Abstract
Currently, the use of antivenoms is the only available treatment for envenomation caused by venomous animals namely, snake, scorpion, spider, tick and jelly fish. Antivenoms are generally produced in large animals, mostly in horses. A large percentage of the population is allergic to horse proteins. Several animals are known to be resistant to snakebites and the antihemorrhagic and anti-lethal components have been isolated from sera of opossum, mongoose, meerkat and hedgehog, as well as from venomous and non-venomous snakes. Anti-lethal factor named Lethal Toxin Neutralizing Factor (LTNF) has been isolated in purity from opossum (Didelphis virginiana) serum by high pressure liquid chromatography (HPLC). The molecular weight of LTNF is 63 kDa, and it does not form precipitation with venoms or toxins by immunodiffusion. Death due to intraperitoneal (IP) injection of a predetermined lethal dose of venom from major families of snakes, for instance Crotalidae, Elapidae, Viperidae and Hydrophiidae, is prevented in mice by subsequent IP inoculation of LTNF. Furthermore, LTNF neutralizes the lethality of scorpion and bee venoms and toxins from various animals, plants and bacteria. Thus, natural LTNF from opossum serum has potential as a universal therapy for envenomation caused by animals, plants and bacteria.
Opossum; Didelphis virginiana; lethal toxin neutralizing factor
ANTI-LETHAL FACTOR FROM OPOSSUM SERUM IS A POTENT ANTIDOTE FOR ANIMAL, PLANT AND BACTERIAL TOXINS
B. V. LIPPS
 CORRESPONDENCE TO:
B. V. LIPPS - Ophidia Products Inc., 11320 South Post oak, Suite 203, Houston, Texas 77035, USA.
CORRESPONDENCE TO:
B. V. LIPPS - Ophidia Products Inc., 11320 South Post oak, Suite 203, Houston, Texas 77035, USA.
1 Ophidia Products, Inc. - Houston, Texas - USA ABSTRACT: Currently, the use of antivenoms is the only available treatment for envenomation caused by venomous animals namely, snake, scorpion, spider, tick and jelly fish. Antivenoms are generally produced in large animals, mostly in horses. A large percentage of the population is allergic to horse proteins.
Several animals are known to be resistant to snakebites and the antihemorrhagic and anti-lethal components have been isolated from sera of opossum, mongoose, meerkat and hedgehog, as well as from venomous and non-venomous snakes.
Anti-lethal factor named Lethal Toxin Neutralizing Factor (LTNF) has been isolated in purity from opossum (Didelphis virginiana) serum by high pressure liquid chromatography (HPLC). The molecular weight of LTNF is 63 kDa, and it does not form precipitation with venoms or toxins by immunodiffusion.
Death due to intraperitoneal (IP) injection of a predetermined lethal dose of venom from major families of snakes, for instance Crotalidae, Elapidae, Viperidae and Hydrophiidae, is prevented in mice by subsequent IP inoculation of LTNF. Furthermore, LTNF neutralizes the lethality of scorpion and bee venoms and toxins from various animals, plants and bacteria. Thus, natural LTNF from opossum serum has potential as a universal therapy for envenomation caused by animals, plants and bacteria.
KEY WORDS: Opossum, Didelphis virginiana, lethal toxin neutralizing factor.INTRODUCTION
Several warm blooded animals show remarkable resistance to snakebites (9). Menchaga and Perez (7) isolated and purified an antihemorrhagic protein from the serum of opossum (Didelphis virginiana) with a molecular weight 68,000 daltons. Catanese and Kress (3) isolated a serum metalloproteinase inhibitor, homologous to human alpha 1B-glycoprotein, from opossum (Didelphis virginiana) which they designated "Oprin." Oprin inhibited snake venom metalloproteinases. They concluded that the presence of Oprin in opossum serum may partially account for the resistance of this marsupial to the localized effects of rattlesnake envenomation. Perales et al. (9) isolated an anti-bothropic fraction (ABF) from the sera of several South American Didelphidae (Didelphis marsupialis, Philander opossum and Lutreolina crassicaudata). They found that ABF has anti-Bothrops jararaca venom activity when tested on mice. The ABF from all three of these Didelphidae have the same sequence of the first five amino acids, L K A M D, from the N-terminal. In addition, antihemorrhagic factors have been isolated from mongoose, and from the non-venomous snake Dinodon semicarinatus, as well as from the venomous snakes Trimeresurus flavoviridis (11), Vipera palestinae (8,10), and Crotalus atrox (12). The properties of the antihemorrhagic factors from the above-mentioned sources have been reviewed by Domont et al. (4). According to these authors, the protective antihemorrhagic and anti-neurotoxic factors have some common characteristics:
i) They are acid proteins with isoelectric points ranging from 4 to 5.4.
ii) Their molecular masses vary from 52 to 90 kDa with one exception of 780 kDa.
iii) They do not show proteolytic activity.
iv) They are thermostable glycoproteins.
v) There are no precipitation lines formed between the neutralizing proteins and the venoms by immunodiffusion, strongly suggesting that the serum protective factors are not immunoglobulins. In addition, the lower molecular weights, with one exception, confirm that these proteins can not be immunoglobulins.
The object of this research is to provide a composition consisting of a natural lethal toxin neutralizing factor (LTNF) derived from opossum serum that will serve as a relatively universal treatment for envenomation caused by animal, plant and bacterial toxins (6). Moreover, the use of LTNF will avoid the horse serum sickness. Thus, LTNF can become an alternative treatment for various snakebites requiring extremely low concentrations of foreign protein in comparison to the currently used massive amounts of antivenom. Currently, antivenoms produced in horses provide the only available treatment for snakebite, even though some people are hypersensitive to horse proteins. Antivenom treatment for snakebite has been in use for almost a century without much improvement. The only considerations being that antivenoms for snake species prevalent in any given region should be available, and that the correct antivenom should be used. In order to administer the appropriate specific antivenom, the victim or physician must identify the offending snake, which is impossible in many cases. Treatment of snakebites would be greatly simplified, if a drug could be found which would overcome the problems associated with antivenoms. A drug which neutralizes the toxicity of venoms from all major species would be a breakthrough. In the words of the 19th century scientist Fayrer "To conceive of an antidote to snake poison, one must imagine a substance so subtle as to follow, overtake and neutralize the venom in the blood -- Such a substance has still to be found" (5). That substance is non-toxic LTNF derived from the serum of opossum, which is resistant to snakebite.
MATERIALS AND METHODS
FRACTIONATION OF OPOSSUM SERUM. The active Lethal Toxin Neutralizing Factor (LTNF) was separated from the serum of opossum (Didelphis virginiana). The serum was diluted 1:4 with 0.01 M phosphate buffer (PBS) and centrifuged to sediment insoluble debris. Approximately 20 mg of serum protein was fractionated on HPLC from Toso Co. (Japan) using an anion exchange column from Polymer Laboratories (UK). The fractionation process was maintained at 20ºC. A plurality of fractions were eluted according to relative ionic charge using gradient Trizma-HCl buffer at pH 7.3. The Toso HPLC automatically mixes water and 1.0 M Trizma-HCl buffer in the range 0.01 M to 1.0 M. (Figure 1). All the fractions were dialyzed against water to remove excess of salt.
High pressure liquid chromatography profile of opossum serum using an anion exchange column. Peak number six represents the lethal toxin neutralizing factor LTNF.
Venom
Family
Lethality µg/mouse
Crotalidae
300
Viperidae
20
Elapidae
20
Elapidae
10
Hydrophiidae
20
Scorpion
10
Apis (Bee)
1
Toxin
Source
Lethality µg/ mouse
Naja n. kaouthia
5
Crotalus durissus terrificus
10
Naja n. kaouthia
20
Oxyuranus s. scutellatus
5
Ricinus communis
1
Clostridium botulinum
0.5
Actinopyga agassizi
20
RESULTS
In order to measure the inhibition of toxicity, mice were injected with a predetermined lethal dose of the respective venoms in 0.1 ml volume IP. Immediately, the control mice were injected with 0.5 ml PBS and the experimental mice with the same volume of opossum serum. For each experimental category three mice were used.
The results in Table 1 show that opossum serum is rendering protection against lethal doses of various snake venoms from all families of venomous snakes. The control mice died within 24 hours. As little as 0.5 ml of opossum serum is adequate to cause neutralization and render protection against envenomation, which otherwise would be lethal.
Opossum serum was fractionated into immunoglobulin and albumin components by saturated ammonium sulfate treatment. The precipitated immunoglobulin component was dissolved in 0.05 M PBS to the starting volume of the serum. After dialysis, both albumin and immunoglobulin components were tested versus lethal doses of various snake venoms. The mice were injected with predetermined lethal doses of snake venoms followed by inoculation with 0.5 ml of albumin or immunoglobulin components. The results are shown in Table 2.
The neutralizing effect of the opossum serum components albumin and immunoglobulin on snake venoms.
The results in Table 2 clearly show that the protective factor resides in the albumin component of the opossum serum to neutralize the lethality of the venoms from C. atrox, N. n. kaouthia V. russellii and O. scutellatus. Thus, the neutralization of the toxic effects of venoms is not due to antigen antibody reaction.
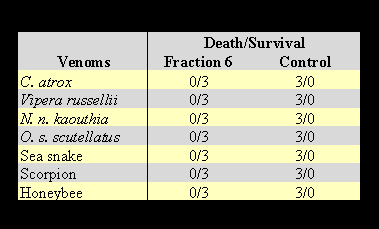
Opossum serum was fractionated on a liquid phase fractionating system. HPLC fractionation yielded nine fractions which were dialyzed and concentrated using a Spectrum (USA) dialysis apparatus. The protein concentration for each fraction was measured on a spectrophotometer using a protein assay kit from Bio-Rad company (USA). Each fraction was adjusted to 1 mg/ml concentration of the protein content. Each fraction was mixed with an equal volume lethal dose of venom from C. atrox. In this case, 200 µg of the protein from each fraction was tested on mice versus a predetermined lethal dose of C. atrox venom. One of the fractions showing anti-lethal activity for C. atrox venom is LTNF. The identified active fraction of LTNF was concentrated and refractionated on HPLC by a second run under identical conditions for further purification. The obtained homogeneous LTNF was used for these studies. Two hundred micrograms of the purified fraction of LTNF (Figure 1) was mixed with an equal volume of the predetermined lethal doses of snake venoms for injection into mice. The results are shown in Table 3.
The results in Table 3 clearly indicate that LTNF shows the ability to neutralize the toxic lethal effects of venoms of snake, scorpion and bee venoms. The molecular weight of natural LTNF was demonstrated to be 63 kDa, corresponding to the albumin component of the opossum serum (Figure 2).
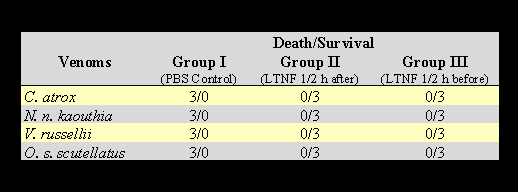
The neutralizing effect of LTNF was tested in mice half an hour before and after the injection of lethal dose of venoms, as documented in Table 4. The mice were divided into three groups for each venom. The mice in group III were injected with 200µg of LTNF in 0.5 ml volume, half an hour before the lethal dose of venom was given. The mice in group II were injected with LTNF half an hour after venom injection. The mice in group I were given 0.5 ml of PBS after venom injection. The results are shown in Table 5.
The results in Table 4 clearly show the strong anti-lethal activity of LTNF identified in opossum serum. LTNF is effective in neutralizing the effect of venoms when given half an hour before or after venom injection. The anti-lethal activity of LTNF was exhibited when it was administered half an hour before venom injection, therefore, it can be used as a preventive measure, especially for snake handlers, etc.
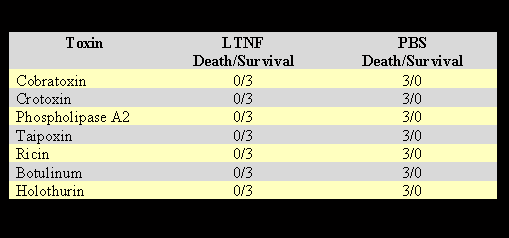
After injecting the predetermined lethal dose of toxins derived from animals, plants, and bacteria, the mice were given 200µg of LTNF in 0.5 ml volume by IP route. The control mice were given 0.5 ml PBS. The results are shown in Table 5.
The results in Table 5 show that TNF neutralizes the lethal toxic effects of various snake venom toxins: crotoxin, cobratoxin, PLA2, and, the most potent taipoxin. Furthermore, LTNF neutralizes the lethal effects of ricin, one of the most toxic plant-derived toxins, and also the bacterial toxins botulinum and holothurin.
DISCUSSION
Snakebite continues to be a major medical problem among rural communities in developing countries. The experimental preparation of anti-Naja serum by Calmette in 1894 (2) led Vital Brazil (1898) (1) to make the first polyvalent serum against Bothrops jararaca and Crotalus durissus terrificus for therapeutic use. Since then, equine antivenoms are in use for the treatment of snakebites, although side effects associated with the sensitivity to equine protein occur in about 70% of all treatments. Apparently, the risk of death from serious snakebite envenoming is greater than the risk of the side effects of antivenom therapy.
Non-equine antivenom would be a comfort to those especially sensitive to horse proteins. Recently, UK based Therapeutic Antibodies Inc. has produced antivenom in sheep to overcome the horse protein allergy problem. However, 20% of the treated patients in clinical trials also showed serum sickness to sheep antivenom.
Currently, Wyeth-Ayerst is the sole producer of antivenom made in horses for the treatment of snakebites in North and Central America. For worldwide needs, antivenoms are made for use against the venoms of snakes prevalent in any given the region. As a result, there are numerous antivenoms made by more than 70 different producers. Wyeth antivenoms are made against the venoms of species of snakes prevalent in North and Central America, for instance: C. atrox, C. durrisus terrificus, and Agkistrodon bothrops. Thus, Wyeth's antivenom is effective only for the snakebites of these species.
On the other hand, LTNF is effective against the venoms of all species of snakes. Therefore, LTNF can become a universal treatment for snakebites. Furthermore, LTNF is effective against scorpion and bee venoms, plant-derived ricin and bacterial toxin botulinum. Therefore, LTNF can become a universal treatment for toxins derived from animals, plants, and bacteria. In the standard treatment for snakebites, massive amounts of antivenom are administered for effectiveness, even though a large part of the population is hypersensitive to antivenom made in horses. Under such conditions, LTNF will be a most favorable replacement. It is further anticipated that the invention of LTNF has military applications due to the variety of unknown exposures that can occur under military conditions.
ACKNOWLEDGEMENTS
Opossum serum was provided by Dr. Mark Peckham of the Houston Zoo.
REFERENCES
01 BRAZIL V.
La defense contre lóphidisme. São Paulo: Poccai & Weiss, 1914. 319p.
02 CALMETTE A. Contribution a l'etude du venin des serpents. Immununisation et traitement de l'envenemation. Ann. Inst. Pasteur, 1894, 8, 275-91.
03 CATANESE JJ., KRESS LF. Isolation from opossum serum of a metalloproteinase inhibitor homologous to human alpha 1 B-glycoprototein. Biochemistry, 1992, 31, 410-8.
04 DOMOND GB., PERALES J., MOUSSATCHE H. Natural anti-snake venom proteins. Toxicon, 1991, 29, 1183-94.
05 FAYRER J. Deaths from snakebites: a trial condensed from the sessions report. Ind. Med. Gaz., 1869, 4, 156.
06 LIPPS BV., LIPPS FW. Embodiments of natural and synthetic lethal toxin neutralizing factors and their utility as treatment for envenomation. US patent, 1996, 5, 576, 297.
07 MENCHAGA JM., PEREZ JC. The purification and characterization of an antihemorrhagic factor in opossum (Didelphis virginiana) serum. Toxicon, 1981, 19, 623-32.
08 OVADIA M. Purification and characterization of an antihemorrhagic factor from the serum of the snake Vipera polestinae. Toxicon, 1978, 16, 661-72.
09 PERALES J., MOUSSATCHE H., MARANGONI S., OLOVERA B., DOMONT GB. Isolation and partial characterization of an anti-bothropic complex from the serum of South American Didelphidae. Toxicon, 1994, 32, 1237-49.
10 PEREZ J., HAWS W., GARCIA V., JENNINGS B. Resistance of warm-blooded animals to snake venoms. Toxicon, 1978, 16, 375-83.
11 TOMIHARA Y., KAWAMURA Y., YONAHA K., ZOZAKI M., YAMAKAWA M., YOSHIDA C. Neutralization of hemorrhagic snake venoms by sera of Trimeresurus flavoviridis (Habu), Herpestes edwardsii (mongoose) and Dinodon semicarninatus (Akamata). Toxicon, 1990, 28, 989-91.
12 WEISSENBERG G., FLEMINGER G., KOCHVA E. Antihemorrhagic factors from the blood serum of western diamondback rattlesnake, Crotalus atrox. Toxicon, 1991, 29, 807-18.
- 02 CALMETTE A. Contribution a l'etude du venin des serpents. Immununisation et traitement de l'envenemation. Ann. Inst. Pasteur, 1894, 8, 275-91.
- 03 CATANESE JJ., KRESS LF. Isolation from opossum serum of a metalloproteinase inhibitor homologous to human alpha 1 B-glycoprototein. Biochemistry, 1992, 31, 410-8.
- 04 DOMOND GB., PERALES J., MOUSSATCHE H. Natural anti-snake venom proteins. Toxicon, 1991, 29, 1183-94.
- 05 FAYRER J. Deaths from snakebites: a trial condensed from the sessions report. Ind. Med. Gaz., 1869, 4, 156.
- 06 LIPPS BV., LIPPS FW. Embodiments of natural and synthetic lethal toxin neutralizing factors and their utility as treatment for envenomation. US patent, 1996, 5, 576, 297.
- 07 MENCHAGA JM., PEREZ JC. The purification and characterization of an antihemorrhagic factor in opossum (Didelphis virginiana) serum. Toxicon, 1981, 19, 623-32.
- 08 OVADIA M. Purification and characterization of an antihemorrhagic factor from the serum of the snake Vipera polestinae Toxicon, 1978, 16, 661-72.
- 09 PERALES J., MOUSSATCHE H., MARANGONI S., OLOVERA B., DOMONT GB. Isolation and partial characterization of an anti-bothropic complex from the serum of South American Didelphidae. Toxicon, 1994, 32, 1237-49.
- 10 PEREZ J., HAWS W., GARCIA V., JENNINGS B. Resistance of warm-blooded animals to snake venoms. Toxicon, 1978, 16, 375-83.
- 11 TOMIHARA Y., KAWAMURA Y., YONAHA K., ZOZAKI M., YAMAKAWA M., YOSHIDA C. Neutralization of hemorrhagic snake venoms by sera of Trimeresurus flavoviridis (Habu), Herpestes edwardsii (mongoose) and Dinodon semicarninatus (Akamata). Toxicon, 1990, 28, 989-91.
- 12 WEISSENBERG G., FLEMINGER G., KOCHVA E. Antihemorrhagic factors from the blood serum of western diamondback rattlesnake, Crotalus atrox Toxicon, 1991, 29, 807-18.
Publication Dates
-
Publication in this collection
16 Apr 1999 -
Date of issue
1999