase from Humicola sp. Enzyme and Microbial Technology, v. 41, 558-564 (2007).
Nemukula, A., Mutanda, T., Wilhemi, B. S. and Whiteley, C. G., Response surface methodology: Synthesis of short chain fructooligosaccharides with a fructosyltransferase from Aspergillus aculeatus. Bioresource Technology, v. 100, 2040-2045 (2009).
Ó'Fágáin, C., Review: Enzyme stabilization - recent experimental progress. Enzyme and Microbial Technology, v. 33, 137-149 (2003).
Ó'Fágáin, C., Stabilizing Protein Function. Springer Verlang, Berlin (1997).
Papinutti, L., Dimitriu, P. and Forchiassin, F., Stabilization studies of Fomes sclerodermeus laccases. Bioresource Technology, v. 99, 419-424 (2008).
Ruth, D. M., Buckley, S. J., O'Connor, B. F. O. andÓ'Fágáin, C., Solvent and thermal stability, and pH kinetics, of praline-specific dipeptidyl peptidase IV-like enzyme from bovine serum. Enzyme and Microbial Technology, v. 41, 307-311 (2007).
Sadana, A., Enzyme deactivation. Biotechnology Advances, v. 6, 349-446 (1998).
Santos, A. M. P., Oliveira, M. G. and Maugeri, F., Modeling thermal stability and activity of free and immobilized enzymes as a novel tool for enzyme reactor desing. Bioresources Technology, v. 98, 3142-3148 (2007).
Šikšnis, V., Mozhaev, V. V., Galkantaite, N., Melik-Nubarov, N. S., Denis, G., Martinek, K., Unusual (zig-zag) temperature dependence of the rate constant for irreversible inactivation of hydrophilized enzymes. Collection of Czechoslovak Chemical Communications, v. 55, 1366-1371 (1990).
Tanriseven, A. and Aslan, Y., Immobilization of Pectinex Ultra SP-L to produce fructooligosaccharides. Enzyme and Microbial Technology, v. 36, 550-554 (2005).
Trevan, M. D., Immobilized Enzymes - an Introduction and Applications in Biotechnology. John Wiley & Sons, New York (1980).
(Submitted: August 31, 2010 ; Revised: December 22, 2010 ; Accepted: March 22, 2011)
- Aguiar-Oliveira, E. and Maugeri, F., Characterization of the immobilized fructosyltranferase from Rhodotorula sp. International Journal of Food Engineering, v. 6, I. 3 (2010). DOI 10.2202/15563758.1894.
- Alvarado-Huallanco, M. B. and Maugeri-Filho, F., Kinetics and modeling of fructooligosaccharide synthesis by immobilized fructosyltransferase from Rhodotorula sp. Journal of Chemical Technology & Biotechnology, (2010). DOI 10.1002/jctb.2477.
- Becktel, W. and Schellman, J. A., Protein Stability Curves, Biopolymers. v. 26, 1859-1877 (1987).
- Bhatti, H. N., Rashid, M. H., Asgher, M., Nawaz, R., Khalid, A. M., Perveen, R., Chemical modification results in hyperactivation and thermostabilization of Fusarium solani glucoamylase. Canadian Journal of Microbiology, v. 53, 177-185 (2007).
- Blanch, H. W. and Clark, D. S., Biochemical Engineering. Marcel Dekker, New York (1997).
- Bolivar, J. M., Mateo, C., Rocha-Martin, J., Cava, F., Berenguer, J., Fernadez-Lafuente, R. and Guisan, J. M., The adsorption of multimeric enzymes on very lowly activated supports involves more enzyme subunits: Stabilization of a glutamate dehydrogenase from Thermus Thermophilus by immobilization on heterofunctional supports. Enzyme and Microbial Technology, v. 44, 139-144 (2009).
- Bromberg, A., Marx, S. and Frishman, G., Kinetic study of the thermal inactivation of cholinesterase enzymes immobilized in solid matrices. Biochimica et Biophysica Acta, v. 1784, 961-996 (2008).
- Catana, R., Eloy, M., Rocha, J. R., Ferreira, B. S., Cabral, J. M. S., Fernandes, P., Stability evaluation of an immobilized enzyme system for inulin hydrolysis. Food Chemistry, v. 101, 260-266 (2007).
- Chen, W. and Liu, C., Production of β-fructofuranosidase by Aspergillus japonicus Enzyme and Microbial Technology, v. 18, I. 2, 153-160 (1996).
- Cornish-Bowden, A., Fundamentals of Enzyme Kinetics. Portland Press, London (1995).
- Doran, P. M., Bioprocess Engineering Principles. Academic Press, New York (2002).
- Dosztányi, Z., Fiser, A. and Simon, I., Stabilization Centers in Proteins: Identification, Characterization and Predictions. Journal of Molecular Biology, v. 272, 597-612 (1997).
- Fogler, H. S., Elements of chemical reaction engineering. 3rd Ed., Prentice Hall, New Jersey (1999).
- Gibson, G. R., Probert, H. M., Loo, J. V., Rastall, R. A. and Roberfroid, M. B., Dietary modulation of the human colonic microbiota: updating the concept of prebiotcs. Nutrition Research Reviews, v. 17, 259-275 (2004).
- Hernalsteens, S. and Maugeri, F., Purification and characterisation of a fructosyltransferase from Rhodotorula sp. Applied Microbiology Biotechnology, v. 79, 589-596 (2008).
- Hidaka, H., Hirayama, M. and Sumi, N., A fructooligosaccharide-producing enzyme from Aspergillus niger ATCC20611. Agricultural and Biological Chemistry, v. 52, I. 5, 1181-1187 (1998).
- Lumry, R. and Eyring, H., Conformation changes of proteins. The Journal of Physical Chemistry, v. 58, 110-120 (1954).
- Lüscher, M., Erdin, C., Sprenger, N., Hochstrasser, U., Boller, T., Wiemken, A., Inulin synthesis by a combination of purified fructosyltransferases from tubers of Heliantus tuberosus FEBS Letters, v. 385, 39-42 (1996).
- Maugeri, F. and Aguiar-Oliveira, E., Método para imobilização de enzimas utilizando suporte sólido inorgânico. University of Campinas (UNICAMP), Patent: PI 070683-1, Brazil Office (2007).
- Maugeri, F. and Hernalsteens, S., Screening of yeast strain for fructosylating activity. Journal of Molecular Catalysis B: Enzymatic, v. 49, I. 1-6, 43-49 (2007).
- Nadeem, M., Perveen, R., Javed, M. R., Nadeem, H. and Rashid, M. H., Kinetic and thermodynamic properties of novel glucoamylase from Humicola sp. Enzyme and Microbial Technology, v. 41, 558-564 (2007).
- Nemukula, A., Mutanda, T., Wilhemi, B. S. and Whiteley, C. G., Response surface methodology: Synthesis of short chain fructooligosaccharides with a fructosyltransferase from Aspergillus aculeatus Bioresource Technology, v. 100, 2040-2045 (2009).
- Ó'Fágáin, C., Review: Enzyme stabilization - recent experimental progress. Enzyme and Microbial Technology, v. 33, 137-149 (2003).
- Ó'Fágáin, C., Stabilizing Protein Function. Springer Verlang, Berlin (1997).
- Papinutti, L., Dimitriu, P. and Forchiassin, F., Stabilization studies of Fomes sclerodermeus laccases. Bioresource Technology, v. 99, 419-424 (2008).
- Ruth, D. M., Buckley, S. J., O'Connor, B. F. O. andÓ'Fágáin, C., Solvent and thermal stability, and pH kinetics, of praline-specific dipeptidyl peptidase IV-like enzyme from bovine serum. Enzyme and Microbial Technology, v. 41, 307-311 (2007).
- Sadana, A., Enzyme deactivation. Biotechnology Advances, v. 6, 349-446 (1998).
- Santos, A. M. P., Oliveira, M. G. and Maugeri, F., Modeling thermal stability and activity of free and immobilized enzymes as a novel tool for enzyme reactor desing. Bioresources Technology, v. 98, 3142-3148 (2007).
- Tanriseven, A. and Aslan, Y., Immobilization of Pectinex Ultra SP-L to produce fructooligosaccharides. Enzyme and Microbial Technology, v. 36, 550-554 (2005).
- Trevan, M. D., Immobilized Enzymes - an Introduction and Applications in Biotechnology. John Wiley & Sons, New York (1980).
Publication Dates
-
Publication in this collection
01 Sept 2011 -
Date of issue
Sept 2011
History
-
Received
31 Aug 2010 -
Reviewed
22 Dec 2010 -
Accepted
22 Mar 2011





















