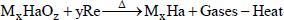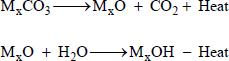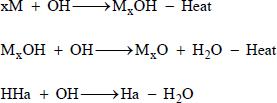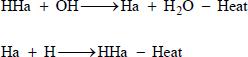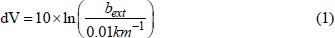Abstract
Since the phase out of Halon extinguishers in the 1980s, hot aerosol fire suppression technology has gained much attention. Unlike traditional inert gas, foam, water mist and Halon fire suppression agents, hot aerosol fire extinguishing agents do not need to be driven out by pressurized gases and can extinguish class A, B, C, D and K fires at 30 to 200 g/m3. Generally, hot aerosol fire extinguishing technology has developed from a generation I oil tank suppression system to a generation III strontium salt based S-type system. S-type hot aerosol fire extinguishing technology greatly solves the corrosion problem of electrical devices and electronics compared to potassium salt based generation I & II hot aerosol fire extinguishing technology. As substitutes for Halon agents, the ODP and GWP values of hot fire extinguishing aerosols are nearly zero, but those fine aerosol particles can cause adverse health effects once inhaled by human. As for configurations of hot aerosol fire extinguishing devices, fixed or portable cylindrical canisters are the most common among generation II & III hot aerosol fire extinguishers across the world, while generation I hot aerosol fire suppression systems are integrated with the oil tank as a whole. Some countries like the U.S., Australia, Russia and China, etc. have already developed standards for manufacturing and quality control of hot aerosol fire extinguishing agents and norms for hot aerosol fire extinguishing system design under different fire protection scenarios. Coolants in hot aerosol fire suppression systems, which are responsible for reducing hot aerosol temperature to avoid secondary fire risk are reviewed for the first time. Cooling effects are generally achieved through vaporization and endothermic chemical decomposition of coolants. Finally, this review discussed areas applying generation I, II or III hot aerosol fire suppression technologies. The generation III hot aerosol fire extinguishing system is most applicable, especially in areas involving delicate electrical and electronic equipments. Nonetheless, developing a much cleaner, non-corrosive and highly efficient hot aerosol fire suppression system is still needed.
Keywords:
Aerosol forming agent; K-type; S-type; Aerosol forming agent cooling
INTRODUCTION
Hot aerosol fire extinguishing technology was introduced and developed since 1960s, based on pyrotechnics. Because of the problem of ozone depletion caused by using Halon extinguishing agents which contain bromofluoroalkanes (Andrzej & Tsang, 1997Andrzej, W. M., Tsang, W., Halon Replacements: Technology and Science. ACS Symposium Series, Washington DC, US (1997).), many countries joined the Montreal Protocol. This Protocol announced protective actions for the ozone layer in 1987 and promoted the phase out movement to phase out Halon extinguishants.
Compared to Halon extinguishants, other traditional extinguishants like foam, inert gas and dry powders are usually not as efficient as Halons. Those traditional extinguishants are also often stored in pressurized containers.
Extinguishing technology based on dry powders or particles dispersed in a solution is called cold aerosol technology. These dry and fine powders can be alkaline or alkaline earth metal carbonates, phosphates, etc. Particles dispersed in solution can be sodium, potassium or magnesium chlorides, lithium iodide, potassium acetate, sodium hydroxide, potassium ferrocyanide (Korobeinichev et al., 2012Korobeinichev, O. P., Shmakov, A. G., Shvartsberg, V. M., Chernov, A. A., Yakimov, S. A., Koutsenogii, K. P. and Makarov, V. I., Fire suppression by low-volatile chemically active fire suppressants using aerosol technology. Fire Safety Journal, 51, 102-109 (2012).), and PCCs (phosphorus containing compounds) (Jayaweera et al., 2005Jayaweera, T. M., Fisher, E. M., Fleming, J. W., Flame suppression by aerosols derived from aqueous solutions containing phosphorus. Combustion and Flame, 141(3), 308-321 (2005).), etc. Table 1 shows the comparison of hot aerosol fire suppression technology with other common fire extinguishing technologies.
Comparison of suppression mechanisms of common extinguishing agents with hot aerosol technology.
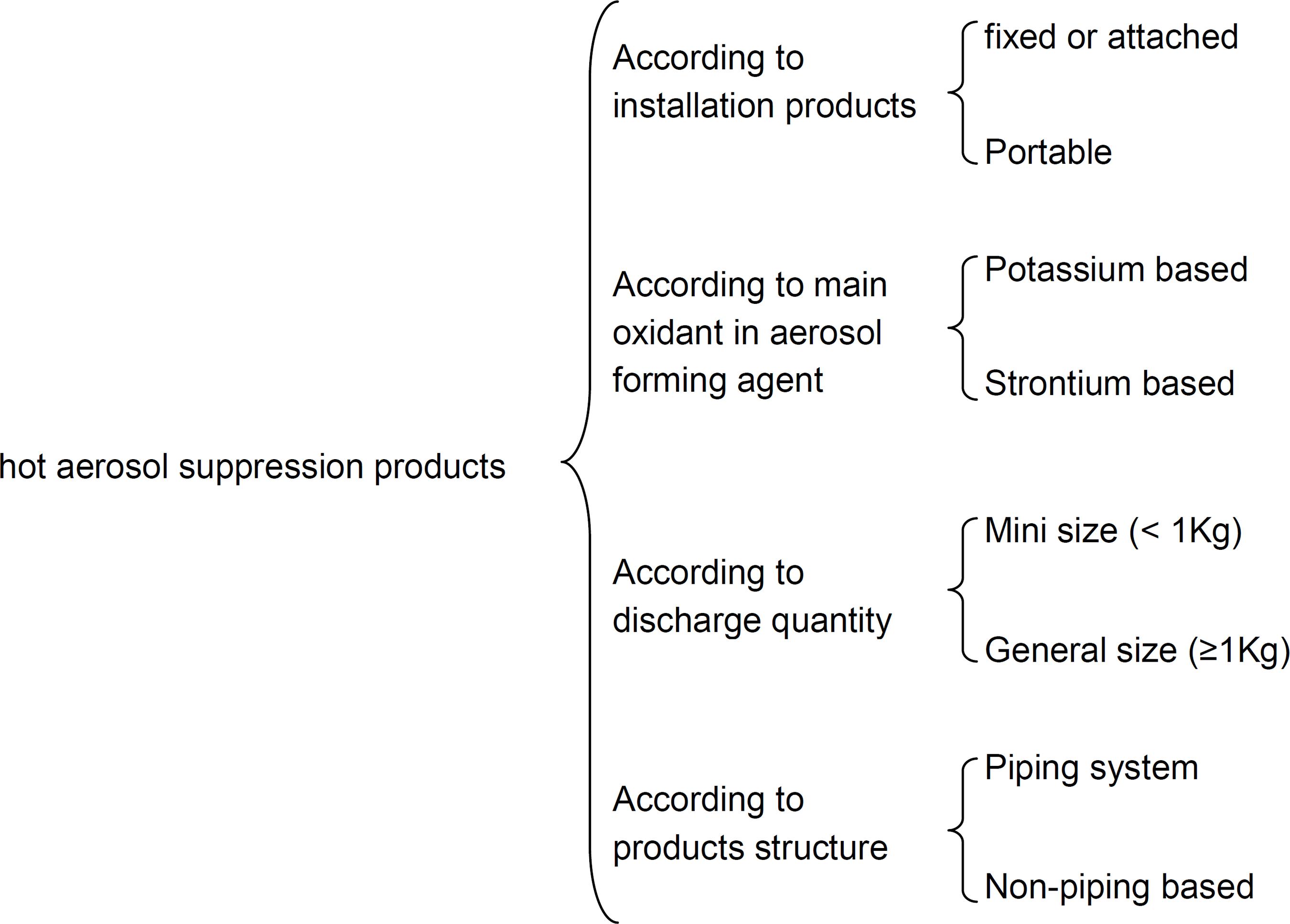
For hot aerosol technology, the main ingredients in the fire extinguishing aerosol forming agent are alkaline or alkaline earth metal nitrates, which act as oxidants in hot aerosol generating process, and different kinds of reductants. Classifications of hot aerosol suppression products can be seen in Figure 1.
Hot aerosol fire extinguishing agents have good efficiency in fire extinguishing and they do not need to be stored in a pressurized container since they do not need to be driven out by pressurized inert gases. A block of hot aerosol fire extinguishing agent sits in a canister with openings in the cap, and a fuse attached to the canister ignites the aerosol forming agent to generate hot aerosol, which can be driven out automatically. Hot aerosol forming agents have low ODP (Ozone Depletion Potential) and GWP (Global Warming Potential) value (Kwon & Kim, 2013Kwon, K. and Kim, Y., Extinction effectiveness of pyrogenic condensed-aerosols extinguishing system. Korean J. Chem. Eng., 30(12), 2254-2258 (2013).). So research and development of such new fire suppression technology have been given much attention, especially in developed countries.
Fire Extinguishing Mechanism of Hot Aerosol Agents
Different from traditional or cold aerosol extinguishing agents, in hot aerosol extinguishing systems, hot aerosol colloids with diameters from 10-9 to 10-7 meters are generated through redox (reducingoxidizing reaction) of a hot aerosol forming agent. Those colloids have diameters much smaller than 4 × 10-6 m at which Brownian motion starts to occur (Fu et al., 2001Fu, S., Yao, H., Physical Chemistry. High Education Press, Beijing (2001).) and hence it gives the hot aerosol particles a high diffusive ability and a long suspension time in the fire zone to fight fire. Basically, the mechanism of hot aerosol extinguishing agents to extinguish fire is both physical and chemical.
Physical Fire Extinguishing Processes
Because aerosol particles have high surface to volume ratios, when those particles enter the fire zone, they attract fire supporting radicals such as hydrogen and hydroxyl radicals onto their surfaces by multiple layer physical adsorption, which is the premise for chemical adsorption to reduce particle surface energy and stop the burning chain reaction (Yang, 2009Yang, J., Fire extinguishment machanism and influence of aerosol fire extinguishment agent. J. Southern Yangtze Uni. (Nat. Sci. Ed.), 2(3), p. 303 (2009).). Also, Agafonov et al., (2004)Agafonov, V. V., Kopylov, S. N., Sychev, A. V., Uglov, V. A., Zhyganov, D. B., The mechanism of fire suppression by condensed aerosols. Proceedings of the 15th HOTWC, Albuquerque, NM (2004). found that gases generated by redox, like nitrogen, water vapor and carbon dioxide, can choke the fire by diluting surrounding oxygen and reduce the fire zone temperature (see Figure 2). Physical extinguishing processes contribute less than chemical fire extinguishing processes unless when the fire is near extinction (Larson, 2003Larson, E. R., Halogenated flame extinguishantssome additional support for the physical mechanism. J. Fire Sci., 23, 93-98 (2005).).
Temperature reductions by some aerosols on flame temperature of hydrogen-air mixture; 1: 100% KOH, 2: 50% KOH + 50% K2CO3, 3: 100% K2CO3, 4: 100% K2O, 5: 50% K2CO3 + 20% N2 + 30% CO2, 6: 40% CO2 + 60% N2, 7: 100% KCl, 8: 50% KCl + 20% CO2 + 30% N2.
Chemical Fire Extinguishing Processes
Combustion of the oxidant with the reductant generates microparticles such as alkaline or alkaline earth oxides, hydroxides or chlorides, which can recombine with the fire supporting radicals like hydroxyl and hydrogen radicals, etc. in the fire zone (Agafonov et al., 2004Agafonov, V. V., Kopylov, S. N., Sychev, A. V., Uglov, V. A., Zhyganov, D. B., The mechanism of fire suppression by condensed aerosols. Proceedings of the 15th HOTWC, Albuquerque, NM (2004).; Williams & Fleming, 1999Williams, B. A., Fleming, J. W., Suppression Mecha- nisms of Alkaline Metal Compounds. HOTWC, Albuquerque, NM (1999).). Figure 3 shows how sodium hydroxide particles reduce fire supporting radical concentration.
Sodium hydroxide concentrations vs. radical concentration reduction in methane-air mixture.
Redox product species are dependent on the anion group in the oxidant and, if the anion group is NO3 -, then the following reaction happens:
If the anion group is HaOz - (Ha can be Cl, Br or I), then the redox becames:
where M can be Na, K, Mg, Ca, Sr or Al and Re refers to reductants like sugers (glucose, sucrose, alpha-lactose, etc.) or derivatives of amides (dicyanamide, dicyandiamide), guanidines (nitroguanidine) or cellulose (nitrocellulose). Also, reductants can be pure carbon powders or charcoal, or even reactive metal powders like aluminum and magnesium powders. Magnesium or ammonium oxalate can also be auxiliary reductants.
The gases are primarily CO2, N2, and H2O vapor and with trace quantity of ammonia, NOx and CO if the anion group is nitrate in the oxidant. Gases can contain halogen acid if the anion group is HaOz-. CO2, H2O vapor, ammonia, NOx or even trace quantities of hydrogen cyanide can exist if the reductants are cyanoguanidines, dicyandiamide or nitroguanidine (Zhang & Tan et al., 1997Zhang, J. B., Tan, Z. C., Meng, S. H., Li, S. H., Zhang, L. M., Heat capacity and thermal decomposition of dicyandiamide. Thermochimica Acta, 307, p. 11 (1997).; Block, 1953Block, J. L., The thermal decomposition of nitroguanidine. NAVORD Report 2705 (1953).). Reductants like metal powders will be oxidized into oxides and become the solid phase in the aerosol.
Next a decomposition reaction starts in the fire zone after redox of the oxidant whose anion group is NO3-decomposition reaction temperature depends on particle size and the element property, e.g., sodium carbonate decomposes at temperature above 1397 ºC (Wanigarathne et al., 2000Wanigarathne, P. C., Krauss, R. H., Chelliah, R. J., Davis, R. J., Fire Suppression by Particles Containing Metallic Compounds. HOTWC, Albuquerque, NM (2000).).
Then a fire supporting radical recombination process starts.
Depletion of hydrogen (Birchall, 1970; Williams & Fleming, 1999):
Depletion of hydroxyl:
Depletion of oxygen:
Those recombining reactions can be either heterogeneous or homogeneous because volatilization of small particles (< 5 μm) in a flame is possible. When M is the element potassium, a third party acting like a catalyst is involved in the recombination reaction (Birchall, 1970Birchall, J. D., On the mechanism of flame inhibition by alkaline metal salts. Combust. Flame., 14, p. 85 (1970).).
For the anion group HaOz-, the fire supporting radical recombination processes are almost same. (Birchall, 1970Birchall, J. D., On the mechanism of flame inhibition by alkaline metal salts. Combust. Flame., 14, p. 85 (1970).):
Depletion of hydrogen:
Depletion of hydroxyl:
Depletion of oxygen:
And for an oxidant with the anion group HaOz-, Ha radicals also participate in hydrogen and hydroxyl radical recombination processes (Sheison et al., 1989Sheinson, R. S., Penner-Hahn, J. E. and Indritz, D., The physical and chemical action of fire suppressants. Fire Safety Journal, 15(6), 437-450 (1989).):
When the radical recombination chain reaction terminates, halogen radicals are converted into halides with the least harm to the environment:
From the above reactions, the hot aerosol particles produce crucial alkaline or alkaline earth metal oxides and hydroxides through the aerosol forming agent combustion to recombine with fire supporting radicals continuously. The whole process continues until most fire supporting radicals are recombined and thus the fire is extinguished (Yang & Fu, 2000Yang, R. J., Properties and fire extinguishment mechanism of pyrotechnic aerosol. Initiators and Pyrotechnics, 4(85), 1-6 (2000).).
Since the cation and anion groups in the oxidants can be different, the fire extinguishing efficiency varies in different aerosol forming agents. Basically, the fire supporting radical binding affinity of different metal element compounds with the same anion group follow the sequence: K> Na > Sr > Ca> Mg> Al. Salts composed of different anions and the same metal cation have fire extinguishing efficiencies following the sequence: oxides> cyanides> iodides> bromides> chlorides> sulphates> phosphates (Guo & Yue, 2008Guo, H. B., Yue, D. K., Aerosol Fire Extinguishing Technology. Chemical Industry Press, Beijing (2008).).
Sulphates and phosphates are used mainly in cold aerosol and dry powder technology, and cyanides can be used as an additive in the hot aerosol forming agent to enhance fire extinguishing efficiency. However, potassium hexacyanoferrate (II), which generates potassium cyanide when it decomposes thermally (Kunrath et al., 1978Kunrath, J. I., Müller, C. S. and Frank, E., Thermal decomposition of potassium hexacyanoferrate(II) trihydrate. J. Therm. Anal. Calorim., 14(3), 253-264 (1978).), is rarely used in hot aerosol fire suppression technology even though its fire extinguishing efficiency is high (Korobeinichev et al., 2010Korobeinichev, O. P., Shmakov, A. G., Chernov, A. A., Bol'shova, T. A., Shvartsberg, V. M., Kutsenogii, K. P. and Makarov, V. I., Fire suppression by aerosols of aqueous solutions of salts. Combustion, Explosion, and Shock Waves, 46(1), 16-20 (2010).).
HAZARDS CAUSED BY HOT AEROSOL EXTINGUISHANTS
Since hot aerosol particles have sizes between 10-9 to 10-7 m, so they are called PM 2.5 particulates whose aerodynamic diameters (dp) are no larger than 2.5 μm. Akeredolu pointed out that fine particles (dp < 2.5μm) can deposit on the bronchi walls in the bronchial tree (Akeredolu, 1996Akeredolu, F. A., Environmental Engineering Notebook. Department of Chemical Engineering, Obafemi Awolowo University, Ile-Ife, Nigeria (1996).), causing chronic respiratory diseases and acute respiratory diseases (Jimoda, 2012Jimoda, L. A., Effects of Particulate Matter on Human Health, the Ecosystem, Climate and Materials: A Review. Series: Working Living Environ. Prot., 9(1), p. 27 (2012).). Figure 4 shows the penetration of different size particulates in the human lung.
Moreover, in the gas phase of the hot aerosol, there can be NOx, CO, CO2 and hydrocarbons (Zhu et al., 2013Zhu, C. G., Wang, J., Xie, W. X., Zheng, T. T. and Lv, C., Improving strontium nitrate-based extinguishing aerosol by magnesium powder. Fire Technol., 1-11, online: <http://link.springer.com/article/10. 1007%2Fs10694-013-0361-6> (2013).
http://link.springer.com/article/10. 100...
). Especially NOx and CO can cause susceptibility to respiratory pathogens, reduction in the ability of the circulatory system to transport O2, impairment of performance on tasks requiring vigilance and aggravation of cardiovascular diseases (Stern et al., 1984Stern, A. C., Turner, D. B., Boubel, R. W., Fundamental of Air Pollution. Academic Press, London (1984).), so strict regulations have been made in countries like the U.S., Russia, Australia and China to limit the maximum concentration of hazardous gases released by hot aerosol forming agent. Another problem caused by hot aerosol is the reduction in visibility in the fire zone since both fine particulates and gases in the hot aerosol can absorb and scatter light. Ying et al. (2004)Ying. Q., Mysliwiec. M., Kleeman, M. J., Source apportionment of visibility impairment using a three-dimensional source-oriented air quality model. Environ Sci. Technol., 38, p. 1089 (2004). reported a visual index, deciview (dV) as:
where 0.01 km-1 is the approximate extinction coefficient due to Rayleigh scattering in a pristine atmosphere and bext is the extinction coefficient that controls the change in the appearance of objects with distance. bext varies with the wavelength. The deciview scale is near zero for a near pristine atmosphere and increases as visibility impairment increases. Also, Ying et al. (2004)Ying. Q., Mysliwiec. M., Kleeman, M. J., Source apportionment of visibility impairment using a three-dimensional source-oriented air quality model. Environ Sci. Technol., 38, p. 1089 (2004). estimated the aerosol extinction coefficient ba,ext and the aerosol scattering coefficient ba,scat for the dimensionless scattering and extinction coefficients for the homogeneous and core-and-shell configuration as:
where i refers to the emission source for primary particles, j refers to size, n is the number of primary particle source categories, m is the number of particles of different sizes. N and r are the number concentration and radius of the particles, respectively. Qs and Qe are the dimensionless scattering and extinction coefficients, respectively (Jimoda, 2012Jimoda, L. A., Effects of Particulate Matter on Human Health, the Ecosystem, Climate and Materials: A Review. Series: Working Living Environ. Prot., 9(1), p. 27 (2012).).
Besides adverse effects on human health and impairment to environment visibility, large numbers of corrosive particles such as oxides, hydroxides and carbonates of alkaline metals together with some acidic NOx and CO2 gases present in the hot aerosol can damage delicate electronics and electrical equipment or treasured archives and antiques with the existence of water droplets (Zhu & Wang et al., 2013Zhu, C. G., Wang, J., Xie, W. X., Zheng, T. T. and Lv, C., Improving strontium nitrate-based extinguishing aerosol by magnesium powder. Fire Technol., 1-11, online: <http://link.springer.com/article/10. 1007%2Fs10694-013-0361-6> (2013).
http://link.springer.com/article/10. 100...
), but this problem can be largely solved by substituting alkaline metals with alkaline earth metals like magnesium and strontium since their oxides, hydroxides and carbonates are insoluble. Meanwhile, high temperature aerosol produced by redox process is inevitable and hot aerosol sometimes can reach 1200 K or above at the canister nozzle (Zhu et al., 2013Zhu, C. G., Wang, J., Xie, W. X., Zheng, T. T. and Lv, C., Improving strontium nitrate-based extinguishing aerosol by magnesium powder. Fire Technol., 1-11, online: <http://link.springer.com/article/10. 1007%2Fs10694-013-0361-6> (2013).
http://link.springer.com/article/10. 100...
), so hot aerosol may bring potential risks of a secondary fire. Coolants must be installed in the canister together with hot aerosol forming agent. Also, a hot aerosol shockwave happens due to release of large quantities of hot gases to the fire zone from a narrow canister space. This blast may damage the infrastructure in a room, but can also be helpful for sweeping off the fire. In countries which have standards for hot aerosol fire suppression unit installation, infrastructure damage caused by a hot aerosol blast has not been reported.
Finally, ALT (Atmospheric Lifetime), ODP and GWP values of hot aerosol forming agents are negligible (Kwon & Kim, 2013Kwon, K. and Kim, Y., Extinction effectiveness of pyrogenic condensed-aerosols extinguishing system. Korean J. Chem. Eng., 30(12), 2254-2258 (2013).).
INGREDIENTS OF HOT AEROSOL EXTINGUISHING AGENTS
In chosing the major components for the oxidants and reductants in hot aerosol forming agents, deliquescence and hydration degree of the oxidants and reductants matter first. In addition, in the choice of reductants, the quantity and species of gases generated by the reductant after redox also matter. Additives are also important in manufacturing, storage and fire extinguishing performance of hot aerosol forming agents. For example, stearates are anti-deliquescence additives, and ammonium nitrate can be added as a minor oxidant and a redox booster, etc. Binders like epoxy, melamine, polysaccharide, phenol aldehyde or rubbers, etc. shape the hot aerosol forming agent into a firm and hard solid.
Choice of Oxidants in Hot Aerosol Forming Agents
As mentioned above, a good oxidant should not be deliquescent and contain no or few hydrates. After redox, the aerosol particle sizes, which are important for fire extinguishing, depend on the oxidant species.
Unfortunately, many oxidants like nitrates and halogenic acid salts are deliquescent and most of them contain hydrates, which make them hard to be ignited. However, less deliquescent or anhydrous oxidants can still be found.
For nitrates, Group IA element salts are all anhydrous and, for Group IIA elements, anhydrous nitrates of Be, Mg, Ca, Sr and Ba can be prepared.
However, Be(NO3)2 and Ba(NO3)2 are very toxic, so nitrates of Mg, Ca and Sr are left for consideration. The stability sequence for anhydrous nitrates of Mg, Ca and Sr is Mg(NO3)2< Ca(NO3)2< Sr(NO3)2 and the deliquescence tendency sequence is Mg(NO3)2> Ca(NO3)2> Sr(NO3)2; as a result, strontium nitrate is superior. As for Group IIIA elements, anhydrous aluminum nitrate is very unstable, and its non-anhydrous form is nonahydrated. Nitrates of the rest of the Group IIIA elements are either toxic or highly hydrated, so they are not ideal oxidants. The nitrate of a Group IVA element is Pb(NO3)2 and it is toxic. The nitrate of Group VA element is Bi(NO3)3, which is a pentahydrate, not ideal for combustion.
Most nitrates of transition metal elements are hydrated and toxic and their anhydrous nitrates are very unstable and deliquescent, so they are not suitable to be major oxidants. But some nitrates of transition elements like zinc and copper nitrate can be additives in hot aerosol forming agents to enhance the fire extinguishing efficiency (Zhou, 2013Zhu, C. G., Wang, J., Xie, W. X., Zheng, T. T. and Lv, C., Improving strontium nitrate-based extinguishing aerosol by magnesium powder. Fire Technol., 1-11, online: <http://link.springer.com/article/10. 1007%2Fs10694-013-0361-6> (2013).
http://link.springer.com/article/10. 100...
; Ji & Wei, 2013Ji, T. and Wei, T., Fire extinguishing composition of copper salts. WO2013023576 A1 (2013).).
Back to Group IA elements, for fire extinguishing efficiency (See Figure 5) the sequence is Cs> Rb> K> Na> Li (Agafonov et al., 2004Agafonov, V. V., Kopylov, S. N., Sychev, A. V., Uglov, V. A., Zhyganov, D. B., The mechanism of fire suppression by condensed aerosols. Proceedings of the 15th HOTWC, Albuquerque, NM (2004).; Williams & Fleming, 1999Williams, B. A., Fleming, J. W., Suppression Mecha- nisms of Alkaline Metal Compounds. HOTWC, Albuquerque, NM (1999).), and KNO3 is a common chemical which only becomes hygroscopic at 95% relative humidity (Schönherr, 2002Schönherr, J., Foliar Nutrition Using Inorganic Salts: Laws of Cuticular Penetration. ISHS Acta Horticulturae 594, Merano, Italy (2002).), so it is the best oxidant for hot aerosol forming agents, and still being widely used in hot aerosol fire extinguishing technology.
Comparison of alkaline metal aerosol influence on hydrogen concentration in a flame of a mixture of hydrogen and oxygen.
The halogenic acid salts, perchlorates, perbromates, periodates or chlorates, bromates and iodates of Group IA elements are all anhydrous but deliquescent and highly reactive. Aerosols of MxHa are less efficient in fire extinguishing than MxO (Birchall, 1970Birchall, J. D., On the mechanism of flame inhibition by alkaline metal salts. Combust. Flame., 14, p. 85 (1970).), so halogenic acid salts are less popular than nitrates as oxidants in hot aerosol forming agents, but still some hot aerosol forming agents contain perchlorates as auxiliary or minor oxidants. Most halogenic acid salts of Group IIA, IIIA or transition metals are either highly hydrated or hygroscopic, and they are relatively expensive, so they are seldom used in hot aerosol fire extinguishing technology except for being used as auxiliary additives. Common oxidants used are listed in Table 2.
Choice of Reductants in Hot Aerosol Forming Agent
Hot aerosol fire extinguishing technology is derived from pyrotechnics. Fuels used in pyrotechnics like metals, carbohydrates or carbons are also used as reductants in hot aerosol forming agents. A good reductant in hot aerosol forming agents should be little or non-toxic, reactive and cheap, and it should produce incombustible gases as much as possible, because incombustible gases produced by reductants are important for carrying the aerosol particles and also help fire killing. Such reductants include carbohydrates like lactose, sucrose and derivatives of cellulose, or charcoal and carbon black. Derivatives of guanidines such as dicyanamide, nitroguanidine are also good reductants, which can produce large quantities of nitrogen when combusted. Nitrogen gas is clean and good for fire extinguishing (Petronella & Leenders, 2003Petronella, A., Leenders, M., Gas-generating preparation and use thereof in an air bag. EP 0844223 B1 (2003).), so derivatives of guanidine are often chosen as reductants in hot aerosol forming agents, but hydrogen cyanide gas can be formed during decomposition of cyanoguanidine (Zhang et al., 1997Zhang, J. B., Tan, Z. C., Meng, S. H., Li, S. H., Zhang, L. M., Heat capacity and thermal decomposition of dicyandiamide. Thermochimica Acta, 307, p. 11 (1997).).
Synthetic reductants like derivatives of tetrazoles, which can produce large quantities of nitrogen gas, can be considered as well. Especially 5-amino tetrazole and guanidinium azotetrazolate can be used since they are relatively stable if cautions are taken (Wang et al., 2010Wang, Q., Li, J. Z., Yu, H. J., Fu, X. L., Fan, X. Z. and Ji, P. Y., Review on azotetrazolate nonmetal salts. chinese J. Energ. Mater., 5, 592-598 (2010).). However, derivatives of tetrazoles are still seldom used as reductants in hot aerosol forming agents due to strict safety issues.
Metal powders can be used as reductants, too. Sometimes binders or polymers like polytetrafluoroethylene, dibutyl-phthalate, or dioctyl sebacate, together with thermoplastic formaldehyde and phenol polycondensate, can also be reductants (Denisyuk et al., 2003Denisyuk, A. P., Michalev, B. D., Rusin, L., Germanovich, S. Y., Pyrotechnical aerosol-forming fire-extinguishing composite and a method of its production. EP 1341587 A2, (2003).).
Redox processes of hot aerosol forming agents are highly exothermic and raise the risk of secondary fire. By changing reductants, a relatively low combustion heat between 59.8-143.5 J/g aerosol forming agent (as compared to 205.7 J/g aerosol forming agent for the others) is achieved by using cyanuric acid, barbituric acid or hydroxyacetic acid salts of Group IA or IIA elements as reductants (Denisyuk et al., 2003Denisyuk, A. P., Michalev, B. D., Rusin, L., Germanovich, S. Y., Pyrotechnical aerosol-forming fire-extinguishing composite and a method of its production. EP 1341587 A2, (2003).). Common reductants used are listed in Table 3.
Choice of Binders and Additives in Hot Aerosol Forming Agents
Usually, binders can be cellulose derivatives, cellulose ether, a gum, a gel or polyvinyl compounds. Epoxy and phenol aldehyde are two of the most common binders in hot aerosol forming agents; others can be nitrocellulose, hydroxyl-terminated polybutadiene, fluoroplastics, polyethylene, melamine and iditol, etc.
The functions of additives include the stabilization of redox process and enhancing the storage life of hot aerosol forming agents. Additives like carbonates of Group IA and IIA elements or magnesium oxide can slow down and smooth the redox rate, and oxides of transition elements like Fe, Cu, Ni, Mn and Cr can be catalysts for reducing carbon and nitrogen mono oxides in the aerosol gas phase (Zhang et al., 2013Zhang, S. R., Shan, J. J., Zhu, Y. Nguyen, L., Huang, W., Yoshida, H., Takeda, S. and Tao, F., Restructuring transition metal oxide nanorods for 100% selectivity in reduction of nitric oxide with carbon monoxide. Nano Letters, 13(7), 3310-3314 (2013).). For storage, stearates of Group IA and IIA elements can keep the hot aerosol forming agent block dry and prevent it from growing mold.
TYPES AND CHARACTERISTICS OF HOT AEROSOL EXTINGUISHING AGENTS AND DEVICES
Through 40 years of development, hot aerosol extinguishing technology has reached the third generation, which is S-type or G3 hot aerosol extinguishing agent in which the major oxidant is strontium or magnesium salts. Historically, the first generation of hot aerosol fire extinguishing technology is called G1 fire extinguishing technology, which was invented in Tianjin, China, in the 1960s (Liu et al., 2005Liu, Y. H., Jin, H. B., Ye, H. L., The developing history and current status of domestic fire extinguishing agents. Fire Techni. & Products Info., 1, p. 82 (2005).). The second generation or G2 hot aerosol fire extinguishing technology, whose major oxidant is potassium salts, is called K-type hot aerosol extinguishing technology.
Differences and Characteristics of G1, G2 & G3 Aerosol Extinguishing Agents
The extinguishant of G1 fire extinguishing technology contains mostly potassium nitrate with a mass ratio of more than 50%, similar to K-type hot aerosol extinguishing agents.
Different from second and third generation of hot aerosol fire extinguishing technology, the first generation of hot aerosol fire extinguishing technology aims at extinguishing in-tank liquid fires such as oil, alcohol and ketone flames, etc., and the aerosol particles are not fully generated in the extinguisher canister. About 85% of the aerosol generated by G1 fire extinguishing agent are steam and inert gases composed of N2 and CO2. Generally, this technology is deemed as the prototype of today's hot aerosol fire extinguishing technology. The G2 and G3 hot aerosol forming agents are compressed or bound into a cylindrical block during manufacturing. During the redox process, aerosols are fully generated in the canister, and then be pushed out by gases generated in the canister.
Compared to traditional Halon extinguishing agents, G2 or K-type extinguishing agents have a 4-5-fold higher fire extinguishing efficiency (Guo & Yue, 2008Guo, H. B., Yue, D. K., Aerosol Fire Extinguishing Technology. Chemical Industry Press, Beijing (2008).). Generally, the lowest concentration for G2 (K-type) hot aerosol forming agents required to extinguish a 10 cm diameter heptane pan ranges from 30-200 g/m3 (Guo & Yue, 2008Guo, H. B., Yue, D. K., Aerosol Fire Extinguishing Technology. Chemical Industry Press, Beijing (2008).). G3 (S-type) requires at least 100 g/m3, so K-type hot aerosol extinguishing agents have higher fire extinguishing efficiencies compared to the S-type. This is because Group IA elements have a higher reactivity with fire supporting radicals compared to Group IIA elements and oxides of Group IA elements have lower evaporation and dissociation temperatures, which make them become aerosols more easily and more readily bind fire supporting radicals compared to oxides of Group IIA elements.
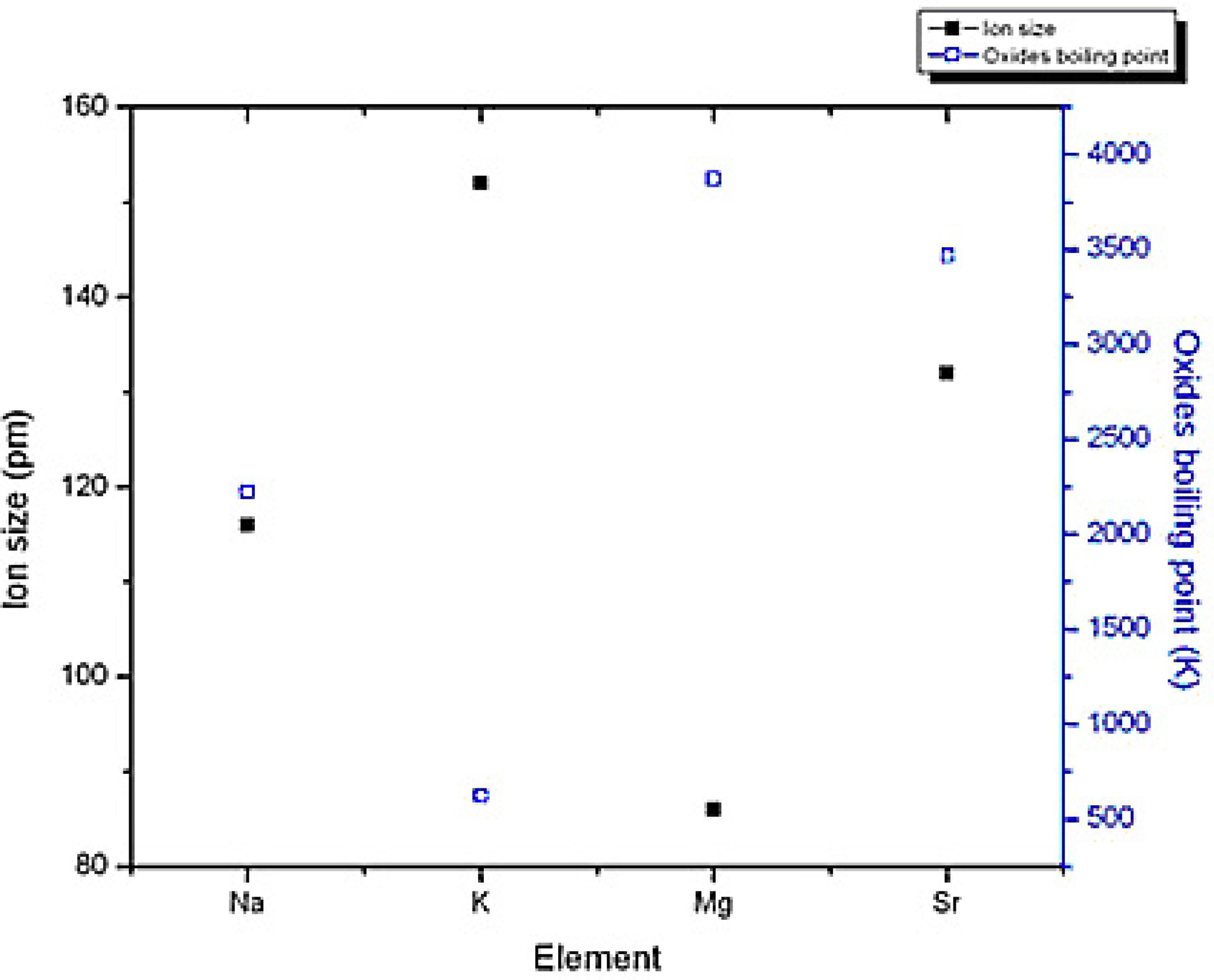
Granularity affects the fire extinguishing efficiency of K and S-type aerosol forming agents greatly and it governs aerosol particle diffusion, aggregation and settlement. When fire extinguishing particles like potassium carbonates and oxides have diameters less than 10 μm, they will evaporate in the flame and then the homogeneous fire inhibition process begins (Baratov et al., 1988Baratov, A. N., Baratova, N. A., Myshak, Y. A., Practice of use of aerosol extinguishing agents obtained by combustion of propellants. AOFST Symposiums 3 (1988).); such fire extinguishing efficiency is very high. Oxides and carbonates of magnesium and strontium (generated from S-type) have smaller diameters, higher densities and evaporation temperatures (see Figure 6).
Ion size (filled square) and oxide boiling point (hollow square) of K-type (Na, K) and S-type (Mg, Sr) elements.
Higher evaporation temperatures of S-type aerosol particles restrict their participation in homogeneous fire inhibition process and reduce their fire extinguishing efficiency.
Moreover, according to the well-known fractal aggregation formula (see Equation (4)) (Meakin, 1983Meakin, P., The Vold-Sutherland and Eden models of cluster formation. J. Colloid Interface Sci., 96(2), p.415-424 (1983).; Jullien & Botet, 1987Jullien, R., Botet, R., Aggregation and Fractal Aggregates. World Scientific, Singapore (1987).), the smaller the diameter dp, the higher the number of particles that aggregate, so S-type aerosol particles aggregate faster than K-type aerosols.
where n is the number of primary particles in an aggregate, dp the diameter of primary particles, Rg the radius of gyration of an aggregate, Df the fractal dimension, and kf the fractal prefactor.
S-type aerosol aggregates with larger volume and heavier mass settle faster and thus suspend for less time in a fire zone to kill the fire according to Stokes' law:
where v is the terminal velocity of spherical particles, r the radius of particles, ρ the density of particles, ρ0 the density of the disperse medium, g the gravitational acceleration and η the dynamic viscosity of the disperse medium. From Einstein's diffusion coefficient equation (Equation (6)), S-type aerosol particles consequently have lower diffusion coefficients than K-type particles. A low diffusion coefficient means that particles cannot spread faster in a confined room to kill the fire in a short time.
where D is the diffusion coefficient, R the ideal gas constant, T the temperature, NA the Avogadro number, η the dynamic viscosity of the disperse medium and r the radius of the particles.
After combustion, the gas to solid mass ratio in K or S-type aerosols ranges from 1:4 to 1: 1.14 and depends on the aerosol forming agents. In K-type aerosol, the main components in the solid phase are bicarbonates and carbonates of relevant Group IA elements, and in S-type, they are oxides, carbonates and hydroxides of Group IIA elements (Fu et al., 2003Fu, Z. M., Huang, J. Y., Yang, R. J., The study of thermal decomposition and combustible characteristic of solid micro particle aerosol fire extinguishant. Fire Techni. & Products Info., 4, p. 46 (2003).). S-type agents precipitates have higher electrical resistivity, which can reach 1012 Ω, since the precipitates are not deliquescent or soluble, while the surface resistivity of K-type precipitates normally does not exceed 105 Ω (Liu & Dong, 2004Liu, J. X., Dong, H. B., Insulation test on the after-discharge precipitate of different kinds of Aerosol. Fire Sci. & Technol., 23(2), p. 168 (2004).). From industrial standards GA 499.1-2004 and GA 499.1-2010, the resistivity of a PVC plate surface covered by aerosol precipitates of the S-type should exceed at least 20 MΩ after 30 min at 35 ºC and 95% humidity.
G1 Fire Extinguishing Devices
G1 hot aerosol fire extinguishing devices can be classified into in-tank or out-tank types. For both in-tank and out-tank configurations, the sprinkler head can be either upward or downward. For diesel, crude oil, heavy oils and petroleum, the in-tank configuration is adopted (Duan et al., 2007Duan, X. L., Zhang, L. L., Zhou, J. D., The application of tank smoke fire extinguishing systems. Xinjiang Petro Sci. & Technol., 17(1), p. 69 (2007).), and for alcohols and ketones, the out-tank configuration is used. See Figure 7 and 8.
Out tank G1 extinguishing device. (Reproduced with permission from Duan et al., 2007Duan, X. L., Zhang, L. L., Zhou, J. D., The application of tank smoke fire extinguishing systems. Xinjiang Petro Sci. & Technol., 17(1), p. 69 (2007).).
In tank G1 extinguishing device. (Reproduced with permission from Duan et al., 2007Duan, X. L., Zhang, L. L., Zhou, J. D., The application of tank smoke fire extinguishing systems. Xinjiang Petro Sci. & Technol., 17(1), p. 69 (2007).).
G1 hot aerosol fire extinguishing technology is convenient in terms of installation and cheap in cost, but the aerosol forming agent storage stability depends on tank sealing. Advanced types developed from G1 became second and third generation (G2 & G3) aerosol fire extinguishing technologies, which have a wide range of applications besides oil tank fire extinguishing.
G2 & G3 Hot Aerosol Fire Extinguishing Devices
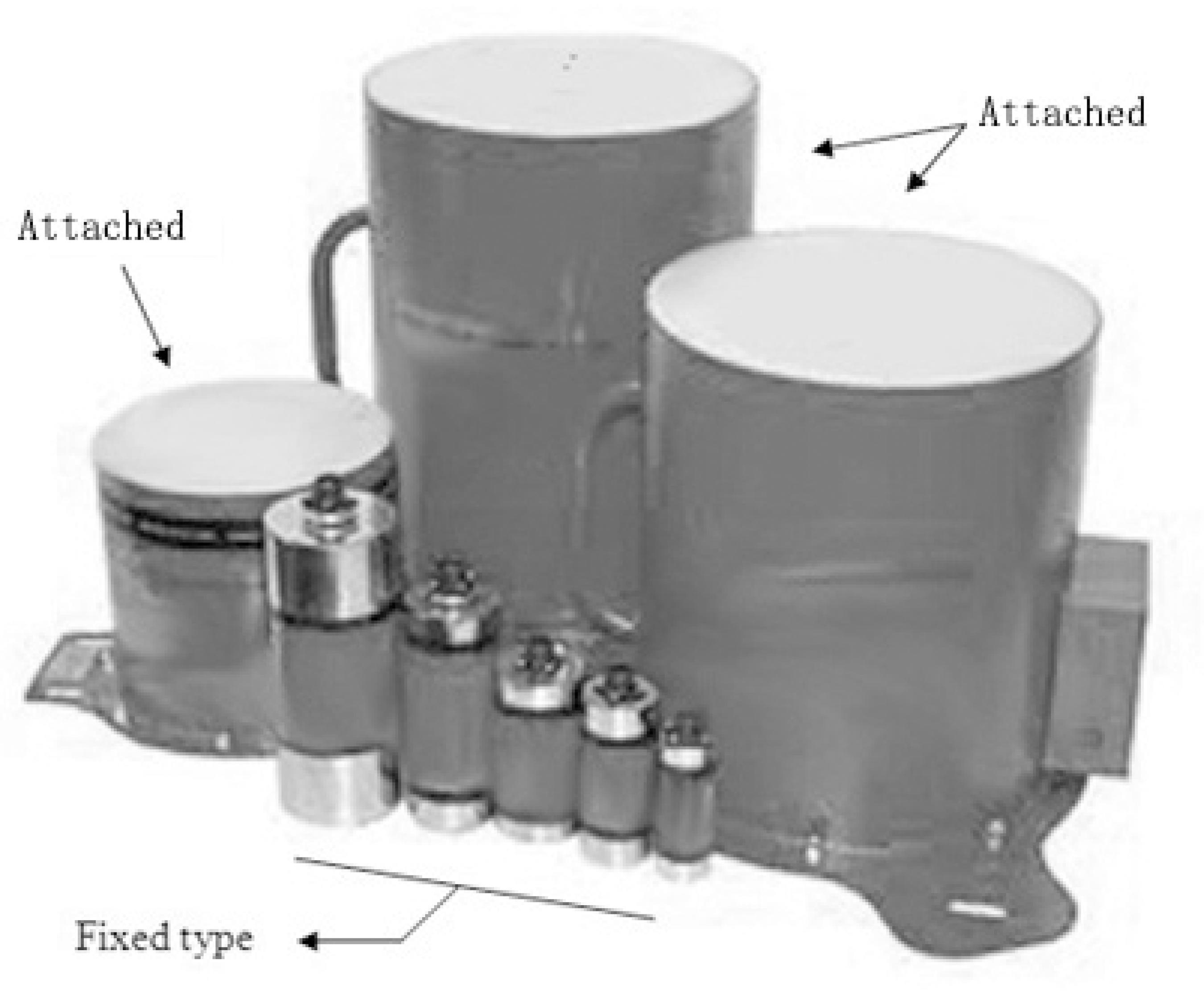
Basically, G2 and G3 fire extinguishing technologies have fixed/attached and portable canister structures. Fixed/attached types (see Figure 9) usually have regular cylinderal canisters of different sizes and the configuration of such canisters can be seen in Figure 10. Portable types include hand-held and grenade configurations. Fixed/attached types are designed for fire extinguishing in a large space like a warehouse, data room, etc. The hand-held type is portable for extinguishing small scale fires close to an operator. The grenade type can enable an operator to throw an aerosol grenade into the fire source from a far distance to extinguish fires and reduces the risks of injury and death of the operator.
Fixed/attached hot aerosol fire extinguishing devices. (Reproduced with permission from Brochure Pyrogen, 2010Brochure Pyrogen, 2010.).
General configuration of hot aerosol canister. (Reproduced with permission from Brochure Pyrogen, 2010Brochure Pyrogen, 2010.).
Portable or hand-held hot aerosol fire suppression devices have the configuration shown in Figure 11. In order to ensure safe holding, an insulating layer is necessary for preventing heat transfer to the outer surface of the canister. The coolant in the canister near to the nozzle can also reduce the risk of being burnt by hot aerosol.
Hand-held hot aerosol device and mounting bracket for storage. (http://www.google.com/patents/EP2441495A1?cl=en).
Usually, the aerosol discharge time of hand-held devices is longer than 15 s and fixed/attached devices have a discharge time depending on the device dimensions. In hand-held devices, the discharge time can be elongated by mixing burning retardants with aerosol forming agents so that the operator has enough time to aim at the fire source before all aerosols are discharged.
For hot aerosol fire extinguishing grenades, a pull ring attached to a pin holds the striker, which hits at a delay tube cartridge; this cartridge provides 6 to 10 s for the operator to throw the grenade into fire source before the ignition of the aerosol forming agents. See Figure 12.
Aerosol grenade configuration (reproduced with permission from Berezovsky and Joukov, 1999Berezovsky, J., Joukov, S., Pyrogen fire suppression grenades. Halon Options Technical Work-ing Conference Proceedings, Albuquerque, NM, US (1999).).
Table 4 compares the differences in G1, G2 and G3 hot aerosol extinguishing technology.
SPECIFICATIONS AND TECHNICAL TERMS OF G1, G2 & G3 HOT AEROSOL FORMING AGENTS
The technical parameters of G1 hot aerosol fire extinguishing agents are different according to in-tank or out-tank type. See Table 5.
G1 fire extinguishing agent technical parameters. (Reproduced with permision from Duan et al, 2007Duan, X. L., Zhang, L. L., Zhou, J. D., The application of tank smoke fire extinguishing systems. Xinjiang Petro Sci. & Technol., 17(1), p. 69 (2007).).
Technical terms for G2 and G3 hot aerosol forming agents are classified into pre-extinguishing and post-extinguishing ones to control the hot aerosol forming agents' performances. See Table 6.
Technical terms for G2 & G3 hot aerosol forming agents* * Testing conditions and evaluation methods are regulated by GA 499.1-2004 and GA 499.1-2010 .
Most pre-fire extinguishing terms are the same for both G2 and G3 hot aerosol forming agents, but different requirements for G2 and G3 can be found in post-fire extinguishing terms, such as effective aerosol concentration for fire extinguishing, precipitate insulation level, residues quantity, etc. G3 hot aerosol forming agents are required to be applicable in places with sensitive electrical and electronics, different from where G2 hot aerosol forming agents are commonly employed. Also, the technical terms of G1 hot aerosol forming agents are different from G2 and G3, due to their specific application in oil tank fire extinguishing. Hence, the technical terms strictly regulate quality control of hot aerosol forming agent manufacturing according to their application fields.
DESIGN AND APPLICATIONS OF HOT AEROSOL EXTINGUISHANT
G1 Hot Aerosol System Design
The fire extinguishing dosage for G1 hot aerosol fire extinguishing agents can be calculated according to Eq. (7):
where M is the dosage (kg); A is the cross-sectional area of the tank (m2); Re is the effective forming agent dosage per storage area of tank (kg/m2) and K is the compensation coefficient.
Here Re and K depend on tank type and diameter. See Table 7.
Re and K value for G1 extinguishing system design. (Reproduced with permission from Duan et al., 2007).
G2 and G3 Hot Aerosol System Design
A modern aerosol fire extinguishing system is composed of fire sensing, alarm, electrical ignition, and discharge units, aiming at fire extinguishing of multiple classes and is designed to be more portable and convenient in assembling, more stable for longtime standby and integrated into a local fire extinguishing network. See Figure 13.
The area or volume protected by hot aerosol extinguishant should be less than 500 m2 or 2000 m3. The working temperature for G2 and G3 hot aerosol forming agents ranges from -20~55 ºC with relative humidity less than 90%, and the protected room should have an opening density less than 0.6%. If the opening density is larger than 0.6%, then blocking devices are needed to ensure a successful fire extinguish (DB 61/368-2005).
For K-type and S-type fire extinguishing agents, dosage for fire extinguishing in a confined area is determined by Eq. (8):
where W is the dosage (kg) and C2 is the effective fire extinguishing concentration (kg/m3) of aerosol forming agent, V is the volume under protection (m3) and Kv is the volume correction coefficient. Kv and C2 depend on fire types and the volume protected. See Table 8.
Areas Applying Hot Aerosol Fire Suppression Technology
Compared to other fire extinguishants, hot aerosol fire extinguishing agents have unique advantages, seen in Table 9. Especially, G2 and G3 hot aerosol fire extinguishing systems have been widely applied in places like communication basements, data analysis rooms, and transportation engine compartments, etc.
But because aerosol particles can have hazardous effects on human health, applications of hot aerosol technology are usually limited to areas like engine cabins, gas turbine cabins, machinery cabins, warehouses, electrical cabins, cable tunnels and computer data processing rooms, etc. where human activities are rare (Zhao et al., 2004Zhao, Y., Zhang, J. W., Jia, L. W., Aerosol fire-extinguishing agent. Tianjin Chemical Industry, 18(2), p. 7 (2004).). See Table 10.
COOLING AGENT FOR HOT AEROSOL FIRE EXTINGUISHERS
Hot aerosol forming agents generate a large amount of heat during redox and may cause secondary fire risks. The temperature of a hot aerosol discharge can vary from 600-1200 degrees Celsius at 1 cm distance from the nozzle of the canister (Song, 2003Song, R. G., Cooling technique of hot aerosol. Fire Techni. & Products Info., 10, p. 17 (2003).), so it is necessary to place coolant inside the extinguisher device.
Currently, there are two typical ways to place coolants. One is to mix coolants with aerosol forming agent ingredients (Qiao et al., 2001Qiao, H. T., Yang, R. J., Li, X. D., Research on lowering the flame temperature of aerosol-generating agent using additives. Chinese J. Explo. & Propell., 2, p. 42 (2001).), and the other more common way is to place perforated metal plates, a beehive-like coolant block or granulized coolant pellets between the aerosol forming agents and the canister nozzle, separating coolants from the aerosol forming agent.
Cooling mechanisms can be physical or chemical. Physical methods cool down hot aerosol temperatures through pure heat absorption by perforated metal plates or metal bars (usually copper bars), but the physical cooling efficiency is low since metals have limited heat capacities. Chemical cooling methods involve the decomposition and phase change of coolants, which adsorbs a large amount of heat. Those coolants can be simple natural material like water containing minerals or simple chemicals like urea or aluminum hydroxide (Qiao et al., 2001Qiao, H. T., Yang, R. J., Li, X. D., Research on lowering the flame temperature of aerosol-generating agent using additives. Chinese J. Explo. & Propell., 2, p. 42 (2001).) or synthetic composites which are thermally unstable. See Table 11.
Coolant packing should have enough porosity to avoid blocking hot aerosol. If blocking occurs, explosion of the extinguisher due to a sudden pressure accumulation in the canister can happen.
For coolants mixed with aerosol forming agent ingredients, if an excessive amount of coolant is added, it may fail to ignite the hot aerosol forming agent. By mixing coolants, the redox rate of the hot aerosol forming agent will be decreased, so mixing is rarely adopted (Hu, 2003Hu, J. G., The cooling technology of hot gas dispersoid. Initiators & Pyrotechnics, 4, p. 33-33 (2003).).
CONCLUSION
In this review, hot aerosol fire extinguishing mechanisms, agent ingredients, extinguisher devices, technical terms, applications and aerosol forming agent coolants are reviewed. Characteristics of hot aerosol fire extinguishing technology were compared with other common fire extinguishing technologies such as cold aerosol, inert gas, Halon and water mist. Basically, hot aerosol fire extinguishing agents have higher fire extinguishing efficiency than inert gas, Halon and water mist fire suppression technology with negligible ODP and GWP values, but health and visible impairment caused by aerosol particles and gases, together with the large amount of heat released during aerosol discharge, are problematic. The ingredients in hot aerosol forming agents are mainly oxidants such as nitrates or halogenic acid salts and reductants like carbohydrates, derivatives of guanidines and cellulose, carbon blacks and metallic powders. Additives in hot aerosol forming agents include binders, combustion adjusters, desiccants and surfactants. The choice of oxidants and reductants determines the type of aerosol forming agent and fire extinguishing behavior. Oxidants containing Group I elements are K-type and oxidants containing Group II elements are S-type. For fire extinguishing efficiency, nitrates are better than halogenic acid salts. Less hydrated oxidants are better than highly hydrated ones and reductants which can produce as much inert gas and water vapor as possible are preferable.
The hot aerosol fire extinguishing process is primarily based on recombination of fire supporting radicals with oxides and halides of metal elements, together with an oxygen dilution and cooling effect caused by gases generated by aerosol forming agent combustion. Oxides are better than halides in fire extinguishing and Group I elements are better than Group II elements in recombination of fire supporting radicals, so K-type hot aerosol forming agents have higher fire extinguishing efficiency than S-type. This also reflects the smaller sizes and evaporation temperatures of K-type aerosol particles, but precipitates of S-type aerosol particles have much higher electrical resistivity which can prevent severe damage to electrical devices and electronics. For both G2 (K-type) and G3 (S-type) hot aerosol fire extinguishing devices, portable or fixed/attached structures are used. Fixed/attached hot aerosol fire extinguishing devices usually have a canister configuration. Portable types include hand-held and grenade types. For G1 (K-type) hot aerosol fire extinguishing technology, device configurations are in-tank or out-tank types. There are differences in technical terms and standards for G1, G2 and G3 hot aerosol forming agent post-extinguishing performances. Differences in their system designs are due to differences in their fire protection application and protection area characteristics such as volume, geometry or opening density. Both G2 and G3 hot aerosol fire extinguishing technologies have wide applications in many sites like engine or cargo compartments, warehouses, cable tunnels etc. On the contrary, G1 hot aerosol fire extinguishing technology is only applied for oil tank fire protection. Moreover, G2 hot aerosol fire extinguishing technology cannot be applied in places where delicate electronics, electrical devices or important archives are present. Though modern integrated hot aerosol fire extinguishing systems are highly automatic and efficient, their application is still limited to areas without human activities since hot aerosols can bring hazards to environment and human health. High temperature aerosols can be cooled chemically by phase changes and thermal decomposition of natural or synthetic coolants. The physical cooling effect is limited. Coolant packing or mixing should not cause clogging of aerosols or failure in ignition of aerosol forming agents.
NOMENCLATURE
REFERENCES
- Aerosol fire extinguishing system, Part 1: Condensed aerosol fire extinguishing device. GA 499.1-2010.
- Aerosol fire extinguishing system, Part 1: Condensed aerosol fire extinguishing device. GA 499.1-2004.
- Agafonov, V. V., Kopylov, S. N., Sychev, A. V., Uglov, V. A., Zhyganov, D. B., The mechanism of fire suppression by condensed aerosols. Proceedings of the 15th HOTWC, Albuquerque, NM (2004).
- Akeredolu, F. A., Environmental Engineering Notebook. Department of Chemical Engineering, Obafemi Awolowo University, Ile-Ife, Nigeria (1996).
- Andrzej, W. M., Tsang, W., Halon Replacements: Technology and Science. ACS Symposium Series, Washington DC, US (1997).
- Back, G., Boosinger, M., Forssell, E., An evaluation of aerosol extinguishing systems for machinery space applications. Fire Technol., 45, p. 4 (2009).
- Baratov, A. N., Baratova, N. A., Myshak, Y. A., Practice of use of aerosol extinguishing agents obtained by combustion of propellants. AOFST Symposiums 3 (1988).
- Berezovsky, J., Joukov, S., Pyrogen fire suppression grenades. Halon Options Technical Work-ing Conference Proceedings, Albuquerque, NM, US (1999).
- Birchall, J. D., On the mechanism of flame inhibition by alkaline metal salts. Combust. Flame., 14, p. 85 (1970).
- Block, J. L., The thermal decomposition of nitroguanidine. NAVORD Report 2705 (1953).
- Brochure Pyrogen, 2010.
- Chen, Z. H., Yang, R. J., An assessment approach for Halon substitute technique. Fire Safety Sci., 12(2), p. 115 (2003).
- Code for design of gas fire extinguishing systems. GB 50370-2005.
- Denisyuk, A. P., Michalev, B. D., Rusin, L., Germanovich, S. Y., Pyrotechnical aerosol-forming fire-extinguishing composite and a method of its production. EP 1341587 A2, (2003).
- Design, construction and acceptance specifications of hot aerosol automatic extinguishing system. DB 61/368-2005.
- Duan, X. L., Zhang, L. L., Zhou, J. D., The application of tank smoke fire extinguishing systems. Xinjiang Petro Sci. & Technol., 17(1), p. 69 (2007).
- Fu, Z. M., Huang, J. Y., Yang, R. J., The study of thermal decomposition and combustible characteristic of solid micro particle aerosol fire extinguishant. Fire Techni. & Products Info., 4, p. 46 (2003).
- Fu, S., Yao, H., Physical Chemistry. High Education Press, Beijing (2001).
- Guo, H. B., Hu, J. G., Zheng, Y. Y., A composition of hot aerosol extinguishing agent. CN 101376049 B (2011).
- Guo, H. B., Yue, D. K., Aerosol Fire Extinguishing Technology. Chemical Industry Press, Beijing (2008).
- Guo, H. B., Zhang, Z. F., Fire-Extinguishing Aerosol Composition for Heavy Current Electric Apparatuses. US 8097667 B2 (2012).
- Hu, J. G., The cooling technology of hot gas dispersoid. Initiators & Pyrotechnics, 4, p. 33-33 (2003).
- Hu, Y. H., Steam hot aerosol fire-extinguishing composition and application method and fire extinguishing device thereof. CN 101376049 A (2009).
- Hu, Y. H., Steam hot aerosol fire-extinguishing composite and application method and fire extinguishing device thereof. CN 101554520 B (2011).
- Jayaweera, T. M., Fisher, E. M., Fleming, J. W., Flame suppression by aerosols derived from aqueous solutions containing phosphorus. Combustion and Flame, 141(3), 308-321 (2005).
- Ji, T. and Wei, T., Fire extinguishing composition of copper salts. WO2013023576 A1 (2013).
- Jimoda, L. A., Effects of Particulate Matter on Human Health, the Ecosystem, Climate and Materials: A Review. Series: Working Living Environ. Prot., 9(1), p. 27 (2012).
- Jullien, R., Botet, R., Aggregation and Fractal Aggregates. World Scientific, Singapore (1987).
- Korobeinichev, O. P., Shmakov, A. G., Chernov, A. A., Bol'shova, T. A., Shvartsberg, V. M., Kutsenogii, K. P. and Makarov, V. I., Fire suppression by aerosols of aqueous solutions of salts. Combustion, Explosion, and Shock Waves, 46(1), 16-20 (2010).
- Korobeinichev, O. P., Shmakov, A. G., Shvartsberg, V. M., Chernov, A. A., Yakimov, S. A., Koutsenogii, K. P. and Makarov, V. I., Fire suppression by low-volatile chemically active fire suppressants using aerosol technology. Fire Safety Journal, 51, 102-109 (2012).
- Kozyrev, V. N., Yemelyanov, V. N., Sidorov, A. I. and Andreev, V. A., Aerosol-forming composition for the purpose of extinguishing fires. US 5,831,209 Washington, DC: U.S. Patent and Trademark Office (1998).
- Kunrath, J. I., Müller, C. S. and Frank, E., Thermal decomposition of potassium hexacyanoferrate(II) trihydrate. J. Therm. Anal. Calorim., 14(3), 253-264 (1978).
- Kwon, K. and Kim, Y., Extinction effectiveness of pyrogenic condensed-aerosols extinguishing system. Korean J. Chem. Eng., 30(12), 2254-2258 (2013).
- Larson, E. R., Halogenated flame extinguishantssome additional support for the physical mechanism. J. Fire Sci., 23, 93-98 (2005).
- Liu, J. X., Dong, H. B., Insulation test on the after-discharge precipitate of different kinds of Aerosol. Fire Sci. & Technol., 23(2), p. 168 (2004).
- Liu, Y. H., Jin, H. B., Ye, H. L., The developing history and current status of domestic fire extinguishing agents. Fire Techni. & Products Info., 1, p. 82 (2005).
- Meakin, P., The Vold-Sutherland and Eden models of cluster formation. J. Colloid Interface Sci., 96(2), p.415-424 (1983).
- NFPA 30: Flammable and combustible liquids code. Edition (2003).
- Pak, Z. P., Zhukov, B. P., Krivosheev, N. A., Zhegrov, E. F., Ivankov, L. D., Mikhailova, M. I., Telepchenkov, V. E., Khalilova, I. B., Rodina, N. A., Chui, G. N., Votyakov, A. G., Agafonov, D. P., Militsyn, J. A., Deruzhinsky, V. I., Aerosol-Producing Fire Extinguishant. CA 2089901 (1998).
- Petronella, A., Leenders, M., Gas-generating preparation and use thereof in an air bag. EP 0844223 B1 (2003).
- Posson, P. L, Clark, M. L., Flame Suppressant Aerosol Generate. US 8182711 B2 (2012).
- Qiao, H. T., Yang, R. J., Li, X. D., Research on lowering the flame temperature of aerosol-generating agent using additives. Chinese J. Explo. & Propell., 2, p. 42 (2001).
- Richardson, A. T., Bennett, J. M., System and Method for Suppressing Fires. US 7455120 B2 (2008).
- Scheuermann, K. P., Modigell, M., Koch, W., Roßkothen, H. J. and Schmidt, J., The use of aerosol fire extinguishing generators at rotogravure fed presses. Proc. Safety Progr., 18(1), 35-41 (1999).
- Schönherr, J., Foliar Nutrition Using Inorganic Salts: Laws of Cuticular Penetration. ISHS Acta Horticulturae 594, Merano, Italy (2002).
- Sheinson, R. S., Penner-Hahn, J. E. and Indritz, D., The physical and chemical action of fire suppressants. Fire Safety Journal, 15(6), 437-450 (1989).
- Shi, Z. R., Characteristics and applications of aerosol fire extinguishing system. Fire Techni. & Products Info., 3, p. 38 (2000).
- Song, R. G., Cooling technique of hot aerosol. Fire Techni. & Products Info., 10, p. 17 (2003).
- Stern, A. C., Turner, D. B., Boubel, R. W., Fundamental of Air Pollution. Academic Press, London (1984).
- Technical specification for smoke fire extinguishing systems. CECS 169: (2004).
- Wang, Q., Li, J. Z., Yu, H. J., Fu, X. L., Fan, X. Z. and Ji, P. Y., Review on azotetrazolate nonmetal salts. chinese J. Energ. Mater., 5, 592-598 (2010).
- Wanigarathne, P. C., Krauss, R. H., Chelliah, R. J., Davis, R. J., Fire Suppression by Particles Containing Metallic Compounds. HOTWC, Albuquerque, NM (2000).
- Williams, B. A., Fleming, J. W., Suppression Mecha- nisms of Alkaline Metal Compounds. HOTWC, Albuquerque, NM (1999).
- http://www.google.com/patents/EP2441495A1?cl=en Portable aerosol fire-fighting device. EP 2441495 A1 (2012).
» http://www.google.com/patents/EP2441495A1?cl=en - Yang, J., Fire extinguishment machanism and influence of aerosol fire extinguishment agent. J. Southern Yangtze Uni. (Nat. Sci. Ed.), 2(3), p. 303 (2009).
- Yang, R. J., Properties and fire extinguishment mechanism of pyrotechnic aerosol. Initiators and Pyrotechnics, 4(85), 1-6 (2000).
- Ying. Q., Mysliwiec. M., Kleeman, M. J., Source apportionment of visibility impairment using a three-dimensional source-oriented air quality model. Environ Sci. Technol., 38, p. 1089 (2004).
- Zhang, J. B., Tan, Z. C., Meng, S. H., Li, S. H., Zhang, L. M., Heat capacity and thermal decomposition of dicyandiamide. Thermochimica Acta, 307, p. 11 (1997).
- Zhang, S. R., Shan, J. J., Zhu, Y. Nguyen, L., Huang, W., Yoshida, H., Takeda, S. and Tao, F., Restructuring transition metal oxide nanorods for 100% selectivity in reduction of nitric oxide with carbon monoxide. Nano Letters, 13(7), 3310-3314 (2013).
- Zhao, Y., Zhang, J. W., Jia, L. W., Aerosol fire-extinguishing agent. Tianjin Chemical Industry, 18(2), p. 7 (2004).
- Zhang, Y. F., Liao, G. X., Zhou, X. M., Pan, R. M., Wang, H., The experimental study of controlling the spout temperature of HEAE. Eng. Sci., 8(3), p. 79 (2006).
- Zhu, C. G., Wang, J., Xie, W. X., Zheng, T. T. and Lv, C., Improving strontium nitrate-based extinguishing aerosol by magnesium powder. Fire Technol., 1-11, online: <http://link.springer.com/article/10. 1007%2Fs10694-013-0361-6> (2013).
» http://link.springer.com/article/10. 1007%2Fs10694-013-0361-6
Publication Dates
-
Publication in this collection
Sept 2015
History
-
Received
19 May 2014 -
Reviewed
15 Sept 2014 -
Accepted
02 Dec 2014