Abstract
The objective of this study was to estimate thermodynamic data, such as standard enthalpy, entropy and Gibbs free energy changes of reaction and, consequently, chemical equilibrium constants, for a reaction system describing the hydrogen production from Liquefied Petroleum Gas (LPG). The acquisition of those properties was made using computational chemistry methods and the results were compared with experimental data reported in the literature. The reaction system of steam reforming of LPG was reported as a set of seven independent reactions involving the chemical species n-C4H10, C3H8, C2H6, C2H4, CH4, CO2, CO, H2O, H2 and solid carbon. Six computational approaches were used: Density Functional Theory (DFT) employing Becke's three parameter hybrid exchange functional, and the Lee-Yang-Parr correlation functional (B3LYP) using the 6-31G++(d,p) basis set and the composite methods CBS-QB3, Gaussian-1 (G1), Gaussian-2 (G2), Gaussian-3 (G3) and Gaussian-4 (G4). Mole fractions of the system components were also determined between 873.15 and 1173.15 K, at 1 atm and a feed with a stoichiometric amount of water. Results showed that the hybrid functional B3LYP/6-31G++(d,p), G3 and G4 theories were the most appropriated methods to predict the properties of interest. Gaussian-3 and Gaussian-4 theories are expected to be good thermodynamic data predictors and the known efficient prediction of vibrational frequencies by B3LYP is probably the source of the good agreement found in this study. This last methodology is of special interest since it presents low computational cost, which is important when more complex molecular systems are considered.
Liquefied Petroleum Gas (LPG); Computational chemistry; Hydrogen; Equilibrium constant; Thermodynamic data
THERMODYNAMICS
Application of computational chemistry methods to obtain thermodynamic data for hydrogen production from liquefied petroleum gas
J. A. SousaI; P. P. SilvaI, II; A. E. H. MachadoIII; M. H. M. ReisI; L. L. RomanieloI; C. E. HoriI, * * E-mail: cehori@ufu.br
IFaculdade de Engenharia Química, Universidade Federal de Uberlândia, Phone/Fax: + (55) (34) 3239-4188, Av. João Naves de Ávila 2121, Campus Santa Mônica, Bloco 1K, CEP 38400-144, Uberlândia - MG, Brazil
IIDepartamento de Engenharia Química, Universidade Federal do Triângulo Mineiro, Av. Doutor Randolfo Borges Júnior 1250, Bairro Univerdecidade, CEP 38064-200 Uberaba - MG, Brazil
IIIInstituto de Química, Universidade Federal de Uberlândia, Av. João Naves de Ávila 2121, Campus Santa Mônica, Bloco 5K, CEP 38400-144, Uberlândia - MG, Brazil
ABSTRACT
The objective of this study was to estimate thermodynamic data, such as standard enthalpy, entropy and Gibbs free energy changes of reaction and, consequently, chemical equilibrium constants, for a reaction system describing the hydrogen production from Liquefied Petroleum Gas (LPG). The acquisition of those properties was made using computational chemistry methods and the results were compared with experimental data reported in the literature. The reaction system of steam reforming of LPG was reported as a set of seven independent reactions involving the chemical species n-C4H10, C3H8, C2H6, C2H4, CH4, CO2, CO, H2O, H2 and solid carbon. Six computational approaches were used: Density Functional Theory (DFT) employing Becke's three parameter hybrid exchange functional, and the Lee-Yang-Parr correlation functional (B3LYP) using the 6-31G++(d,p) basis set and the composite methods CBS-QB3, Gaussian-1 (G1), Gaussian-2 (G2), Gaussian-3 (G3) and Gaussian-4 (G4). Mole fractions of the system components were also determined between 873.15 and 1173.15 K, at 1 atm and a feed with a stoichiometric amount of water. Results showed that the hybrid functional B3LYP/6-31G++(d,p), G3 and G4 theories were the most appropriated methods to predict the properties of interest. Gaussian-3 and Gaussian-4 theories are expected to be good thermodynamic data predictors and the known efficient prediction of vibrational frequencies by B3LYP is probably the source of the good agreement found in this study. This last methodology is of special interest since it presents low computational cost, which is important when more complex molecular systems are considered.
Keywords: Liquefied Petroleum Gas (LPG); Computational chemistry; Hydrogen; Equilibrium constant; Thermodynamic data.
INTRODUCTION
During the last few decades, computer simulations have become more relevant as a tool for acquisition of knowledge and for process decisions. The simulations make feasible the study of complex theoretical models in different areas of science such as physics and chemistry (Ribeiro and Greca, 2003). In addition, the continuous development of computer and processing technologies is disseminating the use of computer simulations in all fields.
Starting in the 1960´s, the fundaments of Classical Physics and Quantum Chemistry were slowly described and implemented in computer models. This field developed rapidly during the last two decades of the 20th century. The continuous progress led to the creation of a new field of study for the scientists that investigate matter and its properties and interactions: the Computational Chemistry.
The optimization of operational conditions of a process usually needs the values of equilibrium constants of all reactions present. These data allow the calculation of mole fractions of all species present at equilibrium, conditions which lead to the maximum theoretical conversions. Sometimes there is a lack of experimental energies of formation (ΔH0f and ΔG0f). Although there has been continuous progress in the publication of experimental data, there are still a very large number of substances for which no thermodynamic data are available (Fringant et al., 1995). One of the causes for this lack of data is the complexity of experimental setups required to obtain thermodynamic data.
In this context, considering the large improvement of computer hardware technologies and Computational Chemistry in the last decade and the lack of experimental data, the use of computational chemistry methods could be a valuable tool to obtain thermodynamic data.
Several researches have been published in the literature applying computational chemistry to predict thermodynamic parameters of interesting reactions. Ramos et al. (2012) used quantum chemical methods to evaluate thermodynamic and kinetic parameters for a set of reactions involved in the oxidation pathway of phenol by reactive oxygen species.
Hydrogen has been attracting great interest as a clean energy source. Generally, hydrogen can be produced by processes such as steam reforming, partial oxidation and autothermal reforming of hydrocarbons. Hydrogen can also be obtained from the reforming of liquefied petroleum gas (LPG), which is is a commercial gas that is easily transported and stored on-site (Laosiripojana and Assabumrungrat, 2006).
Therefore, the objective of this study was to estimate the standard enthalpy (ΔH0r), entropy (ΔS0r) and Gibbs free energy (ΔG0r) changes of reaction and, consequently, the chemical equilibrium constants (K) for the reaction system of hydrogen production from LPG using computational chemistry methods.
Computational Models
Calculations of properties that depend on the electronic distribution are reliable in computational chemistry due to the explicit representation of electrons in this methodology.
The B3LYP exchange-correlation functional (from "Becke, 3-parameter, Lee-Yang-Parr") is based on Density Functional Theory (DFT), so all the properties of a given system are calculated based on its electronic density. Theoretically, compared to methods such as Hartree-Fock (HF), for instance, the consideration of correlation effects increases its prediction capacity. On the other hand, this leads to a higher computational cost when compared to the HF method. In the DFT approach, the total system energy is composed of four contributions: the kinetic energy, ET, the potential energy, EV, which takes into account the positions and charges of the atomic nuclei that form the system (Kohn and Sham, 1965), the term that represents the electron-electron repulsion, EJ, and the exchange energy EXC. The term EXC is calculated using hybridization between HF and DFT theories as a simplified way to calculate several properties such as atomization energies, bond lengths and vibrational frequencies.
The combination of high theoretical level methods and a small data bases with other methods with lower levels of theory and a larger data bases originated the ab initio pos-Hartree-Fock methods. These composite methods are widely used to estimate thermodynamic properties and usually give better agreement with experimental data. However, there are some disadvantages such as relatively higher computational costs and memory requirements, which can make it impossible to analyze larger electronic systems.
The oldest and most widely used composite methods are the Gaussian-n or Gn methods of Pople, Curtiss, and co-workers (Pople et al., 1989; Curtiss et al., 1990; Curtiss et al., 1991; Curtiss et al., 1998; Curtiss et al., 2007). The goal of these methods was to create a procedure that could be applied to any molecular or atomic system and estimate well experimental energetic data, for instance, relative conformational energies, atomization energies, enthalpies of formation, ionization potentials, electron affinities and proton affinities, to near-chemical accuracy. Considering the Gaussian-n methods, the first hybrid method was known as G1 (Pople et al, 1989; Curtiss et al., 1990), which included diffuse and polarization functions and residual correlation effects in the calculations of the system energy. After that, a new method, known as G2, was proposed with the objective of correcting some of the problems detected in G1 theory (Curtiss et al., 1991). The G2 method corrects some G1 considerations concerning diffuse and polarization function additivity and also some parameters to correct the system electronic energy. The G3 theoretical procedure, which is also based on ab initio molecular-orbital theory, modifies G2 theory in several ways, including a new sequence of single point energy calculations using different basis sets, a new formulation of the higher level correction, a spin-orbit correction for atoms, and a correction for core correlation (Curtiss et al., 1998). The fourth in the Gaussian-n series of quantum chemical methods (G4) is based on a sequence of single point energy calculations (Curtiss et al., 2007). G4 theory modifies Gaussian-3 (G3) by an extrapolation procedure to obtain the Hartree-Fock limit for inclusion in the total energy calculation, the d-polarization sets are increased to 3d for the first-row atoms and to 4d for the second-row atoms, with reoptimization of the exponents for the latter, the QCISD(T) method is replaced by the CCSD(T) method for the highest level of correlation treatment, optimized geometries and zero-point energies are obtained with the B3LYP density functional and two new higher level corrections are added to account for deficiencies in the energy calculations.
CBS models are unique in their use of the N-1 asymptotic convergence of second-order MØller-Plesset pair energies calculated from pair natural orbital expansions to extrapolate to the complete basis set (CBS) limit. In CBS models, a series of calculations are made on a defined geometry, and a complete basis set model chemistry includes corrections for basis set truncation errors. Accuracy in structure and energy of the system requires convergence in basis set size and in the degree of correlation; the dilemma is that both expansion of the basis set and increasing the degree of correlation increase the cost of calculation. The philosophy of implementation is that, instead of using additive corrections to account for the limitations of the basis set, as in the Gn methods, results for different levels of theory are extrapolated to the CBS limit. The five-step CBS-QB3 series of calculations starts with a geometry optimization at the B3LYP level, followed by a frequency calculation to obtain thermal corrections, zero-point vibrational energies, and entropic information. According to Ochterski et al. (1996), the CBS-QB3 model chemistry is reliable, offers small improvements in the mean absolute and root-mean-square errors, and suffers little penalty in speed.
METHODOLOGY
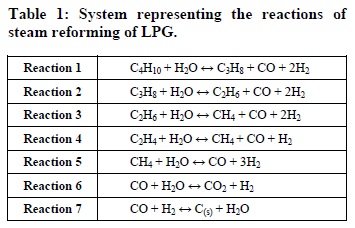
According to the literature (Laosiripojana and Assabumrungrat, 2006), the species present in a reaction system for the steam reforming of LPG are C4H10, C3H8, C2H6, C2H4, CH4, CO, CO2, H2, H2O and solid carbon (C). A set of 7 linearly independent reactions was chosen to represent the system. These reactions are presented in Table 1.
The Gaussian 09W® computational package was used to perform the calculations. Each molecule was pre-optimized using the semi-empirical AM1 method. The achieved structure was used as the first step in the next calculations. After this, the optimized structure and the energy of each molecule were calculated using the B3LYP hybrid functional with the 6-31G++(d,p) basis set, the Gaussian-n methods (G1, G2, G3, and G4), and the CBS-QB3 model chemistry.
The calculations were performed at constant pressure (1 atm) and at different temperatures (from 873.15 to 1173.15 K). This temperature range was chosen according to experimental data published in the literature (Liu et al., 2001 and Avci et al., 2004).
The standard Gibbs free energy changes of reaction at different temperatures ( ) were calculated from the values of the electronic energy (Ee) plus the thermal corrections (Gcorr), taken directly from the simulation output file. This was made by taking the difference between the products and reactants considering their stoichiometric coefficients. Equation (1) describes these calculations at a given temperature.
) were calculated from the values of the electronic energy (Ee) plus the thermal corrections (Gcorr), taken directly from the simulation output file. This was made by taking the difference between the products and reactants considering their stoichiometric coefficients. Equation (1) describes these calculations at a given temperature.
where  is the standard Gibbs free energy change of the reaction at temperature T, ν is the stoichiometric coefficient of the chemical species and the terms (ε0+Gcorr) correspond to the sum of the electronic and thermal free energies.
is the standard Gibbs free energy change of the reaction at temperature T, ν is the stoichiometric coefficient of the chemical species and the terms (ε0+Gcorr) correspond to the sum of the electronic and thermal free energies.
It is important to note that carbon, C(s), is in the solid state in Reaction 7. In this case, an alternative methodology was applied to obtain the thermochemical parameters for the solid carbon. The relative deviations between theoretical quantities and those derived from experimental data for the standard enthalpy, entropy and Gibbs free energy changes of the water-gas shift reaction and reforming of methane were used for the calculation of arithmetic means. These two reactions were taken as references due to the similarity between the chemical species related to Reaction 7. These means, in turn, were admitted as the respective discrepancies between theoretical and reference data in the reduction of carbon monoxide reaction. Then, the standard enthalpy, Gibbs free energy and entropy changes for solid carbon were set up such that the calculated deviations were found.
Using experimental data, the standard Gibbs free energy change of reaction was calculated from the standard Gibbs free energy change of formation of the compounds taking part in the reaction. The obtained values are referred to as reference data.
In order to calculate the equilibrium constants, the data obtained from computational chemistry methods and from experimental energies of formation were used according to Equation (2).
where K is the equilibrium constant of reaction at temperature T and R is the ideal gas constant.
The effect of temperature on the standard Gibbs free energy change of reaction obtained from experimental data was calculated using Equation (3).
where  and
and  and are the standard Gibbs free energy and enthalpy of formation, respectively, at the reference temperature T0, and ΔCp0 is the standard heat-capacity change of the reaction.
and are the standard Gibbs free energy and enthalpy of formation, respectively, at the reference temperature T0, and ΔCp0 is the standard heat-capacity change of the reaction.
The reference temperature was taken as 298.15 K. The standard heat-capacity change of reaction (ΔCp0) is defined by Equation (4) (Smith et al., 2001):
where νi is the stoichiometric coefficient of the chemical species i and  is the standard constant-pressure heat capacity of the chemical species i.
is the standard constant-pressure heat capacity of the chemical species i.
Experimental data for standard properties changes of formation at 298.15 K and a correlation for the heat capacity of gases in the ideal-gas state as a function of the temperature, as well as the parameters of this correlation, were taken from Smith et al. (2001).
After obtaining the chemical equilibrium constants for each reaction of the LPG steam reforming system, they were used to calculate the mole fractions of each species present at equilibrium. Thus, for a reaction j:
where  is the standard fugacity of species i (the reference state is the ideal gas fugacity at atmospheric pressure (1 atm) - at this condition,
is the standard fugacity of species i (the reference state is the ideal gas fugacity at atmospheric pressure (1 atm) - at this condition,  =1),
=1),  is the fugacity of species i in the mixture and is the stoichiometric coefficient of chemical species i in reaction j. For each component, the fugacity in the mixture can be expressed in terms of fugacity coefficient
is the fugacity of species i in the mixture and is the stoichiometric coefficient of chemical species i in reaction j. For each component, the fugacity in the mixture can be expressed in terms of fugacity coefficient  , the mole fraction (yi) and the pressure (P), as shown in Equation (6):
, the mole fraction (yi) and the pressure (P), as shown in Equation (6):
As the system was considered to be at atmospheric pressure and high temperatures, species in the gas phase can be considered as an ideal gas. This condition leads to
The system of seven non-linear equations was solved using a program developed in Maple® 8, with a few restrictions on the search range for the extent of reaction. The data obtained by Zeng and coworkers (2010) were used in order to validate the program and errors below 3% for H2 yields were found.
The calculations were performed using a computer with an Intel Dual Core® 1.73 GHz processor, 2GB of RAM, 2 MB of cache memory L2 and hard drive of 380 GB.
Deviations between calculated and experimental data for the standard enthalpy and entropy changes of reaction were considered in order to check the accuracy of the proposed computational methods. These deviations were calculated according to Equation (8):
where xi and yi are respectively calculated and experimental thermodynamic parameters at each considered temperature. Means and standard deviations of those values were calculated and reported in this paper. Standard deviations (SD) were also reported in order to check the calculated average regarding temperature variations.
The average deviations of the data obtained by different computational methods were submitted to hypothesis tests. Since the number of points was limited (n<30), a t-student distribution was used and the level of significance was 5% in all tests.
RESULTS AND DISCUSSION
The standard enthalpy changes of reaction at the considered temperatures obtained for the LPG steam reforming system with the B3LYP hybrid functional with the 6-31G++(d,p) basis set, the G-n methods and the CBS-QB3 model chemistry are shown in Figures 1 to 7 , as well as the reference data computed using Equation (3) and experimental data at 298.15 K available in Smith et al. (2001).
All computational methods predict well the endothermic nature of butane, propane and ethane reforming (Reactions 1 to 3 of Table 1), as shown in Figures 1 to 3 . In addition, the behavior of having an approximately constant standard enthalpy variation in these reactions was also correctly predicted by these methods.
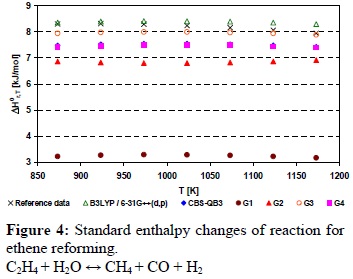
Figure 4 shows the standard enthalpy changes for the reaction between ethene and water (Reaction 4 of Table 1) estimated by the proposed computational methods and from experimental data. Again the endothermic behavior of the reactions was described by the proposed computational methods.
The standard enthalpy changes of reaction calculated for the steam reforming of methane (Reaction 5 of Table 1) are presented in Figure 5. Once more, the proposed methods predicted well the endothermic nature of the reaction.
Figure 6 shows the results obtained for the water-gas shift reaction (Reaction 6 in Table 1), showing that the proposed computational methods predicted the exothermic nature of this transformation.
Reaction 7 describes the formation of solid carbon and gaseous hydrogen from carbon monoxide and water. Results presented in Figure 7 show that there is an agreement between predicted and reference data. Thus, besides the accuracy of the proposed computational models, the alternative methodology used to predict the thermochemical data of solid carbon seems to have succeeded well.
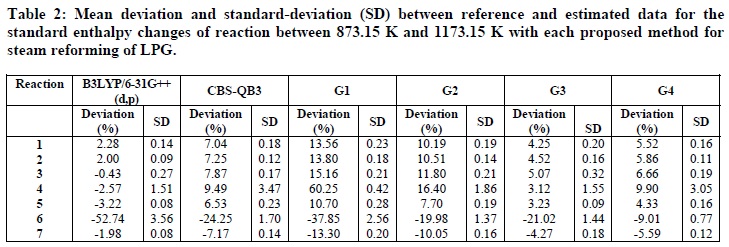
Table 2 presents the average deviations between calculated and experimental data (Equation (5)), as well as the standard deviations related to the average over temperature of the standard enthalpy changes of reaction calculated by each computational method. The hybrid functional B3LYP/6-31G++(d,p) presented the lowest deviations for almost all the reactions analyzed, except for Reaction 6 (the water-gas shift reaction). For this reaction, the most reliable method was G4. Larger deviations were observed for all the computational methods for Reaction 6. This behavior may be attributed to the presence of carbon dioxide. When the vibrational frequencies of this species were analyzed, it was found that frequencies of π bonds present on this molecule was not as overestimated as others. The overestimation of vibrational frequencies by B3LYP is widely reported (Foresman and Frisch, 1996; Marstokk et al., 2000), so the hybrid functional B3LYP/6-31G++(d,p) probably was not efficient to represent the double bounds present in the carbon dioxide molecule.
Hypothesis tests for the deviations obtained with the proposed computational methods were performed. All the methods showed statistically significant differences at p < 0.05 for standard enthalpy changes for Reactions 1, 2, 3, and 6. For Reaction 4, CBS-QB3 and G4 methods presented statistically different mean deviations (p = 0.04), however, since the p-value is very close to the significance level of the test (5%), a greater number of data would be required for a more reliable inference. For Reaction 5, the B3LYP/6-31G++(d,p) and G3 methods are statistically equivalent, and other methods showed a statistically significant difference between their mean deviations (p> 0.05).
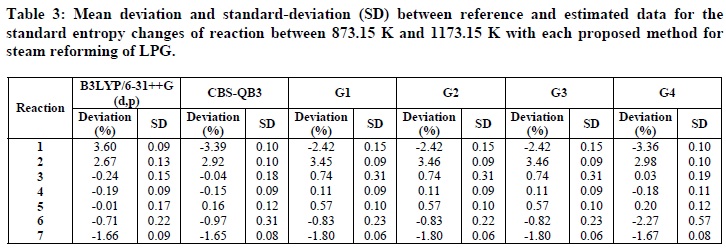
The analysis of the standard entropy changes calculated for the reactions of LPG reforming showed small deviations from the ones obtained from experimental data. Table 3 presents the average % deviations, as well as the standard deviations for the standard entropy changes calculated by each computational method. As can be observed, in general the deviations are low, demonstrating that the computational methods may be considered as good predictors of standard entropy changes for this reaction system. For Reactions 2, 5, 6, and 7, the smallest errors were achieved by the functional B3LYP/6-31G++(d,p), although a statistical analysis showed that, within a confidence interval of 95%, there are no significant differences between B3LYP/6-31G++(d,p), CBS-QB3 and G4 methods for Reaction 7. For Reaction 1, the G1, G2, and G3 methods presented the smallest errors. For Reaction 3, the smallest errors were achieved by the CBS-QB3 and G4 methods (statistically equivalent within a confidence interval of 95%).
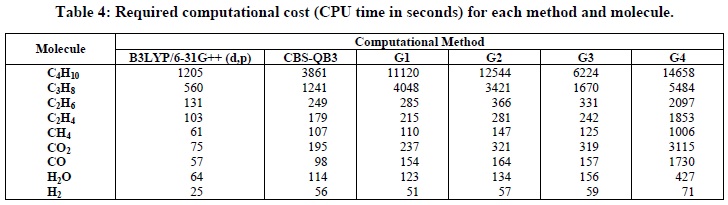
Table 4 presents the required computational cost (CPU time in seconds) for each method and molecule at 298.15 K. The functional B3LYP/6-31G++(d,p) was the least expensive for all considered molecules. As expected, molecules with a larger number of electrons required more CPU time. In this way, considering the trade-off between accuracy and computational cost, the B3LYP/6-31G++(d,p) approach is the most suitable for predicting thermochemical data of the considered reactions. For the CO2 molecule, the required computational time using the G4 method is considerably lager than with other methods, evidencing the suitability of the G4 method for estimating the CO2 molecule electronic structure and, as a consequence, Reaction 6.
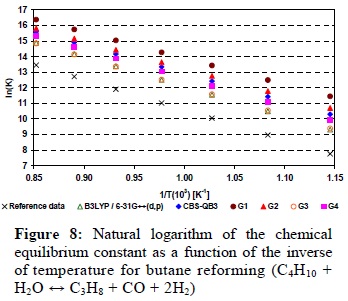
Figures 8 to 14 present the results obtained for the reaction equilibrium constants (Equation (2)) as a function of temperature. All the data obtained for Reactions 1 to 5 showed that these reactions are favored by an increase in temperature, as can be seen in Figures 8 to 12 . Therefore, hydrogen production would also be favored by the use of higher temperatures. This result is in agreement with literature reports that show the use of high temperatures in the reforming of hydrocarbons for the production of hydrogen (Laosiripojana and Assabumrungrat, 2006). Figures 8 to 10 show that Reactions 1 to 3 (butane, propane and ethane reforming) have very similar behavior regarding their equilibrium constants. This is due to the fact that the changes in standard enthalpy and entropy are quite similar for these reactions.
The deviations from experimental data follow this same pattern. For butane reforming (Reaction 1), B3LYP/6-31G++(d,p) presented the best result, with a deviation of 1.60 (±0.32), but that method is statistically equivalent to G3 (p=0.65), with a deviation of 1.71 (±0.60). Regarding Reaction 2 - propane reforming - statistical equivalence was also found for the B3LYP/6-31G++(d,p) and G3 methods (p=0.19), with deviations of, respectively, 0.04 (±0.68) and 0.33 (±0.0.31). The DFT approach - represented by the hybrid functional B3LYP/6-31G++(d,p) - provided the best result when the reaction of ethane reforming was analyzed, with a deviation of 0.09 (±0.0.16).
Fig. 11 shows that ethene reforming has a smaller dependence with temperature when compared with the first three reactions. So, this reaction will be much less favored by a temperature increase than Reactions 1, 2, 3, or even 5. All the methods predict correctly that behavior and, once more, B3LYP/6-31G++(d,p) was the best predictor, with a deviation of 0.02 (±0.04).
Data calculated by different methods for steam reforming of methane are shown in Fig. 12 . As observed for other reforming reactions, the production of hydrogen is favored by an increase in temperature. G3 presented the smallest deviation (0.90 (±0.68)) but it is statistically equivalent (p=0.54) to B3LYP/6-31G++(d,p) (deviation of 1.12 (±0.72)).
Fig. 13 shows that all quantum-mechanical methods predicted correctly the decrease in conversion of the water-gas shift reaction with the increase of temperature. The smallest deviation was found for the G4 method, 0.40 (±0.31), and the highest one for B3LYP/6-31G++(d,p): 2.83 (±0.1.87). The higher deviation found with the B3LYP approach was probably due to problems with the carbon dioxide electronic structure. For the ethene reforming reaction as well, the overall deviation was higher than those observed for the other reactions. As was the case for carbon dioxide, the vibrational frequencies of the π bond of ethylene were not as overestimated as those of other bonds. Therefore, once again, the hybrid functional B3LYP/6-31G++(d,p) probably was not adequate to represent the double bounds present in this molecule.
Computational methods together with the proposed methodology for solid carbon also represented well the behavior of Reaction 7 (reduction of carbon monoxide), as presented in Fig. 14 . Deviations showed that B3LYP/6-31G++(d,p) (0.18 (±0.57)) and CBS-QB3 (0.61 (±1.78)) were statistically equivalent (p=0.42). Overall, G3 and B3LYP/6-31G++(d,p) presented the best prediction capabilities and, for all reactions but the water-gas shift one, the G1 method presented the highest deviations.
Mole fractions of the major chemical species in the LPG reforming system at equilibrium, calculated from reference data of energies of formation and also by B3LYP/6-31G++(d,p) and G3, are presented in Fig. 15. n-Butane, propane, ethane, and acetylene mole fractions were lower than 1x10-5 and so they were omitted. Regarding CH4, CO2, CO, H2O and H2 quantities, G3 results showed a better accuracy than the hybrid functional B3LYP. Probably, this was due to the higher discrepancy between reference and theoretical data for Reaction 6 (water-gas shift reaction) simulated by the DFT approach. In general, better agreement was found at higher temperatures.
As predicted, when equilibrium constants were analyzed, hydrogen production is favored by higher temperature. However, as one can see in Fig. 15, the increase in H2 amount above 1000 K is very small. Since thet CO mole fraction increases as the CO2 mole fraction decreases with increasing temperature, the H2 mole fraction stabilization is probably a consequence of the reverse of the water-gas shift reaction. Above 973.15 K, there is a significant reduction in H2 produced, indicating that LPG steam reforming should be carried out at around 950 to 1050 K.
CONCLUSIONS
Based on the results presented, it can be verified that computational chemistry methods were able to predict thermodynamic properties of the LPG steam reforming system. It was shown that the use of methods with higher levels of complexity did not always lead to more precise data, as could be observed when B3LYP/6-31G++(d,p) and G1 or G2 were compared. Using the functional B3LYP with an adequate basis set can provide good agreement between predicted and experimental data with a low computational cost, which is very convenient for more complex molecular systems. It can be pointed out that there is a close dependence between the results obtained and the molecular structure proposed for each molecule present in the reaction system. The computational methods were not capable of predicting the thermodynamic properties of the reaction that represents formation of solid carbon. This was due to the electronic structure proposed for solid carbon, so an alternative methodology had to be developed for this chemical species. On the other hand, when an adequate structure was proposed for a given molecule, in general, there was good agreement between simulated and reference data regarding the thermodynamic properties of the studied reactions, in particular, the standard entropy changes of reaction. Mole fractions of the species present in LPG steam reforming were calculated from predicted equilibrium constants and, especially for B3LYP/6-31G++(d,p) and G3 a good agreement between theoretical and reference data was found. In general, higher temperatures led to better accuracy between reference and simulated mole fractions for all chemical species. Probably due to the greater discrepancy found for B3LYP/6-31G++(d,p) predictions regarding Reaction 6, the G3 method showed lower deviations when mole fractions were evaluated. From these results, it can be concluded that the use of computational chemistry methods to predict thermodynamic properties can be very promising and a very good alternative for the prediction of thermodynamic properties of substances when experimental data are not available.
ACKNOWLEDGMENTS
The authors wish to acknowledge CAPES, CNPq and FAPEMIG for the financial support.
(Submitted: January 31, 2012 ; Revised: September 19, 2012 ; Accepted: September 19, 2012)
- Avci, K. A., Trimm, D. L., Aksoylu, A. E., Onsan, Z. I., Hydrogen production by steam reforming of n-butane over supported Ni and Pt-Ni catalysts. Applied Catalysis A: General, 235, 235-240 (2004).
- Curtiss, L. A., Jones, C., Trucks, G. W., Raghavachari, K., Pople, J. A., Gaussian-1 theory of molecular energies for second-row compounds. Journal of Chemical Physics, 4, 2537-2545 (1990).
- Curtiss, L. A., Raghavachari, K., Trucks, G. W., Pople, J. A., Gaussian-2 theory for molecular energies of first- and second-row compounds. Journal of Chemical Physics, 94, 7221-7230 (1991).
- Curtiss, L. A., Rachavachari, K., Redfern, P. C., Rassolov, V., Pople, J. A., Gaussian-3 (G3) theory for molecules containing first and second-row atoms. Journal of Chemical Physics, 18, 7764-7776 (1998).
- Curtiss, L. A., Redfern, P. C., Rachavachari, K., Gaussian-4 theory. Journal of Chemical Physics, 126 084108/1-084108/12 (2007).
- Foresman, J. B., Frisch, E., Exploring Chemistry with Electronic Structure Methods. 2nd Ed., Gaussian Inc., Pittsburgh, (1996).
- Fringant, C., Tvaroska, I., Mazeau, K., Rinaldo, M., Desbrieres, J., Hydration of α-maltose and amylase: Molecular modeling and thermodynamics study. Carbohydrate Research, 278, 27-41 (1995).
- Kohn, W., Sham, L. J., Self-consistent equations including exchange and correlation effects. Physical Review, 14, 1133-1138 (1965).
- Laosiripojana, N., Assabumrungrat, S., Hydrogen production from steam and autothermal reforming of LPG over high surface area ceria. Journal of Power Sources, 158, 1348-1357 (2006).
- Liu, S., Xu, L., Xie, S., Wang, Q., Partial oxidation of propane to syngas over nickel supported catalysts modified by alkali metal oxides and rare-earth metal oxides. Applied Catalysis A, 211, 145-152 (2001).
- Marstokk, K-M., Mollendal, H., Samdal, S., Microwave spectrum, conformational equilibrium, 14N quadrupole coupling constants, dipole moment, vibrational frequencies and quantum chemical calculations for acrylamide. Journal of Molecular Structure, 524, 69-85 (2000).
- Ochterski, J. W., Peterson, G. A., Jr., J. A., M., A complete basis set model chemistry. Extension to six or more heavy atmos. Journal of Chemical Physics, 15, 2598-2607 (1996).
- Pople, J. A., Head-Gordon, M., Fox, D. J., Raghavachari, K., Curtiss, L. A., Gaussian-1 theory: A general procedure for prediction of molecular energies. Journal of Chemical Physics, 90, 5622-5629 (1989).
- Ramos, B., Farah, J. P. S., Teixeira, A. C. S. C., Estimating reaction constants by ab initio molecular modeling: A study on the oxidation of phenol to catechol and hydroquinone in advanced oxidation processes. Brazilian Journal of Chemical Engineering, 29, 113-120 (2012).
- Ribeiro, A., Greca, I. M., Computer simulations and modeling tools in chemical education: A review of published literature. Química Nova, 26, 542-549 (2003).
- Smith, J. M., van Ness, H. C., Abbott, M. M., Introduction to Chemical Engineering Thermodynamics. 6 Ed., McGraw Hill: Singapore, (2001).
- Zeng, G., Tian, Y., Li, Y., Thermodynamic analysis of hydrogen production for fuel cell via oxidative steam reforming of propane. International Journal of Hydrogen Energy, 25, 6726-6737 (2010).
Publication Dates
-
Publication in this collection
01 Mar 2013 -
Date of issue
Mar 2013
History
-
Received
31 Jan 2012 -
Accepted
19 Sept 2012 -
Reviewed
19 Sept 2012







 and, therefore:
and, therefore: