Abstract
Dairy industry effluent is characterized by high chemical oxygen demand (COD) and other pollution load. This study addresses the elimination of organic compounds in dairy wastewater using electrocoagulation (EC), electrooxidation (EO) and a synergistic combination of EC and EO. The removal of COD, color and turbidity was investigated at various current intensities by using different electrodes and at various electrolysis times. Results show that EC is a relatively quick process (6 min), which is very effective in removing colloidal and suspended particles, as seen in changes in turbidity and color, but it is effective to eliminate only half of the COD. However, EO reduces 40% of COD in a practical time of about 30 min. To improve the removal of COD we proceeded to combine the two processes. Thus, the coupled process eliminates 60% of COD in a practical amount of time (21 min), removes the colloidal and suspended particles and eliminates color, turbidity, phosphorus, K+ and NTK. The use of the seed toxicity test allows evaluating the quality and effectiveness of the studied effluent treatment system. Seed irrigated with raw waste or treated dairy effluent show no phytotoxicity.
Keywords:
Dairy wastewater; Electrocoagulation; Electrooxidation; Combined electrocoagulation-electrooxidation treatment
INTRODUCTION
Industrial wastewater is now one of the major sources of water pollution. Apart from organic carbon, wastewater also contains significant amounts of organic/inorganic nutrients like nitrogen, protein, ammonia, nitrate, and phosphate, including biorefractory organic compounds which resist conventional treatment techniques (Narkis et al., 1979Narkis, N., Rebhum, M., Sheindorf, C. H., Denitrification at various carbon to nitrogen ratios. Water Res., 13, 93-98 (1979).).
Agro-industries, like the dairy industry, generate large amounts of wastewaters, which are difficult to treat properly because of their complex behavior. Dairy waste effluents are concentrated in nature. The main contributors of organic charge to these effluents are carbohydrates, proteins and fats originating from milk (Vidal et al., 2000Vidal, G., Carvalho, A., Mendez, R., Lema, J. M., Influence of the content in fats and proteins on the anaerobic biodegradability of dairy wastewaters. Bioresour. Technol., 74, 231-239 (2000).; Bensadok et al., 2011Bensadok, K., El Hanafi, N., Lapicque, F., Electrochemical treatment of dairy effluent using combined Al and Ti/Pt electrodes system. Desalination, 280, 244-251 (2011).).
Recently, electrochemical techniques are gaining importance for the treatment of wastewater containing organic and inorganic compounds such as phenols (Comninellis and Nerini, 1995Comninellis, C., Nerini, A., Anodic oxidation of phenol in the presence of NaCl for wastewater treatment. J. Appl. Electrochem., 25, 23-28 (1995).; Kallel et al., 2009Kallel, M., Belaid, C., Boussahel, R., Ksibi, M., Montiel, A., Elleuch, B., Olive mill wastewater degradation by Fenton oxidation with zero-valent iron and hydrogen peroxide. J. Hazard. Mater., 163(2-3), 550-554 (2009).; Belaid et al., 2013Belaid, C., Khadraoui, M., Mseddi, S., Kallel, M., Elleuch, B., Fauvarque, J. F., Electrochemical treatment of olive mill wastewater: Treatment extent and effluent phenolic compounds monitoring using some uncommon analytical tools. J. Environ. Sci., 25(1), 220-230 (2013).), tannins (Murugananthan et al., 2007), dyes (Muthukumar et al., 2007Muthukumar, M., Thalamadai Karuppiah, M., Bhaskar Raju, G., Electrochemical removal of CI Acid orange 10 from aqueous solutions. Sep. Purif. Technol., 55, 198-205 (2007).; Bhaskar Raju et al., 2008Bhaskar Raju, G., Thalamadai Karuppiah, M., Latha, S. S., Parvathy, S., Prabhakar, S., Treatment of wastewater from synthetic textile industry by electrocoagulation- electrooxidation. Chem. Eng. J., 144, 51-58 (2008).) and hexavalent chromium (Lakshmipathiraj et al., 2008Lakshmipathiraj, P., Bhaskar Raju, G., Raviatul Basariya, M., Parvathy, S., Prabhakar, S., Removal of Cr (VI) by electrochemical reduction. Sep. Purif. Technol., 60, 96-102 (2008).). The application of electrochemical methods for the removal of organic pollutants has some advantages compared with chemical or biological methods.
Chemical oxidation methods can be used for the oxidative decomposition of many organic pollutants, but these methods require large amounts of reactive chemical reagents. Biological and electrochemical methods have little negative effects on the environment, because these techniques do not involve the use of harmful reagents.
Among the different electrochemical methods, the most applied technologies include the oxidation of organic pollutants by cathodic generation of hydrogen peroxide (Sanchez-Sanchez et al., 2007Sanchez-Sanchez, C. M., Expósito, E., Casado, J., Montiel, V., Goethite as a more effective iron dosage source for mineralization of organic pollutants by electro-Fenton process. Electrochem. Commun., 9, 19-24 (2007).; Nidheeshand Gandhimathi, 2012Nidheesh, P. V., Gandhimathi, R., Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination, 299, 1-15 (2012).), anodic oxidation using different electrodes (Panizza et al., 2001Panizza, M., Michaud, P. A., Cerisola, G., Comninellis, C., Electrochemical treatment of wastewaters containing organic pollutants on boron-doped diamond electrodes: Prediction of specific energy consumption and required electrode area. Electrochem. Commun., 3, 336-339 (2001).; Iniesta et al., 2002Iniesta, J., Expósito, E., Gonzalez-Garcia, J., Montiel, V., Aldaz, A., Electrochemical treatment of industrial wastewater containing phenols. J. Electrochem. Soc., 149, 57-62(2002).), cathodic removal of metals (Fu and Wang, 2011Fu, F., Wang, Q., Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage., 92, 407-418 (2011).) and electrocoagulation (Valero et al., 2008Valero, D., Ortiz, J. M., Expósito, E., Montiel, V., Aldaz, A., Electrocoagulation of a synthetic textile effluent powered by photovoltaic energy without batteries: Direct connection behavior. Sol Energy Mater. Sol. Cells, 92, 291-297 (2008).; Linares-Hernández et al., 2010Linares-Hernández, I., Barrera-Díaz, C., Bilyeu, B., Juárez-GarcíaRojas, P., Campos-Medina, E., A combined electrocoagulation-electrooxidation treatment for industrial wastewater. J. Hazard. Mater., 175, 68-694 (2010).; Emamjomeh and Sivakumar, 2010; Valero et al., 2011Valero, D., Ortiz, J. M., García-García, V., Expósito, E., Montiel, V., Aldaz, A., Electrocoagulation of wastewater from almond industry. Chemosphere, 84, 1290-1295 (2011).).
Electrocoagulation (EC) has been in use for water production or wastewater treatment (Matveevich, 2000Matveevich, V. A., Electrochemical methods of natural and waste water purifying. Elektronnaya Obrabotka Materialov, 5, 103-114 (2000).). It is finding more applications using either aluminum, iron or hybrid Al/Fe electrodes. EC is efficient in removing suspended solids as well as oil and greases. It is very effective in coagulating the colloids found in natural water, reducing the turbidity and color (Can et al., 2003Can, O. T., Bayramoglu, M., Kobya, M., Decolorization of reactive dye solutions by electrocoagulation using aluminum electrodes. Industrial and Industry Chemical Research, 42, 3391-3396 (2003).; Daneshvar et al., 2006Daneshvar, N., Oladegaragoze, A., Djafarzadeh, N., Decolorization of basic dye solutions by electrocoagulation: An investigation of the effect of operational parameters. J. Hazard. Mater., 129, 116-122 (2006).). It can be used to remove iron ions, silicates, humus, dissolved oxygen, etc. (Chen, 2004Chen, G., Electrochemical technologies in wastewater treatment. Sep. Puri. Technol., 38, 11-41 (2004).). EC has been employed in treating wastewaters from textile (Can et al., 2006Can, O. T., Kobya, M., Demirbas, E., Bayramoglu, M., Treatment of the textile wastewater by combined electrocoagulation. Chemosphere, 62,181-187 (2006).; Phalakornkule et al., 2010Phalakornkule, C., Polgumhang, S., Tongdaung, W., Karakat, B., Nuyut, T., Electrocoagulation of blue reactive, red disperse and mixed dyes, and application in treating textile effluent. J. Environ. Manage., 91, 918-926 (2010).), tannery (Benhadji et al., 2011Benhadji, A., Ahmed, M. T., Maachi, R., Electrocoagulation and effect of cathode materials on the removal of pollutants from tannery wastewater of Rouïba. Desalination, 277, 128-134 (2011).), food industries (Tezcan et al., 2006Tezcan, Ü., Ugur, S., Kopraral, A. S., Ögütveren, Ü. B., Electrocoagulation of olive mill wastewaters. Sep. Purif. Technol., 52, 136-141 (2006).; Valero et al., 2014Valero, D., García-García, V., Expósito, E., Aldaz, A., Montiel, V., Electrochemical treatment of wastewater from almond industry using DSA-type anodes: Direct connection to a PV generator. Sep. Purif. Technol., 123, 15-22 (2014).), catering (Chen et al., 2000Chen, G., Chen, X., Yue, P. L., Electrocoagulation and electroflotation of restaurant wastewater. J. Environ. Eng., 126(9), 858-863 (2000).), petroleum, tar sand and oil shale wastewater (Renk, 1988Renk, R. R., Electrocoagulation of tar sand and oil shale wastewaters. Ener. Prog., 8, 205-208 (1988).), municipal sewage (Pouet and Grasmick, 1995Pouet, M. F., Grasmick, A., Urban wastewater treatment by electrocoagulation and flotation. Water Sci. Technol., 31, 275-283 (1995).), chemical fiber wastewater (Lin and Lin, 1998Lin, S. H., Lin, C. S., Reclamation of wastewater effluent from a chemical fiber plant. Desalination, 120, 185-195 (1998).), oily wastewater (Pazenko at al., 1985Pazenko, T. Ya., Khalturina, T. I., Kolova, A. F., Rubailo, I. S., Electrocoagulation treatment of oil-containing wastewaters. J. Appl. USSR, 58, 2383-2387 (1985).), nitrite (Abuzaid et al., 1999Abuzaid, N. S., Al-Hamouz, Z., Bukhari, A. A., Essa, M. H., Electrochemical treatment of nitrite using stainless steel electrodes. Water Air Soil Pollut., 109, 429-442(1999).), and dye stuff (Ogutveren and Koparal, 1992Ogutveren, U. B., Koparal, S., Electrochemical treatment of water containing dye-stuffs: Anodic oxidation of congo red and xiron blau 2RHD. Int. J. Environ. Stud., 42, 41-52 (1992).) from wastewater.
In those cases where EC is not able to reduce COD to below discharge limits, it is compulsory to use an additional treatment.
Several papers showed that Electrooxidation (EO) was an effective method for reducing the pollutant load of water containing soluble organic matter (Valero et al., 2014Valero, D., García-García, V., Expósito, E., Aldaz, A., Montiel, V., Electrochemical treatment of wastewater from almond industry using DSA-type anodes: Direct connection to a PV generator. Sep. Purif. Technol., 123, 15-22 (2014).). Moreover, due to the growing development of more efficient electrodes, electrochemical oxidation is able to destroy several practically recalcitrant organic pollutants such as polyphenols and fatty acids (Belaid et al., 2013Belaid, C., Khadraoui, M., Mseddi, S., Kallel, M., Elleuch, B., Fauvarque, J. F., Electrochemical treatment of olive mill wastewater: Treatment extent and effluent phenolic compounds monitoring using some uncommon analytical tools. J. Environ. Sci., 25(1), 220-230 (2013).). Titanium-based boron-doped diamond film electrodes (Ti/BDD) show high activity and give reasonable stability. Its industrial application calls for the production of Ti/BDD anodes of large size at reasonable cost and durability. On the other hand, EO systems are not designed for treating water containing high amounts of suspended solids. Therefore, wastewater containing suspended solids should be first treated, for example by EC, to remove those solids before sending the wastewater to an EO treatment system.
The objective of this study is to compare the effectiveness of EC and EO methods for pollutant removal from dairy effluent. Once the optimal conditions of each method were identified, we combine them to produce synergistic effects and to obtain the best conditions for complete pollutant removal, allowing water recovery and reuse for agricultural purposes.
METHODOLOGY
Water Physicochemical Analysis
The dairy effluent sample was collected from the wastewater treatment plant of a Tunisian dairy industry and kept at 4 ºC before use. The actual wastewater treatment plant receives 600 m3 of effluents every day and it consists of a primary clarifier, biological activated sludge reactors and secondary clarifier.
The pH and electric conductivity of untreated and treated wastewaters were determined using a pH meter (Model EA940, Orion, USA) and a conductivity meter (Model WTW LF 90), respectively. Chemical oxygen demand (COD) was determined according to the Knechtel (1978)Knechtel, R. J., A more economical method for the determination of chemical oxygen demand. Water Pollut. Control, Fed May/June, 25-29 (1978). standard method. Biochemical oxygen demand (BOD5) was determined by the manometric method using a digital respirometer BSB-Controller, Model 620 T (WTW). The phosphorus concentration in aqueous media was determined according to Hanson (1973)Hanson, N. W., Official standardized and recommended methods of analysis. The Society for Analytical Chemistry, London (1973)..
Electrocoagulation Reactor
Electrocoagulation was performed in a cylindrical reactor with a working volume of 1 L. All the experiments were conducted at a constant temperature of 25 ºC. The electrochemical cell has two aluminum plates, one serving as a cathode and the other as anode (Figure 1). The spacing between electrodes was 5mm. A direct current variable from 0 to 10 A was supplied from a current generator, type CLES 35-10 (Laboratory Power Supply, Convergie, France).
The mechanism of EC is based on generating polyvalent metal cations (Al 3+) directly into the wastewater by anodic dissolution of the aluminum electrode, as a result of a current imposed to the electrodes.
The eletrochemical reactions involved in the reactor can be written as:
At the cathode:
At the anode:
In the solution:
The H2 produced as a result of the redox reaction may remove dissolved organics or any suspended materials by flotation and this phenomenon is one of the advantages of the EC process (Yildiz, 2008Yildiz, S. Y., Optimization of Bomaplex Red CR-L dye removal from aqueous solution by electrocoagulation using aluminum electrodes. J. Hazard. Mater., 153,194-200 (2008).).
In order to enhance the process performance, the effect of the density of current has been explored. Four densities of current were chosen for this study: 0.6 A/dm2, 1.2 A/dm2, 1.8 A/dm2 and 2.4 A/dm2 with an application time of 15 min. To follow the progress of the treatment, samples of 10 mL were periodically taken from the beaker, then decanted for 30 min to separate sludge produced during the process.
Electrooxidation
Electrooxidation was performed in a cylindrical reactor with a working volume of 500 mL. All the experiments were conducted at a constant temperature of 25 ºC. The reactor cell is composed of two cylindrical electrodes made of expanded platinized titanium and the electrodes were separated by 0.5 cm (Figure 2). To keep the homogenization of the effluent a small pump with a flow rate of 2 mL/min was used. During the EO process, two phenomena can be observed. In the first case, the electrode is active and participates directly in the oxidation of the organic product by adsorption of the pollutant on the surface of the anode and its degradation by electron transfer. In the second case, the electrode contributes to oxidation via hydroxyl radicals formed during electrolysis of water.
Methods of Analysis
The evaluation of the treatments was determined by analysis of the chemical oxygen demand (COD) (Knechtel, 1978Knechtel, R. J., A more economical method for the determination of chemical oxygen demand. Water Pollut. Control, Fed May/June, 25-29 (1978).), biochemical oxygen demand (BOD5), color (OD), turbidity, suspended solids, dry matter and pH, as indicated in the Standard Methods procedures. The color was assayed at 270 nm using a UV/Visible spectrophotometer (Thermospectronic UV1).
Total nitrogen was determined by the Kjeldahl method (Kjeldahl, 1883Kjeldahl, J. Z., A new method for the determination of nitrogen in organic matter. Anal. Chem., 22, 366 (1883).); phosphorus was measured colorimetrically (Hanson, 1973Hanson, N. W., Official standardized and recommended methods of analysis. The Society for Analytical Chemistry, London (1973).) and K, Na and Cl by atomic absorption spectrophotometry (HITACHI Model Z-6100).
Phytotoxicity Test
The phytotoxicity was assessed for the dairy effluent treated (EC, EO and EC+EO) samples mentioned above by using seed germination of pea (Pisum sativum). The phytotoxicity was determined according to the Zucconi test (Zucconi et al., 1981Zucconi, F., Pera, A., De Bertoldi, M., Evaluating toxicity of immature compost. BioCycle, 27-29 (1981).). Ten pea seeds were placed on filter papers in glass Petri dishes with dimensions 110 mm x 20 mm. 10 ml of dairy effluent samples were then uniformly added to each dish. The dish was capped and kept in a dark incubator at 25 ºC temperature for 5 days. 10 ml of tap water were used for the controls, instead of dairy effluent. All samples, including the control, were run in triplicate. All seeds were kept in tap water for approximately 12 h prior to the initiation of the experiments, to accelerate seed growth. A germination index (GI) was calculated by counting the number of germinated seeds and the average sum of the seeds' root elongation in a sample relative to the control. Results were finally expressed as a percentage.
RESULTS AND DISCUSSION
Dairy Effluent Characteristics
The physico-chemical properties of dairy effluent are given in Table 1. The dairy effluent was milky white in color and turbid. The analyses performed on this effluent demonstrate that it is principally characterised by a slightly acidic pH (around 6.55) and an electrical conductivity of around 3.93 mS/cm. Total suspended solid (1065.5 mg/L) is higher when compared to the permissible value. The total solid concentration in the waste effluent represents the colloidal form and dissolved species. It contains large amount of total solids and total dissolved solids, resulting in high BOD and COD. COD and BOD5 values of the effluent were 3069 and 2900 mg/L respectively, compared to their respective standard values of 1000 and 400 mg/L.
Electrocoagulation of Dairy Effluent
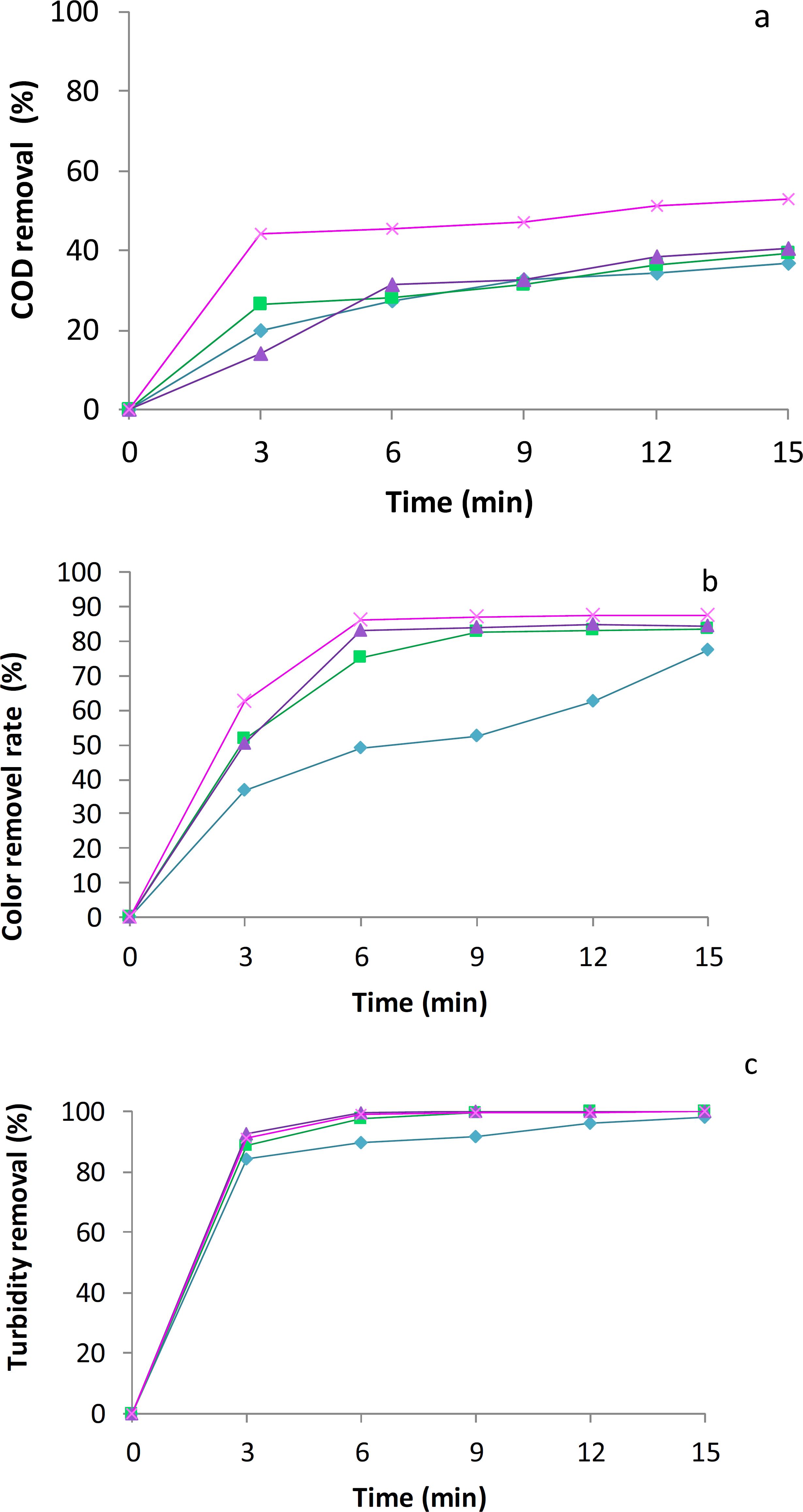
EC experiments have been run with dairy effluent for four current densities, from 0.6 to 2.4 A/dm2 and with aluminum electrodes (anode and cathode), Figure 3 (a, b and c) represents the effects of the electrolysis time on COD, turbidity and color removal efficiencies for various current densities using Al electrode materials. As shown in Fig. 3, when the current density was increased from 0.6 to 2.4 A/dm2, the COD removal increased from 19.6 to 53%. As the current density increased, the electrolysis time needed to achieve similar efficiencies decreased. As seen in Fig. 3, 31.51% of COD removal was obtained at the current density of 1.8 A/dm2 in 6 min. However, 31.19% of COD removal was reached at current densities of 1.2 A/dm2 in 9 min.
Effect of electrocoagulation on (a) COD, (b) color and (c) turbidity reduction as a function of electrolysis time and current density. Current density and electrolysis time (pH 6.6; Al-Al electrodes). 0.6 A/dm2 (♢), 1.2 A/dm2 (□), 1.8 A/dm2 (Δ), 2.4 A/m2 (x).
At the current density of 2.4 A/dm2, 53% of COD removal was achieved with an electrolysis time of 15 min. Likewise, 87.52% of color removal was attained at the current density of 2.4 A/dm2 in 15 min. Similar color removal efficiencies of 86.84% and 87.52% were obtained at a current density of 2.4 A/dm2 in 9 and 12 min, respectively. This expected behavior can be explained by the fact that the treatment efficiency was mainly affected by charge loading, as reported by others (Chen et al., 2000Chen, X., Chen, G. H., Yue, P. L., Separation of pollutants from restaurant wastewater by electrocoagulation. Sep. Purif. Technol., 19, 65-76 (2000).; Adhoum and Monser, 2004Adhoum, N., Monser, L., Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem. Eng. Process., 43, 1281-1287 (2004).). Low COD (Inan et al., 2004Inan, H., Dimoglo, A., Simsek, H., Karpuzcu, M., Olive mill wastewater treatment by means of electro-coagulation. Sep. Purif. Technol., 36, 23-31(2004).) and color (Kim et al., 2002Kim, T. H., Park, C., Shin, E. B., Kim, S., Decolorization of disperse and reactive dyes by continuous electrocoagulation process. Desalination, 150, 165-175 (2002).) removals may be attributed to the presence of several recalcitrant inorganic compounds, complex components, and other undesirable impurities in the wastewaters. During electrolysis, the visual observation shows that the milky white color of the dairy effluent turned to white clear, and then gradually disappeared to obtain finally an almost colorless solution.
More to the point, the results showed that, for current densities ≥1.2 A/dm2, abatement of the pollution variables occurs in two phases as follows:
-
− The first phase of decreasing pollution during which the COD, the color and the turbidity removal yields increase with the current density. This can be explained by the fact that Al3+ production resulting from the anode dissolution increases with current density according to Faraday's law.
-
− The second phase represents the stationary phase. This phase is reached after 6 min of treatment. For this period, a further increase in aluminum concentration has no further effect on the treatment.
In general, Figure 3 for COD, turbidity and color removals versus electrolysis time demonstrated an increasing trend with the increase of current density. Our results show also an increase of pH, which passes from 6.6 to 6.9, and this can be explained by formation of OH by the reduction of water at the cathode. The pH has a considerable effect on the performance of the EC process (Kobya et al., 2003Kobya, M., Can, O. T., Bayramoglu, M., Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J. Hazard. Mater., 100, 163-178 (2003).). In a previous study conducted by Adhoum and Monser (2004)Adhoum, N., Monser, L., Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem. Eng. Process., 43, 1281-1287 (2004)., the highest COD and color removal efficiencies were obtained in acidic medium, at pH values in the limits of 4.0-6.0. However, very poor removals are found either at low (<2.0) or high pH (>10). This behavior was attributed to the amphoteric character of Al(OH)3 that does not precipitate at pH less than 2.0. However, high pH will increase Al(OH)3 solubility and lead to the formation of soluble Al(OH)4- which is useless for water treatment. Can et al. (2003)Can, O. T., Bayramoglu, M., Kobya, M., Decolorization of reactive dye solutions by electrocoagulation using aluminum electrodes. Industrial and Industry Chemical Research, 42, 3391-3396 (2003). have reported that an acidic or neutral initial medium is beneficial for low electrical energy consumption.
Electrooxidation of Dairy Effluent
Electrooxidation is an effective method for degradation of organic pollutants. Effect of current intensity was also investigated in the range of 1.1 A/dm2 to 1.8 A/dm2 at an initial pH of 6.6 and at constant flow rate of wastewater. As shown in Figure 4, increasing current intensity led to the decrease of turbidity, color and COD following Faraday's law (Prentice, 1991Prentice, G., Electrochemical Engineering Principles. Prentice- Hall, Singapore (1991).).
Effect of Electrooxidation on color (a), Turbidity (b) and COD (c) reduction as a function of electrolysis time and current density. 1.1 A/dm2 (□), 1.4 A/dm2 (Δ), 1.8 A/dm2 (x).
For higher current densities, the treatment can be carried out with high efficiency: turbidity of the waste could be nearly removed (+91.61%) after 60 min. At the same current intensity of 1.8 A/dm2, 59.46% and 42% of color and COD were reduced within 60 min electrolysis time. The decrease of color and COD is attributed to the destruction of organic contaminants in wastewater when the EO was implemented. These results showed that the treatment process by EO required a relatively longer reaction time than EC.
The EO process also allowed the elimination of odors caused by the high pollution load of the effluent. Piya-areetham et al. (2006)Piya-areetham, P., Shenchunthichai, K., Hunsom, M., Application of electrooxidation process for treating concentrated wastewater from distillery industry with a voluminous electrode. Water Research, 40, 2857-2864 (2006). show that the reduction mechanism of organic contaminants by EO can occur directly on anodes by generating physically adsorbed active oxygen (adsorbed hydroxyl radicals, OH*) or chemisorbed active oxygen (oxygen in the oxide lattice, MOx+1). The physically adsorbed active oxygen can cause the complete combustion of organic compounds (R), and the chemisorbed active oxygen can participate in the formation of selective oxidation products, as shown in Eqs. (4) and (5).
Generally, OH* is more effective for pollutant oxidation than O in MOx+1 (Chen, 2004Chen, G., Electrochemical technologies in wastewater treatment. Sep. Puri. Technol., 38, 11-41 (2004).).
In summary, the results show that the time to reduce the COD to about 40% with EO takes about 27 min, while EC (Figure 4) takes less than 6 min. Hence, EC is a fast but an incomplete process and EO is a slow process and can improve the efficiency of treatment; coupling the two processes offers a practical hybrid.
Combined Electrocoagulation-Electrooxidation
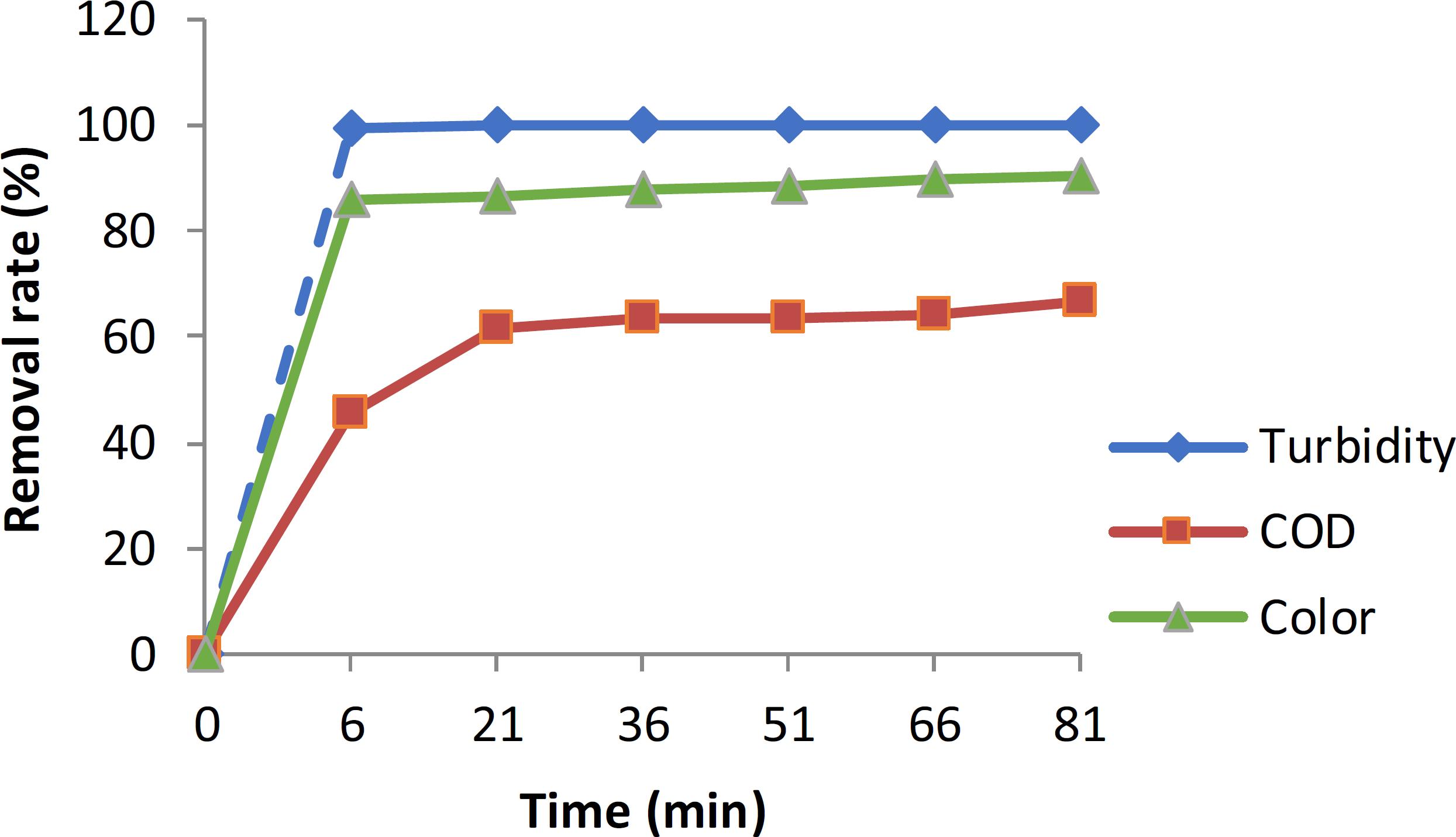
The optimal conditions for the EC and EO processes were determined and then the coupled treatment was applied. Figure 5 show that EC is a suitable technique to eliminate suspended solids and flocculate particles. The time to reduce the COD to about 40% with EC takes about 6 min with a current density 0.6A/dm2, then EO oxidizes the remaining organics up to 60%. Table 2 summarizes the results of the treatments. Note that, in addition to reducing COD, it also greatly reduced the BOD5, turbidity, fatty matter, phosphorus, K+ and NTK.
Combined treatment: electrocoagulation and electrooxidation processes. The current density used was 0.6 for EC and 1.4 A/dm2 for EO and the pH of the water sample was 6.6.
Impact of Dairy Effluent on Seed Germination
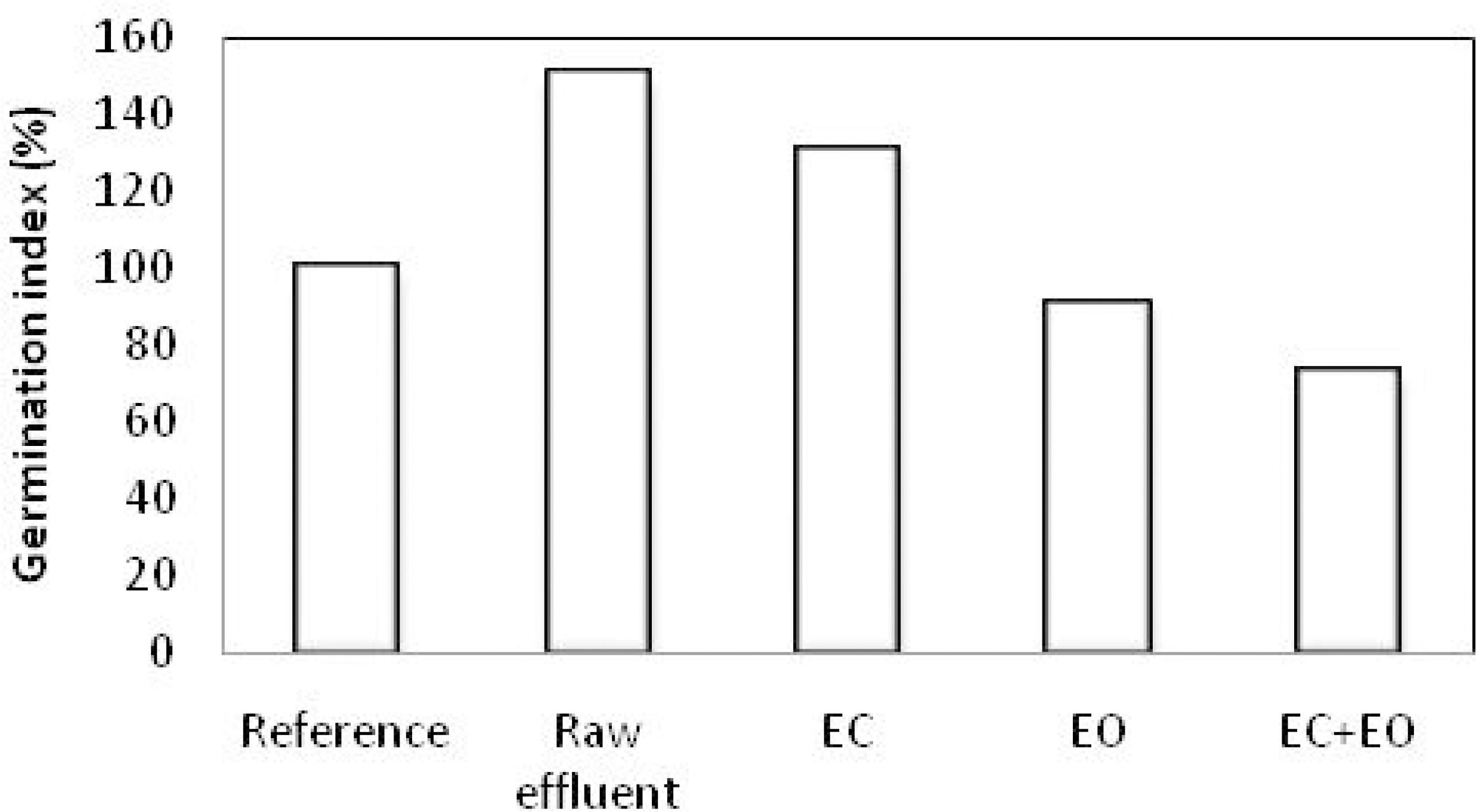
Seed germination of pea (Pisum sativum) was used to evaluate the feasibility reuse of treated dairy wastewater in irrigation. The seed germination index (GI) is a good indicator for phytotoxicity (Brewer and Sullivan, 2003Brewer, L., Sullivan, D., Maturity and stability evaluation of composted yard trimmings. Comp. Sci. Util., 11, 96-112 (2003).). The results showed that the germination index for raw dairy wastewater was 151% (Figure 6), while seed germination of the treated effluents was 131%, 91% and 73%, respectively, for EC, EO and combined treatment.
According to Abdullahi et al. (2008)Abdullah, Y. A., Akunna, J. C., White, N. A., Hallett, P. D., Wheatley, R., Investigating the effects of anaerobic and aerobic post-treatment on quality and stability of organic fraction of municipal solid waste as soil amendment. Bioresour. Technol., 99, 8631-8636 (2008)., a seed germination index (GI) greater than 70% is considered non-phytotoxic, while a GI of less than 70% is considered phytotoxic. Hence, Figure 6 suggests that both raw waste and treated effluent are non-phytotoxic. Our results demonstrate clearly the beneficial effect of dairy effluent on seed germination of Pisum sativum (GI>70). However, seed irrigated with wastewater treated by EO and the combined treatment have relatively lower germination rates than raw effluent and EC. More to the point, results show that the use of the EO treatment is expected to be insufficient because of the formation of organic oxidation intermediates which may be more refractory to oxidation treatment than the parent compounds. Moreover, the oxidation intermediates may be more toxic than the parent compounds, thus increasing the final toxicity/phytotoxicity.
CONCLUSIONS
EC, EO and hybrid processes were employed for the treatment of dairy industry effluent. The operating parameters such as electrolysis time and current density were examined to maximize COD, color and turbidity removal of the effluent with minimal power consumption. The percentages of color, turbidity and COD removal for the EC, EO and hybrid processes were compared. The result showed that the hybrid technique was more effective than EC or EO alone.
The maximum percentage turbidity, color and COD removals of 100%, 90.4% and 66.4%, respectively, were observed at 21 min for an initial COD concentration of 3850 ppm, initial pH 6 and current density 3 A/dm2. This technology will work in the wastewater treatment process due to the significant improvement over the conventional system. The use of the seed toxicity test allowed evaluating the quality and effectiveness of the studied effluent treatment system. Seed irrigated with raw waste or treated dairy effluent show no phytotoxicity. However, seed irrigated with wastewater treated by EO and by the combined treatment have relatively low germination rates compared to raw effluent and effluent treated with EC. The EO treatment is expected to be insufficient because of the formation of organic oxidation intermediates which may be more refractory to oxidation treatment than the parent compounds.
REFERENCES
- Abdullah, Y. A., Akunna, J. C., White, N. A., Hallett, P. D., Wheatley, R., Investigating the effects of anaerobic and aerobic post-treatment on quality and stability of organic fraction of municipal solid waste as soil amendment. Bioresour. Technol., 99, 8631-8636 (2008).
- Abuzaid, N. S., Al-Hamouz, Z., Bukhari, A. A., Essa, M. H., Electrochemical treatment of nitrite using stainless steel electrodes. Water Air Soil Pollut., 109, 429-442(1999).
- Adhoum, N., Monser, L., Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem. Eng. Process., 43, 1281-1287 (2004).
- Belaid, C., Khadraoui, M., Mseddi, S., Kallel, M., Elleuch, B., Fauvarque, J. F., Electrochemical treatment of olive mill wastewater: Treatment extent and effluent phenolic compounds monitoring using some uncommon analytical tools. J. Environ. Sci., 25(1), 220-230 (2013).
- Benhadji, A., Ahmed, M. T., Maachi, R., Electrocoagulation and effect of cathode materials on the removal of pollutants from tannery wastewater of Rouïba. Desalination, 277, 128-134 (2011).
- Bensadok, K., El Hanafi, N., Lapicque, F., Electrochemical treatment of dairy effluent using combined Al and Ti/Pt electrodes system. Desalination, 280, 244-251 (2011).
- Bhaskar Raju, G., Thalamadai Karuppiah, M., Latha, S. S., Parvathy, S., Prabhakar, S., Treatment of wastewater from synthetic textile industry by electrocoagulation- electrooxidation. Chem. Eng. J., 144, 51-58 (2008).
- Brewer, L., Sullivan, D., Maturity and stability evaluation of composted yard trimmings. Comp. Sci. Util., 11, 96-112 (2003).
- Can, O. T., Bayramoglu, M., Kobya, M., Decolorization of reactive dye solutions by electrocoagulation using aluminum electrodes. Industrial and Industry Chemical Research, 42, 3391-3396 (2003).
- Can, O. T., Kobya, M., Demirbas, E., Bayramoglu, M., Treatment of the textile wastewater by combined electrocoagulation. Chemosphere, 62,181-187 (2006).
- Chen, G., Chen, X., Yue, P. L., Electrocoagulation and electroflotation of restaurant wastewater. J. Environ. Eng., 126(9), 858-863 (2000).
- Chen, G., Electrochemical technologies in wastewater treatment. Sep. Puri. Technol., 38, 11-41 (2004).
- Chen, X., Chen, G. H., Yue, P. L., Separation of pollutants from restaurant wastewater by electrocoagulation. Sep. Purif. Technol., 19, 65-76 (2000).
- Comninellis, C., Nerini, A., Anodic oxidation of phenol in the presence of NaCl for wastewater treatment. J. Appl. Electrochem., 25, 23-28 (1995).
- Daneshvar, N., Oladegaragoze, A., Djafarzadeh, N., Decolorization of basic dye solutions by electrocoagulation: An investigation of the effect of operational parameters. J. Hazard. Mater., 129, 116-122 (2006).
- Emamjomeh, M. M., Sivakumar, M., Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manage., 90, 1663-1679 (2009).
- Fu, F., Wang, Q., Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage., 92, 407-418 (2011).
- Hanson, N. W., Official standardized and recommended methods of analysis. The Society for Analytical Chemistry, London (1973).
- Inan, H., Dimoglo, A., Simsek, H., Karpuzcu, M., Olive mill wastewater treatment by means of electro-coagulation. Sep. Purif. Technol., 36, 23-31(2004).
- Iniesta, J., Expósito, E., Gonzalez-Garcia, J., Montiel, V., Aldaz, A., Electrochemical treatment of industrial wastewater containing phenols. J. Electrochem. Soc., 149, 57-62(2002).
- Kallel, M., Belaid, C., Boussahel, R., Ksibi, M., Montiel, A., Elleuch, B., Olive mill wastewater degradation by Fenton oxidation with zero-valent iron and hydrogen peroxide. J. Hazard. Mater., 163(2-3), 550-554 (2009).
- Kim, T. H., Park, C., Shin, E. B., Kim, S., Decolorization of disperse and reactive dyes by continuous electrocoagulation process. Desalination, 150, 165-175 (2002).
- Kjeldahl, J. Z., A new method for the determination of nitrogen in organic matter. Anal. Chem., 22, 366 (1883).
- Knechtel, R. J., A more economical method for the determination of chemical oxygen demand. Water Pollut. Control, Fed May/June, 25-29 (1978).
- Kobya, M., Can, O. T., Bayramoglu, M., Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J. Hazard. Mater., 100, 163-178 (2003).
- Lakshmipathiraj, P., Bhaskar Raju, G., Raviatul Basariya, M., Parvathy, S., Prabhakar, S., Removal of Cr (VI) by electrochemical reduction. Sep. Purif. Technol., 60, 96-102 (2008).
- Lin, S. H., Lin, C. S., Reclamation of wastewater effluent from a chemical fiber plant. Desalination, 120, 185-195 (1998).
- Linares-Hernández, I., Barrera-Díaz, C., Bilyeu, B., Juárez-GarcíaRojas, P., Campos-Medina, E., A combined electrocoagulation-electrooxidation treatment for industrial wastewater. J. Hazard. Mater., 175, 68-694 (2010).
- Matveevich, V. A., Electrochemical methods of natural and waste water purifying. Elektronnaya Obrabotka Materialov, 5, 103-114 (2000).
- Murugananthan, M., Bhaskar Raju, G., Prabhakar, S., Removal of tannins and polyhydroxy phenols by electrochemical techniques. J. Chem. Technol. Biotechnol., 80, 1188-1197 (2005).
- Muthukumar, M., Thalamadai Karuppiah, M., Bhaskar Raju, G., Electrochemical removal of CI Acid orange 10 from aqueous solutions. Sep. Purif. Technol., 55, 198-205 (2007).
- Narkis, N., Rebhum, M., Sheindorf, C. H., Denitrification at various carbon to nitrogen ratios. Water Res., 13, 93-98 (1979).
- Nidheesh, P. V., Gandhimathi, R., Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination, 299, 1-15 (2012).
- Ogutveren, U. B., Koparal, S., Electrochemical treatment of water containing dye-stuffs: Anodic oxidation of congo red and xiron blau 2RHD. Int. J. Environ. Stud., 42, 41-52 (1992).
- Panizza, M., Michaud, P. A., Cerisola, G., Comninellis, C., Electrochemical treatment of wastewaters containing organic pollutants on boron-doped diamond electrodes: Prediction of specific energy consumption and required electrode area. Electrochem. Commun., 3, 336-339 (2001).
- Pazenko, T. Ya., Khalturina, T. I., Kolova, A. F., Rubailo, I. S., Electrocoagulation treatment of oil-containing wastewaters. J. Appl. USSR, 58, 2383-2387 (1985).
- Phalakornkule, C., Polgumhang, S., Tongdaung, W., Karakat, B., Nuyut, T., Electrocoagulation of blue reactive, red disperse and mixed dyes, and application in treating textile effluent. J. Environ. Manage., 91, 918-926 (2010).
- Piya-areetham, P., Shenchunthichai, K., Hunsom, M., Application of electrooxidation process for treating concentrated wastewater from distillery industry with a voluminous electrode. Water Research, 40, 2857-2864 (2006).
- Pouet, M. F., Grasmick, A., Urban wastewater treatment by electrocoagulation and flotation. Water Sci. Technol., 31, 275-283 (1995).
- Prentice, G., Electrochemical Engineering Principles. Prentice- Hall, Singapore (1991).
- Renk, R. R., Electrocoagulation of tar sand and oil shale wastewaters. Ener. Prog., 8, 205-208 (1988).
- Sanchez-Sanchez, C. M., Expósito, E., Casado, J., Montiel, V., Goethite as a more effective iron dosage source for mineralization of organic pollutants by electro-Fenton process. Electrochem. Commun., 9, 19-24 (2007).
- Tezcan, Ü., Ugur, S., Kopraral, A. S., Ögütveren, Ü. B., Electrocoagulation of olive mill wastewaters. Sep. Purif. Technol., 52, 136-141 (2006).
- Valero, D., García-García, V., Expósito, E., Aldaz, A., Montiel, V., Electrochemical treatment of wastewater from almond industry using DSA-type anodes: Direct connection to a PV generator. Sep. Purif. Technol., 123, 15-22 (2014).
- Valero, D., Ortiz, J. M., Expósito, E., Montiel, V., Aldaz, A., Electrocoagulation of a synthetic textile effluent powered by photovoltaic energy without batteries: Direct connection behavior. Sol Energy Mater. Sol. Cells, 92, 291-297 (2008).
- Valero, D., Ortiz, J. M., García-García, V., Expósito, E., Montiel, V., Aldaz, A., Electrocoagulation of wastewater from almond industry. Chemosphere, 84, 1290-1295 (2011).
- Vidal, G., Carvalho, A., Mendez, R., Lema, J. M., Influence of the content in fats and proteins on the anaerobic biodegradability of dairy wastewaters. Bioresour. Technol., 74, 231-239 (2000).
- Yildiz, S. Y., Optimization of Bomaplex Red CR-L dye removal from aqueous solution by electrocoagulation using aluminum electrodes. J. Hazard. Mater., 153,194-200 (2008).
- Zucconi, F., Pera, A., De Bertoldi, M., Evaluating toxicity of immature compost. BioCycle, 27-29 (1981).
Publication Dates
-
Publication in this collection
Jan-Mar 2017
History
-
Received
22 Jan 2015 -
Reviewed
07 Sept 2015 -
Accepted
22 Sept 2015