Abstract
Death caused by scorpion envenoming (Buthidae family) is a common event in tropical and subtropical countries. Severe scorpion envenoming causes an autonomic storm resulting in a massive release of catecholamines, angiotensin II, glucagon, cortisol, and changes in insulin secretion. As a consequence of these changes in the hormonal milieu, scorpion envenoming results in a syndrome of fuel energy deficits and an inability of the vital organs to utilize the existing metabolic substrates, which causes myocardial damage, cardiovascular disturbances, peripheral circulatory failure, pulmonary oedema, and many other clinical manifestations alone or in combination, producing multi-system-organ-failure (MSOF) and death. Insulin-glucose infusion or antivenom administration through the release of insulin seems to be the physiological basis for the control of the metabolic response when that has become a determinant to survival of scorpion sting victims.
catecholamines; angiotensin II; glucagon; cortisol; insulin; myocardial damage; cardiovascular disturbances; peripheral circulatory failure; pulmonary oedema; multi-system-organ-failure (MSOF)
Review article
The scorpion envenoming syndrome: a different perspective. The physiological basis of the role of insulin in scorpion envenoming
K. R. KRISHNA MURTHY1 CORRESPONDENCE TO:
K. R. KRISHNA MURTHY - Department of Physiology, Seth G.S. Medical College & K.E.M. Hospital, Parel, Mumbai 400 012, India.
CORRESPONDENCE TO:
K. R. KRISHNA MURTHY - Department of Physiology, Seth G.S. Medical College & K.E.M. Hospital, Parel, Mumbai 400 012, India.
1 K. R. KRISHNA MURTHY - Department of Physiology, Seth G.S. Medical College & K.E.M. Hospital, Parel, Mumbai 400 012, India.
ABSTRACT. Death caused by scorpion envenoming (Buthidae family) is a common event in tropical and subtropical countries. Severe scorpion envenoming causes an autonomic storm resulting in a massive release of catecholamines, angiotensin II, glucagon, cortisol, and changes in insulin secretion. As a consequence of these changes in the hormonal milieu, scorpion envenoming results in a syndrome of fuel energy deficits and an inability of the vital organs to utilize the existing metabolic substrates, which causes myocardial damage, cardiovascular disturbances, peripheral circulatory failure, pulmonary oedema, and many other clinical manifestations alone or in combination, producing multi-system-organ-failure (MSOF) and death.
Insulin-glucose infusion or antivenom administration through the release of insulin seems to be the physiological basis for the control of the metabolic response when that has become a determinant to survival of scorpion sting victims.
KEY WORDS: catecholamines, angiotensin II, glucagon, cortisol, insulin, myocardial damage, cardiovascular disturbances, peripheral circulatory failure, pulmonary oedema, multi-system-organ-failure (MSOF).INTRODUCTION
Death caused by scorpion envenoming is a common event in tropical and subtropical countries (1-10) (11-21) (23,24,26-33) (34-43) (44-49) (51-55) (56-65) (66-75). About 800 species of scorpions belonging to 6 families all over the world have been described. The largest family of scorpions is the Buthidae with the most toxic species, such as Androctonus, Buthus, Centruroides, Leiurus quinquestriatus, and Tityus. In spite of zoological differences resulting in venoms of different chemical structure, symptomatology following human envenomation is quite similar and may involve the central nervous system (CNS), the autonomous nervous system, the respiratory tract, the pancreas, and the cardiovascular system in experimental animals (1,8-11,15-17,20,26) (27,32-34,37,49,51-55) (71,73,78,88,91,94-98) (99-106) (107-112) (114-116,119,121,123,142,143,146,150) and scorpion sting victims (3-7,12-14,19) (29-31,38-47) (50-55,57-59) (68-71,76,77,93,113,117,118,120) (124,125,127,128,131-134) (136,137,139,141).
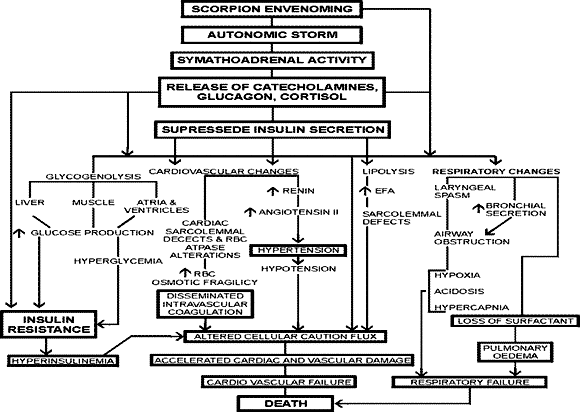
India harbours 99 species of scorpions belonging to all 6 families of scorpions, of these 45 species belong to the Buthidae family. These venomous scorpions are distributed in a few "scorpion districts" throughout India (50). The distribution of different species of scorpions of medical importance in India are shown in Figure 1 and Figure 2. Deaths due to these killer scorpions are reported from the Rayalaseema region of Andhra Pradesh, a few districts in Karnataka, Tamilnadu, Uttar Pradesh, West Bengal, Orissa, Maharashtra, and many other places (140). According to Vachons classification (140), the genus Buthus of the Buthidae family has been split into many genera, several species being reported in the Indian fauna. All those species previously reported under the genus Buthus have been transferred to various genera and at present, not a single species represents the original genus Buthus from India. Signs and symptoms following stings by dangerous scorpions are remarkably similar (2-6,10-15) (16-19,27,29-31) (37-46) (50-55,57-59,69-71) (76,77,79,93,99,100,113) (117,118,120,124,125) (128,130-133,137,139-141,146,150). The probable mechanisms of death due to scorpion envenoming are shown in Figure 3.
There have been many excellent review articles on scorpion envenoming syndrome (51,52,54,55). However, Ismail (51,52,54,55) has not considered the changes in the hormonal environment due to scorpion envenoming and, therefore, the therapeutic role of insulin. Important advances are being made in scorpion envenoming. This review highlights some of these advances. This review has used information on trials obtained from colleagues and data published in literature.
ACUTE MYOCARDITIS AND ECG CHANGES. Acute myocarditis is often missed because of the severity of associated manifestations. Non-specific electrocardiographic (38,39,41,42,46) changes are characteristic of scorpion myocarditis. Ventricular ectopics, low voltage RS-T and T wave changes, ST-T changes, myocardial infarction-like patterns, ST depression, ST elevation, prolonged QT-C, sinus bradycardia, sinus tachycardia, and ventricular premature contractions were some of the features of electrocardiographic changes in scorpion sting victims (2-5,12,13,16-18,19,22) (30-34,38,39,41,42) (44-47,50,53,54,57-59,69) (70,76,77,79,83,113,117,118,120) (123-135,143,144).
In humans, none of the electrocardiograms (ECG) were normal on admission (3-5,19,40,46,50-52,77,93,136). The changes recorded were bizarre, broad, notched, biphasic T waves with ST segment changes (elevation or depression) followed by Q waves (38,39,41,42,46), consistent with an acute myocardial infarction-like pattern (52). Bundle branch block pattern, beat to beat electrical alternation of R wave (40), and disturbances, such as atrial fibrillation, A-V dissociation with accelerated junctional rhythm, APC (atrial premature complex) and VPC (ventricular premature complex), left bundle branch block, and first or second degree heart block were also recorded (41). We have reported almost all of the ECG changes noticed earlier in experimental or human envenoming (95-105) (106-110) (111-116). We have recorded myocardial infarction-like patterns, junctional rhythm, and electrical alternans in experimental scorpion myocarditis, which was also reported in scorpion sting victims (43,105).
CARDIAC ENZYME CHANGES. Envenoming by the scorpion Leiurus quinquestriatus caused an increase in the activity of succinate dehydrogenase (41). A rise in creatine phosphokinase (CPK) activity in mice following envenoming was reported (52). Androctonus amoreuxi venom caused an apparent but not significant increase in serum levels of aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and glucose-6-phosphate dehydrogenase (G6PD) (117). Radmanesh (117) reported normal levels of serum glutamic-oxaloacetic transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), LDH, and creatine phosphokinase (CPK) in patients stung by the scorpion Hemicorpius lepturus. Gajalakshmi (32-34) reported a significant rise in SGPT levels after envenoming by the scorpion Buthus tamulus. Jain et al. (57) reported changes in the levels of SGPT and SGOT enzymes in a 15-year old girl stung by a scorpion. CPK was found to be markedly elevated even 48 h after scorpion sting (51-55). Sofer and Gueron (136) reported high levels of SGOT, total CPK, creatine-MB isoenzyme (CK-MB), and ECG changes consistent with myocardial damage. They also suggested that CK-MB activity is specific and highly sensitive in detecting myocardial damage in children following scorpion envenoming, and that myocardial lesions are too small to cause heart failure in most cases, but these might account for the cardiovascular changes frequently seen in scorpion envenoming.
Depressed left ventricular function was observed in echocardiographic studies in scorpion sting victims (4,71,137). Radionuclide ventriculography (MUGA Scan) showed decreased contraction of the septum and apical wall, and a reduced left ventricular contractility with reduced ejection fraction (118). Gueron et al. (38-46) evaluated the echocardiographic and radionuclide angiographic abnormalities in children after scorpion envenoming by L. quinquestriatus. All the ECG, echocardiographic, radionuclide, and ventriculography studies showed enzymatic evidence of myocardial damage, as indicated by an elevation of SGOT, CPK, MB-PK ratio in scorpion sting victims (4,44,79,118).
Scorpion envenoming resulted in an increase of circulating LDH, SGOT, SGPT, CK-MB, and alpha hydroxy butyrate dehydrogenase (HBDH) enzyme levels (68,114,116) at 60 min, with a further rise 120 min following venom injection. Administration of scorpion antivenom (SAV) at different time intervals after venom injection resulted in a reversal of ECG changes and a reduction in the cardiac enzyme levels (98,114,116).
ELECTROLYTE CHANGES AND ECG IN SCORPION ENVENOMING. The cardiac effects in experimental animals were comparable to the changes observed in human envenoming (52). Scorpion venoms appeared to cause some of their ECG abnormalities through electrolyte changes. This could be seen by the tall, peaked, and slender T waves, and the wide QRS complex characteristic of hyperkalemia, and the prolonged ST segment or QTC interval in addition to the S wave > R wave characteristic of hypocalcemia. Similar effects were seen in the ECG records of more than 600 scorpion sting victims (52). The tall and peaked T wave and the prolonged QTC interval were also recorded in all the victims of yellow scorpion stings (41). The effects of autonomic stimulation usually mask those of electrolyte changes. It seems likely that vagal stimulation potentiates, while sympathetic stimulation masks the effect of electrolyte changes in the ECG recordings seen after scorpion venom injection, since the changes were more rapid in the onset and much more pronounced when the sympathetic actions of the venom were blocked with propranolol or phentolamine (52).
A retrospective study of 223 scorpion sting victims revealed the occurrence of hyponatremia accompanied by normal to elevated levels of serum potassium and lowered serum calcium. B. minax, B. occitanus, and A. amoreuxi venoms caused a significant increase in serum potassium levels (52). We have observed hyperkalemia and hyperglycemia in dogs following B. tamulus venom injection (106).
The following factors may contribute and aggravate the condition of hyperkalemia: a) the deficiency of some of the normal body mechanisms, causing K+ influx and arising primarily from the pronounced and prolonged hyperglycemia, enhanced glycogenolysis, and inhibited glycogenesis from decreased insulin secretion; b) venom-induced release of catecholamines, which in turn, causes K+ efflux from the liver; c) decreased serum Ca++ secondary to its increased deposition in the heart; and d) decreased serum magnesium (52). We have demonstrated a reduction in insulin (68,98,108,109,112,114), hyperglycemia (68,96,98,106-109,112), and enhanced glycogenolysis in the atria, ventricle, liver, and skeletal muscle of rabbits and dogs following scorpion envenoming.
The unopposed hyperkalemia can explain the ECG changes seen in sting victims and experimental animals injected with scorpion venom. Thus, the tall, slender "tented" T waves, the wide slurred bizarre QRS complex, the prolonged QTC intervals, and ST segment elevation can result from hyperkalemia and hypocalcemia, while the prolonged PR and QTC intervals, premature ventricular contractions, ventricular tachycardia, and ventricular fibrillation can result from hypomagnesemia. It is also known that in acute myocardial infarction, ST segment elevation is probably due to a leakage of K+ from damaged myocardial cells into the interstitial fluid, resulting in "localized" hyperkalemia (52).
The relationship between cellular potassium and ECG is described by Schamroth (129). A slight loss of intracellular potassium causes a reduction in the resting membrane potential. This causes alterations in the repolarization process in the ventricle, manifested as T wave changes in the ECG. The ST segment changes in the ECG are due to further reductions in the intracellular potassium level. At the resting membrane potential with a 50% loss of intracellular potassium, the myocardial cell is electrically dead, and is seen as a Q wave in the ECG. The potassium loss reflected in the ECG is probably due to decreased cardiac sarcolemmal Na+ -K+ ATPase activity observed in atria and ventricles of venom-treated rabbits (95).
The hyperkalemia assumption can also explain the reversal of the ECG changes induced by B. tamulus venom in dogs after treatment with insulin alone (112), insulin and sodium bicarbonate (109), or sodium bicarbonate in intensive care treatment of severe cases of envenoming by L. quinquestriatus and A. crassicauda (52).
Insulin is known to stimulate K+ uptake by the skeletal muscle and hepatic cells, while sodium bicarbonate stimulates the movement of extracellular K+ back into cells by effects more complex than the pH-induced K+ redistribution. The antagonism of Ca++ and K+ in the cardiac tissue is well documented in classical physiology. However, it should also be remembered that the electrolyte effects on the ECG can be largely influenced by other hemodynamic actions induced by scorpion venom (52).
SCORPION ENVENOMING AND FREE FATTY ACID (FFA) LEVELS. There was a sudden increase in free fatty acids (FFA) along with a simultaneous reduction in triglyceride levels following envenoming (68,103,108,109,112,114). Increased catecholamines (3), elevated levels of glucagon, cortisol (106), and changes in insulin secretion (68,89,97,98,108,109,112,114,148) might be responsible for the increased FFA levels following envenoming.
EFFECT OF INCREASED FFA ON THE HEART. The use of increased amounts of circulating FFA results in increased oxygen consumption. This could aggravate the ischemic injury to myocardium, predisposing to arrhythmias and heart failure. The elevated FFA also increase the susceptibility of the ventricles to the disorganized electrical behavior and produce ectopic beats in the vulnerable period of cardiac cycle. Under pathological conditions, high levels of FFA produce inhibition of Na+ -K+ -stimulated ATPase activity (148) and sarcolemmal defects (95). The increased FFA, by altering the function of platelets, may increase the tendency to intravascular thrombus and result in disseminated intravascular coagulation (DIC) (27,108).
THE METABOLISM OF NORMAL AND INJURED MYOCARDIUM AND THE ROLE OF INSULIN. Under normal conditions, the myocardium derives its energy from the aerobic metabolism of substrates extracted from the plasma. The most important fuels are FFA, glucose, triglycerides, amino acids, pyruvate, and lactate. At rest, the myocardial extraction of many of these substrates is generally related to their arterial concentrations, but the relative uptake of each compound may be modified by hormones and utilization of other substrates. Thus, glucose transport into the myocardial cell depends on insulin, and increases of plasma insulin concentration enhance the extraction of glucose by the heart. On the other hand, myocardial glucose utilization is negatively correlated with plasma FFA levels, which means that high plasma FFA concentrations inhibit glucose uptake by the heart. In the human heart, a 10% increase of plasma FFA decreases the myocardial extraction of glucose by 17%, while a 10% increase of plasma insulin enhances glucose utilization by an average of 24% (85). We have consistently demonstrated a sudden increase in FFA levels (200% to 300% rise) in experimental scorpion envenoming (68,103,108,109,112,114).
The formation of high-energy adenosine triphosphate (ATP) from FFA and triglyceride fatty acids is strictly dependent on maintained oxygen supply, whereas glucose may produce ATP in the heart muscle also by anaerobic glycolysis. High plasma FFA concentration increases myocardial oxygen consumption without augmenting mechanical performance. In the anoxic myocardium, the synthesis of ATP from FFA is suppressed, and the only way to maintain the energy supply for contraction is by anaerobic metabolism of glucose. However, if utilization is inhibited by FFA, an increase in plasma FFA levels may be disadvantageous to the ischemic myocardium. That this really is the case, is shown by clinical and experimental observations that high plasma FFA levels in patients with myocardial infarction are associated with increased incidence of arrhythmias, and that the elevation of FFA to very high levels may cause arrhythmias, decrease heart contractility, and increase the extent of myocardial damage after experimental coronary occlusion (85).
Elevated FFA levels with incidence of different types of arrhythmias, conduction defects, ischemia, and infarction-like ECG patterns have been shown in experimental scorpion envenoming and in scorpion sting victims (68,95,97,101-103,105,106). We have already reported a reduction in FFA levels, disappearance of different arrhythmias, conduction defects, ischemia, and infarction-like ECG patterns with normal sinus rhythm after insulin administration in experimental envenoming and in scorpion sting victims (68,95,96,98,101-103) (105,106,108,109,114).
Insulin counteracts all these deleterious effects of FFA by: a) inhibiting the catecholamine-induced lipolysis in the adipose tissue, thus reducing the plasma FFA level; b) facilitating the glucose transport to the myocardium and glucose metabolism through different pathways; and c) increasing the intracellular potassium concentration (85).
THE IONIC BASIS OF CARDIAC FAILURE. Since the Na+ -K+ activated ATPase enzyme, which is responsible for maintaining the selective ionic permeability of cardiac cell membranes is Mg++ -dependent, it is not surprising to find that a reduced cellular Mg++ results in cellular loss of K+ and gain in Na+ . The Mg++ -dependent loss of cellular K+ may be due to a failure of Na+ -K+ activated ATPase enzyme, for which Mg++ ATP is the substrate, or an enhanced loss of K+ from the mitochondria (84).
A myocardial loss of K+ has been noted in infarcted areas of the heart subsequent to experimental ligation of the coronary vessels and in patients who died suddenly due to myocardial infarction. Tissue hypoxia overproduction of corticosteroids also cause a loss of tissue Mg++ , and consequently a loss of K+ , and a gain in Na+ . Since the correct proportional distribution of Na+ and K+ is an indisputable requirement for normal electrical stimulation and impulse propagation, it is difficult to escape the conclusion that Mg++ loss precipitates cardiac malfunction (84). Alterations in cardiac sarcolemmal Mg++ -dependent, ouabain sensitive Na+ -K+ ATPase, Mg++ ATPase, and Ca++ ATPase activities, indicating sarcolemmal defects, have been demonstrated in scorpion envenoming (95).
EFFECTS OF ACUTE ISCHEMIA ON MYOCARDIAL METABOLISM. The immediate metabolic changes in the myocardium during acute ischemia is largely determined by the rates of glycolysis and glycogenolysis and, to a lesser extent, of fatty acid availability in relation to the demand for phosphorylation (87).
Glycolysis increases with mild hypoxia, and in areas of profound hypoxia, decreased glycogenolyisis occurs. Hydrolysis of stored triglycerides results from the activation of myocardial lipase with increases in FFA (87). Greater glycogenolysis was observed in atrial and ventricular tissue in response to a smaller dose of scorpion venom compared to lower glycogenolysis with a higher dose of scorpion venom (8,9). In addition, important early systemic changes have been recorded in man in the first hours of the onset of acute myocardial ischemia. These probably result from the anxiety and pain associated with ischemia, including a sustained rise in plasma catecholamines, a marked increase in plasma FFA and plasma cortisol concentrations, a transient elevation in blood glucose, and decreased plasma insulin levels (87).
Plasma FFA are absorbed by tissues in an exponential relationship to their molar binding with plasma albumin, while glucose uptake depends on adequate concentrations of plasma insulin, which is reduced in acute myocardial infarction. In many patients, the increase in plasma FFA concentrations is such that the two main binding sites of albumin are saturated, and from then onwards can be speculated that the ischemic myocardium extracts proportionately more FFA than at lower plasma concentrations. The ischemic myocardium is presented, therefore, with a considerable excess of FFA relative to glucose and, in a severely ischemic zone, the available oxygen may be insufficient for oxidation (87).
MYOCARDIAL VULNERABILITY. While myocardial vulnerability may finally be determined by critical extra- and intracellular gradients in the availability of Ca++ , Mg++ , and K+ , the intracellular concentrations of these ions, are in turn, dependent on factors which influence their transference across the cell and mitochondrial membranes. A decreased availability of Ca++ can interfere with actin-myosin coupling so that contractility is impaired. An excess loss of K+ could alter action potential so that self-perpetuating re-entry currents are established (87). But, what governs these changes?
One possibility is that low concentrations of myocardial Mg++ are associated with arrhythmias and sudden death. Another possibility is that the intra- and extracellular concentrations of these ions are initially adequate for the continuance of physiological functions but that the acute changes that occur in the aerobic metabolism and in the concentration of intracellular substrates, alter the ionic balance critically and lead to a rapid deterioration of function and loss of viability (87).
Accumulation of excess intracellular FFA could have a detergent effect on cell membranes. This accumulation could result from decreased oxidation in the myocardium and catecholamine-induced hydrolysis of stored triglycerides in addition to increased uptake resulting from higher concentrations in arterial blood. The evidence that excess intracellular FFA could adversely influence myocardial metabolism in the presence of acute ischemia is based on studies about the relationship between high plasma FFA with arrhythmias and myocardial function. Elevated concentrations of plasma FFA have been associated with an increased prevalence of serious ventricular arrhythmias and death in man and in dogs. Elevated FFA levels have also been shown to increase myocardial oxygen consumption and, when there is an under-perfusion of the left ventricle, to decrease myocardial contractility. In dogs with experimental myocardial infarction, high plasma FFA levels increased ST elevation and plasma CK levels, suggesting that they may lead to more extensive damage (87).
CAN EXCESS UNOXIDIZED FFA INCREASE MYOCARDIAL VULNERABILITY. Excess unoxidized FFA probably become toxic to the myocardium only when there is acute ischemia. It is difficult to dissociate the toxic effects of increased local FFA concentrations from those of catecholamines, although it is possible to experimentally show that elevations of plasma concentration of FFA can lead to adverse effects on myocardial function in the absence of changes in plasma catecholamine levels. Each of these toxic effects can increase local oxygen consumption and could lead to a critical deficit with changes in lysosomal and mitochondrial integrity, and together they can have a synergistic effect (87).
The biochemical consequences which result from the intracellular accumulation of excess unoxidized FFA or their metabolites could be that some unoxidized FFA soon bind to intracellular proteins. Calcium-fatty acid complexes can develop, and competition for Ca++ will occur simultaneously with protein binding, possibly making less ionic calcium available for passage into the sarcotubular reticulum. This might interfere with tropomyosin-troponin activation of actin-myosin coupling.
Magnesium-fatty acid complexes could result and so any intracellular depletion of Mg ++ would be exaggerated. This might lead to uncoupling of oxidative phosphorylation, possibly by interfering with a magnesium-dependent ATPase system.
Fatty acids may be transported intracellularly in the unesterified form and have specific affinities for certain subcellular structures. Esterification of certain phospholipids of the mitochondrial and cell membranes may be altered when there are excess unoxidized FFA. Different cell membranes have lipoprotein layers with variable permeability for ions, and this could also be changed if the intracellular accumulation of FFA were to have a detergent action leading to cation loss. Excess unoxidized fatty acids or metabolites could alter the stabilization of lysosomal membranes (85).
INITIAL TRANSIENT HYPERTENSION IN SCORPION ENVENOMING. All scorpion venoms cause pronounced hypertension in experimental animals (68,101-106,114) and children (14,28,29,30,39) stung by scorpions. Hypertension from scorpion envenoming is compared with a massive outpouring of catecholamines observed in phaeochromocytoma and is considered one of the causes for the development of scorpion venom-induced cardiac failure and pulmonary oedema (30,31,39,40,43-46,52). Scorpion venom crosses the blood-brain barrier poorly (51,52,55). However, either the CNS or the cardiovascular manifestations could occur first in the early phases of scorpion envenoming syndrome. CNS manifestations, however, always preceded terminal hypotension and cardiac arrest (55). Most scorpion sting victims are infants and children. It is thought that there is a possible incomplete development of the blood-brain barrier in these victims, allowing venom penetration, which results in central action of scorpion venom (51,52).
Scorpion venoms contain protein toxins that prolong the opening of Na+ channels by slowing the inactivation process, the so-called alpha scorpion toxins, resulting in an increase in neuron excitability, leading to repetitive action potentials. This can lead to an increase in transmitter release (49,78). Scorpion venoms also contain protein toxins, the beta scorpion toxins, which cause Na+ channels to open at membrane potentials at which they would normally be closed. These toxins can produce a sustained depolarization of nerve membranes and nerve terminals. This can block action potential conduction in axons and cause an uncontrolled release of transmitters from nerve endings (49,78).
The alpha-type scorpion toxins are isolated from "Old World" scorpion venoms, such as Androctonus australis, Hector, Leiurus quinquestriatus, Buthus tamulus, and Buthus eupeus. The beta-type scorpion toxins are isolated from "New World" scorpion venoms, such as Centruroides suffusus, Centruroides sculpturatus, and Tityus serrulatus (49,78). The combined actions of alpha and beta neurotoxins and potassium channel toxins (charybdotoxin from the "Old World" scorpion Leiurus quinquestriatus)(49) might be responsible for the potentiation of the hypertensive response (52).
HYPOTENSION IN SCORPION ENVENOMING. Hypotension, following the initial transient hypertension, is clinically important and a serious medical problem in severe cases of scorpion envenoming. The mechanisms speculated for the causes of hypotension include a depressive cholinergic effect, a catecholamine depletion syndrome, an exaggerated beta2 vasodilator effect and/or hypovolemia secondary to excessive fluid loss (32-34,39,40,43-46), and partial participation of the kinins and/or prostaglandins in the genesis of terminal hypotension (52). On the other hand, Freire-Maia and Campos (30) believe that hypotension could be due to: 1) apnea and bradycardia, 2) a reflex effect induced by the stimulation of pulmonary oedema, and 3) pulmonary oedema and heart failure. In addition to these, we believe that hypotension is due to an extravasation of fluids caused by angiotensin II (104).
The presence of circulating ATP inhibitors has been demonstrated. These inhibitors tend to increase intracellular sodium and calcium content. The net result of these changes in cell sodium would be a secondary accumulation of free calcium ions in the cytosol via sodium calcium exchange. The dynamic balance between intracellular cyclic adenosine monophosphate (cAMP) and intracellular calcium content is known to determine the degree of vasoconstriction in vascular smooth muscle cells (52). Changes in potassium flux exert secondary effects on calcium and other intracellular cations. Catecholamines directly affect ATPase, and therefore, cellular potassium distribution with alpha receptors, stimulating this process. Insulin deficiency is another factor stimulating these processes. Thus, potassium flux abnormalities could be related to insulin resistance and hypertension. Potassium metabolism itself may be a critical factor linking vasoconstriction, glucose and insulin metabolic abnormalities, and target organ damage (56).
SCORPION ENVENOMING AND HEMOLYSIS. Scorpion envenoming resulted in an increased plasma hemoglobin (plasma Hb) levels (68). The rise in plasma Hb indicates hemolysis, and the highest levels are found when hemolysis takes place predominantly in the blood stream (intravascular hemolysis). The presence of excess Hb in the plasma is a reliable sign of intravascular hemolysis (23). The venom of certain venomous snakes, such as the cobra, and venoms of various insects cause destruction of RBC. Cobra venom releases an enzyme phosphatidase A, which converts lecithin to lysolecithin, a powerful hemolytic and cytolytic substance. Since lecithin is present in erythrocytes, plasma, and all the cells, the entrance of the venom into the body stimulates production of the hemolytic substance. The presence of hyaluronidase, phospholipase A, acetylcholinesterase, alkaline phosphatase, acid phosphatase, 5 nucleotidase, ATP, ribonuclease, and deoxyribonuclease were demonstrated in the venom of Buthus tamulus (1). The presence of intravascular hemolysis, as indicated by plasma Hb, is a new finding in our study. When the rate of hemolysis is excessive, the plasma extracorpuscular hemoglobin can not be converted into bilirubin as quickly as it is released and hemoglobinuria may occur. When the concentration of plasma extracorpuscular hemoglobin exceeds the hemoglobin binding capacity and the tubular reabsorption capacity of the kidney, the excess free plasma Hb will be filtered and excreted in the urine. This could be the cause of hemolysis associated with hematuria due to scorpion stings (117).
SCORPION ENVENOMING AND HEMOCONCENTRATION. Packed cell volume (PCV) was found to be increased after venom injection in animals (68). This could be due to increased secretion of angiotensin II (104). Angiotensin II produces a significant decrease in blood volume by shift of fluid from intravascular to extravascular compartment, leading to peripheral circulatory failure and pulmonary oedema (28).
There is limited evidence that raised hematocrit levels may be associated with insulin resistance. It has been reported that intravenous infusion of insulin in doses that increase plasma insulin levels is associated with an increased transcapillary escape rate of albumin and reduced plasma volume. This may partially explain the association between hematocrit and hyperinsulinemia and insulin sensitivity. Data from clinical and epidemiological studies suggest that insulin resistance is associated with hemorheological derangements, including elevated hematocrit, which is a major determinant of whole blood viscosity (149). Hb levels were found to be increased following venom injection (68). The elevated Hb levels could be due to an increase in PCV. The increased Hb and PCV levels in scorpion sting victims at the time of hospital admission might mislead the clinician, and physicians might mistakenly be given the impression that the complete blood picture of the victim was normal.
Increased sympathetic activity causes elevated renin release by direct stimulation of juxtaglomerular cells. A subsequent increase in angiotensin secretion enhances the ongoing sympathetic nerve output by a direct action on the brainstem and by a blunting of baroreceptor mechanisms (28). Thus, the renin-angiotensin system (45,104) is an important facilitator of ongoing sympathoadrenal traffic (149).
SCORPION ENVENOMING AND HYPERGLYCEMIA. Blood glucose levels were found to be increased, resulting in hyperglycemia after envenoming (68,96,98,106-109,112). This could be due to a massive release of catecholamines (5), increased glucagon, cortisol (98), changes in thyroid hormone levels (96), and changes in insulin secretion (68,97,98,108,109,112,114).
EFFECTS OF AN ACUTE INCREASE IN EPINEPHRINE AND CORTISOL ON CARBOHYDRATE METABOLISM DURING INSULIN DEFICIENCY. Elevations in plasma epinephrine and cortisol levels are associated with scorpion envenoming (98). In diabetic patients, plasma epinephrine and cortisol levels increase during diabetic ketoacidosis. Goldstein et al. (36) demonstrated that an acute physiological rise in the plasma epinephrine level was associated with a transient increase in hepatic glucose production and a sustained fall in glucose clearance, which caused persistent hyperglycemia. The initial increase in glucose production was primarily due to an increase in hepatic glycogenolysis, whereas the later elevation in glucose production was due to a stimulation of gluconeogenesis (36).
Cortisol may be synergestic with other stress hormones. Glucose production was reported to be synergestically enhanced by a combined epinephrine, glucagon, and cortisol infusion, while glucose production was increased in an additive manner by epinephrine and glucagon infusions. Glucagon and cortisol modified lactate gluconeogenesis in an additive rather than a synergestic manner (36).
Small increases in the plasma epinephrine level during insulin deficiency can significantly worsen the resulting hyperglycemia. This occurs as a result of what is probably an additive effect on hepatic glucose production, without any additional change in glucose clearance. The small increase in epinephrine significantly increases the importance of gluconeogenesis, as the period of insulin deficiency becomes prolonged. Small acute physiological increases in cortisol during insulin deficiency, which occur in conjunction with increases in epinephrine, have no significant effect on glucose production or gluconeogenesis (36).
SCORPION ENVENOMING AND GLUCOSE TOXICITY. In virtually all tissues except the brain, glucose, at a fixed insulin concentration, promotes its own utilization in a concentration-dependent manner. The superiority of insulin in stimulating glucose oxidation seems to be explained by its anti-lipolytic effect. Even a small increment in serum insulin concentration promptly suppresses lipolysis, and consequently, the use of FFA for energy production, which in turn, enhances glucose oxidation. In contrast, glucose per se is unable to suppress lipolysis in man (48). The concept that glucose per se (i.e. hyperglycemia) may be a cellular toxin is less well appreciated. The concept of glucose toxicity, a hypothesis, can be stated as follows: Hyperglycemia may cause a generalized desensitization of all cells in the body through the downregulation of the glucose receptors /in the glucose transport system. In muscles and adipocytes, this would be reflected by a defect in insulin action, whereas at the level of beta cells of the islets of Langerhans, this would be manifested by an impairment in insulin secretion (25,48,122).
HEMODYNAMIC ABNORMALITIES IN SHORT-TERM INSULIN DEFICIENCY. In diabetic ketoacidosis, the simultaneous relative insulin deficiency and excessive secretion of counter-regulatory hormones lead to magnified lipolysis and beta oxidation of FFA with a parallel hepatic overproduction and peripheral underutilization of ketone bodies. The clinical characteristics of patients are drowsiness and over-breathing. In addition, signs of circulatory collapse, such as tachycardia, weak pulse, and low blood pressure are normally present (7). Similar clinical manifestations are usually observed in scorpion sting victims.
SCORPION ENVENOMING AND INSULIN LEVELS. Insulin levels, as measured by radioimmunoassay, were significantly suppressed or elevated after venom injection (68,97,98,108,109,112,114).
THE INSULIN/GLUCAGON RATIO. The insulin/glucagon ratio (I/G ratio) may be more important than the levels of individual hormones. A high I/G ratio produces an anabolic state with more nutrient incorporation into peripheral tissues. A high ratio is associated with low levels of cAMP and a respiratory quotient close to 1, indicating that carbohydrates are the predominant energy source (140). When the I/G ratio is low, a catabolic state is produced in which nutrients are mobilized. Scorpion envenoming causes a low I/G ratio(98).
What is "Hyperinsulinemia"?
Hyperinsulinemia is said to exist when plasma insulin levels are inappropriate for the blood glucose estimated simultaneously. When insulin levels are elevated with a normal glucose level, "true hyperinsulinemia" is the most appropriate term, while high insulin levels with elevated blood glucose levels may be referred to as "insulin resistance" (82). Elevated insulin levels were observed 30 min following venom injection (114).
INSULIN-RESISTANT STATE. The relationship between insulin resistance, plasma insulin level, and glucose intolerance is significantly mediated to a significant degree by changes in ambient plasma FFA concentration (25,122). Plasma FFA levels can be suppressed by relatively small increments in insulin concentration. Consequently, an elevation of circulating FFA concentration can be prevented if large amounts of insulin are secreted. If hyperinsulinemia can not be maintained, plasma FFA concentration will not be suppressed normally, and the increase in plasma FFA concentration will result in increased hepatic glucose production. Because these events are taking place in individuals who are quite resistant to insulin stimulated glucose uptake, even small increases in hepatic glucose production are likely to lead to significant hyperglycemia under these conditions (25,122).
The observation of hyperglycemia or euglycemia in the face of concomitant hyperinsulinemia suggests an insulin-resistant state (68,98,108,109,112,114) in scorpion envenoming. Short-term hyperglycemia can induce insulin resistance (48).
CAUSES OF INSULIN RESISTANCE. In any insulin-resistant state, the cause of insulin resistance can be due to an abnormal beta cell secretory product, circulating insulin antagonists, or target tissue defect in insulin action (25,86,122).
Insulin resistance could be caused by a change in the receptor number, hormone-receptor binding characteristics, or post-receptor events. Insulin receptors are probably downregulated by high concentrations of agonist hormone/s. Post-receptor resistance can be caused by other hormones (56). Hormonal antagonists consist of all counter-regulatory hormones, such as growth hormone, cortisol, glucagon, and epinephrine. Increases in circulating levels of glucagon (98), cortisol (98) and catecholamines (3) have been demonstrated in scorpion envenoming.
Insulin resistance may occur because of pre-receptor, receptor, and post-receptor abnormalities (130). Insulin resistance as a result of pre-receptor involves metabolic (elevated counter-regulatory hormonal and non-hormonal) factors. Circulating insulin antagonists as a cause of insulin resistance have been clearly demonstrated in a variety of clinical syndromes. Excess endogenous or exogenous glucocorticoids are often associated with carbohydrate intolerance. Availability of substrates and plasma levels of glucagon glucocorticoids stimulate hepatic glucose production through increased activity of hepatic gluconeogenic enzymes.
In addition, corticosteroids decrease peripheral glucose utilization by diminishing the activity of glucose transporters and inhibiting insulin-mediated translocation of these facilitative transporters. Additionally, glucocorticoids affect insulin receptor affinity and number, decreasing insulin binding to its receptor (130).
States of catecholaminergic hyperactivity antagonize insulin effects through several mechanisms. Catecholamines stimulate hepatic glucose production by direct stimulation of glycogenolysis and gluconeogenesis and indirectly by increasing glucagon secretion. Additionally, catecholamines decrease peripheral glucose disposal both in vitro and in vivo (130).
It has been suggested that hyperlipidemia could impair peripheral glucose utilization by inhibiting glucose uptake and glucolysis secondary to increased cellular fatty acid oxidation rates (130).
The accelerated receptor degradation has been found to be responsible for a decreased number of receptors. The effect of acidic pH to accelerate insulin dissociation from the receptor is markedly reduced, leading to an inhibition of receptor recycling and acceleration of receptor degradation (130).
In addition to animal studies, several in vitro models of insulin resistance suggest defects in the receptor kinase activity. Catecholamines induce a 90% inhibition of the tyrosine kinase activity in isolated human adipocytes in vitro. The suggested cellular mechanism for this inhibitory effect is a change in the affinity of the ATP binding site, secondary to serine phosphorylation of the insulin receptor beta-subunit by a cAMP-dependent kinase (130).
Tissue insensitivity to insulin is an important pathogenic disturbance that contributes to glucose intolerance (25). It should be stressed that the tissues responsible for insulin resistance in the basal state are quite different from those responsible for insulin resistance in the insulin-stimulated state. In the basal state, the liver represents a major state of insulin resistance. This is reflected by an overproduction of glucose despite the presence of fasting hyperglycemia and hyperinsulinemia. In the insulin-resistance state, muscle is the primary tissue responsible for insulin resistance (25).
THE ROLE OF LIPID OXIDATION IN THE PATHOGENESIS OF INSULIN RESISTANCE. The role of lipid oxidation in the pathogenesis of insulin resistance in NIDDM (NIDD non-insulin-dependent diabetes mellitus) has been demonstrated. Increased FFA oxidation restrains glucose oxidation in muscle by altering the redox potential of the cell and inhibiting the several key enzymatic steps within the glycolytic cascade. When FFA is oxidized in excessive amounts, there is an accumulation of acety1 Co-A, a powerful inhibitor of pyruvate dehydroxygenase within the cell. In addition, because of the increased NADH/NAD, the Krebs cycle is slowed, and citrate accumulates. This intermediate is a powerful inhibitor of phosphofructokinase, which leads to product inhibition of the early steps involved in glucose metabolism. G6P (Glucose-6-phosphate) eventually builds up and inhibits hexokinase, leading to a decrease in glucose transport into the cell. This sequence of events impairs both glucose oxidation (direct inhibition of pyruvate dehydrogenase and Krebs cycle) and glucose storage, i.e. glycogen formation (secondary to decreased glucose transport).
Increased FFA oxidation can inhibit glycogen synthatase activity directly by causing a dissociation of its sub-units. Thus, an elevated rate of FFA oxidation can reproduce all major intracellular abnormalities (decreased glucose transport, decreased glycogen synthatase, decreased pyruvate dehydrogenase) and could account for the defects in glucose oxidation and storage. Physiological elevations in plasma FFA concentrations cause a stimulation of FFA oxidation, which in turn, inhibits glucose oxidation and storage. Physiological elevations of FFA levels and lipid oxidation can reproduce the major defects in glucose metabolism that have been described in NIDDM patients (25,122).
An elevated rate of FFA oxidation also has important effects on the hepatic glucose metabolism. In vitro studies have demonstrated that FFA stimulate gluconeogenesis. In addition, if FFA are infused into humans under conditions that stimulate the diabetic state or into obese insulin-resistant subjects, hepatic glucose production is enhanced. The following sequence might explain the relationship between plasma FFA concentrations, lipid oxidation, and glucose metabolism at the level of the liver: 1) increased plasma FFA, by mass action, enhance their cellular uptake, which leads to an increase in lipid oxidation that provides the stimulus for activation of the key enzymes involved in the regulation of gluconeogenesis; 2) at the same time, the augmented rate of lipid oxidation provides a continued source of energy (ATP) and substrate to drive gluconeogenesis; 3) in addition, the uptake of circulating gluconeogenic precursors by the liver is elevated. The inhibitory influence of insulin on gluconeogenesis is much more resistant than its restraining action on glycogenolysis (25,122).
THE ROLE OF INSULIN RESISTANCE IN THE PATHOGENESIS OF HYPERTENSION. Resistance to insulin-stimulated glucose uptake, glucose intolerance, and hyperinsulinemia is characteristic of a certain proportion of patients with hypertension. In addition, these abnormalities in glucose and insulin metabolism do not necessarily improve when hypertension is controlled by commonly used pharmacological approaches to lower blood pressure (122). Abnormalities in glucose and insulin metabolism may play some role in the etiology of high blood pressure, as observed in scorpion envenoming.
INSULIN, A POTENT ANTITHROMBOTIC HORMONE. Insulin is well known for its hypoglycemic effect. However, from some of the recent reports on the understanding of insulin effects, it is gradually becoming more convincing that insulin probably has a preventive role in the development of cardiovascular complications and is, perhaps independent of its well known hypoglycemic effect (60-67).
Platelets are aggregated in the presence of aggregating agents like ADP, l-epinephrine, collagen, thrombin, or platelet-activating factor. Platelet aggregation is an important event in the life-saving process of blood coagulation. On the other hand, hyper-aggregation of platelets and their adhesion at the site of injury leads to thrombogenesis. Acute ischemic heart disease occurs due to the blockade of normal blood circulation as a result of thrombus formation. During aggregation, various pro-aggregation prostaglandins are produced by the platelets themselves. Some of these prostaglandins act as platelet aggregating agents (prostaglandin G2 and prostaglandin H2), but some other prostaglandins, most notably thromboxene A2, is a potent vasoconstricting agent that results in substantial constriction of the blood vessel, thereby inducing further blockade of blood circulation through the artery and aggravating the ischemic condition, thus leading to death (60-67).
Platelet aggregation, which initiates thrombogenesis for the development of acute ischemic heart disease, is counteracted by the inhibition of platelet aggregation by several humoral factors including prostacyclin, blood coagulation factor Xa, and the recently discovered endothelial-derived relaxing factor, nitric oxide (NO). Although insulin does not inhibit platelet aggregation by itself, when added in vitro, in vivo insulin infusion results in the inhibition of platelet aggregation. Inhibition of platelet aggregation is believed to produce beneficial effects on the prevention of thrombosis, and thus, acute ischemic heart disease.
Although l-epinephrine aggregates platelets, it is a rather weak platelet-aggregating agent and acts as an aggregating agent only at supra-physiological concentrations. This weak aggregating agent, however, plays an important role in thrombogenesis, leading to coronary artery disease. This effect of l-epinephrine, which is a consequence of the presence of low amounts of l-epinephrine, profoundly increases platelet sensitivity to the aggregating effects of all other aggregating agents, thereby making platelets more vulnerable to produce thrombosis due to an increased platelet aggregation (60-67).
In acute ischemic heart disease, platelets are hyperactive and resistant to the inhibitory effect of prostacyclin. The failure of prostacyclin to inhibit platelet aggregation is related to the decrease of prostacyclin receptor numbers on the platelet surface. Treatment of platelets in vitro or in vivo with physiological amounts of insulin causes an increase of prostacyclin receptor numbers on the platelet surface, returning them to normal levels, and thereby restoring the sensitivity of these platelets in acute ischemic heart disease to the inhibition of aggregation by prostacyclin due to the increased activation of adenylate cyclase and increased cellular level of cAMP. Furthermore, the synthesis of prostacyclin, the arachidonate derivative from vascular endothelial cells and a major responsible factor for the inhibition of platelet aggregation, is significantly stimulated when endothelial cells are exposed to physiological amounts of insulin. The effect of l-epinephrine, which plays a significant role in the initiation of thrombosis, results from the binding of l-epinephrine to alpha2 adrenergic receptors on the platelet surface. Treatment of platelets with physiological amounts of insulin not only increases prostacyclin receptor numbers on the platelet surface, but also results in the reduction of alpha2 adrenergic receptor numbers on the platelet surface. As such, platelet exposure to insulin prevents l-epinephrine-induced potentiation of platelet aggregation by other aggregating agents. This hormone might play an important role in the prevention of thrombus formation on the vessel wall (60-67).
In vivo insulin infusion resulted in the inhibition of platelet aggregation induced by all known aggregating agents. Prevention of acute thrombosis by insulin is neither related to the blood sugar level nor influenced by the blood sugar level. This anti-thrombotic effect of insulin against acute thrombogenesis is mediated in vivo by the increase of nitric oxide level in blood.
The role of nitric oxide both as a vasodilatory agent and a potent antithrombotic humoral factor are known (60-67).
Why does insulin then fails to protect the development of acute ischemic heart disease in patients in the first place? This question could be answered, at least in part, by the fact that during the acute stage of coronary heart disease patients become hyperglycemic, but the plasma insulin decreases to the level found during fasting conditions, although none of these patients had history of diabetes mellitus. In addition, insulin binding to its receptors on the platelet surface is impaired, mimicking Type II diabetes mellitus status in these patients with coronary artery disease. Mechanism/s for the lack of stimulation of insulin production/secretion by glucose in acute ischemic heart disease in patients without any history of diabetes mellitus remains obscure. However, this impairment of insulin production and its binding to receptors in platelet help in the development of acute ischemic heart disease in these cases when other mechanisms, which also contribute to the development of the disease, fail. Nevertheless, insulin infusion in patients with acute ischemic heart disease has shown to produce significant improvement in the prognostic outcome of the disease (60-67). Scorpion sting victims may die from respiratory failure unrelated to pulmonary oedema and secondary to brain hemorrhage thrombosis or ischemia (15,47,52,58,59,76,77) (101,108,133,134,144).
INCREASED OSMOTIC FRAGILITY OF RBC. An increase in Hb, mean corpuscular hemoglobin concentration (MCHC), PCV, plasma Hb levels, and an increased osmotic fragility of erythrocytes (in vivo) were observed after envenoming (68,114). An increase in RBC osmotic fragility was also observed when blood (in vitro) was incubated with different concentrations of scorpion venom. Abnormal ECG changes following envenoming were reverted after antivenom administration. Scorpion antivenom (SAV) caused a decrease in Hb, MCHC, PCV, and plasma Hb levels in the envenomed animals, and a reversal in osmotic fragility changes of erythrocytes (68,114).
OSMOTIC FRAGILITY CHANGES OF ERYTHROCYTES (IN VIVO) (30-MIN INCUBATION). The osmotic fragility of freshly taken RBC reflects their ability to absorb water without lysis. The behavior of a RBC in hypotonic saline depends on the initial ratio of surface area to volume and not on the absolute size of the cell. The ability of the normal RBC to withstand hypotonicity results from its biconcave shape, which allows a cell to increase its volume by about 70% before the surface membrane is further stretched (24).
The increase in erythrocyte osmotic fragility is thus due to its shape and is independent of the cause of alteration of the shape of RBC (24). On the other hand, resistance to hemolysis by lysolecithin is not influenced by the shape of the cells, as the hemolytic process is chemical in nature (23). Blood pH, temperature, hypertonicity, and viscosity have a complex effect on the osmotic fragility (90).
Lactic acidosis, ketoacidosis, reduction in bicarbonate (HCO3- ) with a simultaneous increase in partial pressure of carbon dioxide (P CO2 ), and reduction in partial pressure of oxygen (P O2) were also observed in scorpion-envenomed animals (109). In cases of scorpion envenoming in experimental studies or human victims, hyperthermia, hypothermia, and hyperglycemia were observed (51,54,68,98,101,106-109,112,114).
Therefore, alterations in temperature, pH, and osmolarity (hyperglycemia) of body fluids in envenomed animals could be responsible for the increased osmotic fragility of RBC. The presence of phospholipase A2 in scorpion venom (1,20,121) has been reported. This enzyme is a powerful hemolytic agent and contributes to the increased osmotic fragility of RBC. In addition, the increase in PCV, Hb, plasma Hb, and MCHC in envenomed animals could also account for the increase in osmotic fragility of erythrocytes.
OSMOTIC FRAGILITY CHANGES OF ERYTHROCYTES (IN VIVO) (24 hour-INCUBATION). A significant increase in osmotic fragility of RBC after incubation at 37º C for 24 hours was observed (68,101,102,110,114). This increase probably reflects a change in volume to surface area ratio of RBC, but the factors which altered this ratio are more complicated than in fresh blood samples processed for osmotic fragility (24). During incubation for 24 hours, RBC metabolism is under stress and the pumping mechanisms may fail (110). The inhibition of cardiac sarcolemmal (95) and erythrocyte Na+ -K+ ATPase, Mg++ ATPase, and Ca++ ATPase activities (95) were demonstrated earlier in envenomed animals. A reduction in the erythrocyte Na+ -K+ ATPase activity has been reported earlier in dogs and rabbits following venom injection and in a scorpion sting victim (110). Pande and Mead (89) have observed an inhibition of Na+ -K+ ATPase activity by elevated FFA. Through their detergent properties, FFA inhibit the activity of this enzyme. An increase in FFA following venom injection has been observed.
The increase in PCV, Hb, plasma Hb, and MCHC might also have contributed to the observed increase in the osmotic fragility of RBC after venom injection. The rise in PCV, Hb, and MCHC after venom injection could be due to hemoconcentration caused by a massive release of catecholamines (3-5,30-36) (37-46) (51,52,55) and angiotensin II (104). Angiotensin II produces a significant decrease in the blood volume and an increase in the extravascular fluid, leading to peripheral circulatory failure and pulmonary oedema (28). Angiotensin II also stimulates the release of catecholamines. Catecholamines and angiotensin II may synergize or amplify each others action, and these my act, at least in part, at similar sites (28), resulting in hematological changes.
IN VITRO STUDIES OF OSMOTIC FRAGILITY. The enzyme phospholipase present in scorpion venom (1,20,121) could be the agent responsible for the increased hemolysis in vitro. This is in contrast to the large number of factors contributing to the increased hemolysis, as discussed above. Certain venoms, such as the cobra venom release the enzyme phosphatidase A, which converts lecithin to lysolecithin, a powerful hemolytic substance (23). Increased osmotic fragility of RBC due to the direct action of scorpion venom in vitro is a new finding. The results indicate that scorpion venom at the concentrations ranging from 0.012 to 0.04 mg/ml of blood resulted in increased osmotic fragility of erythrocytes. This could be the cause of intravascular hemolysis responsible for hematuria (117).
The normal blood volume is 70 ml/kg of body weight (23). The results of in vitro RBC osmotic fragility incubated with venom (0.024 mg of the venom/ml of blood) when extrapolated might infer that the venom causes an increase in osmotic fragility and hemolysis in infants and children weighing between 5 and 30 kg. The amount of venom required to cause these changes in infants could be 8.4 mg. Toxicity caused by scorpion envenoming is dose dependent. However, many factors such as species, venom dose, route of envenomation, and age, weight, and sex of the victim contribute to lethality. The sensitivity of various species to scorpion venom is extremely variable (143,147).
ANTIVENOM ADMINISTRATION. The effect of antivenom in reversing the osmotic fragility changes of erythrocytes is difficult to explain. However, it may be argued that the antivenom will neutralize the effect of venom, and therefore can prevent a further rise in osmotic fragility, but it may not be able to reverse the changes in osmotic fragility of RBC, which has already taken place.
The results of PCV, MCHC, and Hb after antivenom administration showed a definite reversal (68,114). These changes could be due to a fall in (68,114) angiotensin II level, leading to hemodilution. At the same time, an increase in physiologically active insulin levels has been observed (114). Insulin administration resulted in reversal of elevated angiotensin levels in envenomed animals (111). Changes in insulin secretion (hypoinsulin secretion and hyperinsulin secretion at different time intervals following scorpion envenomation), resulting in hyperglycemia have been observed (68). The renin-angiotensin-aldosterone system is involved in scorpion sting victims (45). Changes in potassium levels have been demonstrated after scorpion envenoming (106). Insulin influences the renin-angiotensin-aldosterone system through insulin-induced potassium changes (145). There are also reports suggesting that insulin induces stimulation of Na+ -K+ ATPase activity. The interaction of insulin with its receptor elicits the release of a mediator, which converts the Na+ -K+ ATPase into an activated form, operating with a higher affinity for Na+ on its inner surface. This leads to a transient increase in the rate of active Na+ -K+ transport, establishing a new steady state with a steeper concentration gradient for Na+ across the cell membrane (22).
The maintenance of optimum skeletal muscle function-like excitability, contractile performance, and metabolism is essentially through the rate of active Na+ -K+ transport, with the help of Na+ -K+ ATPase (the Na-K pump) activity. The activity of the transport system located primarily in the sarcolemma, but also in the transversal tubules, is subject to acute regulation by epinephrine, nor-epinephrine, and insulin. Thus, observations of fasciculations, clonus, and tetany-like contractions in the skeletal muscle of envenomed animals could be due to a change in the basal Na+ -K+ ATPase activity (22).
Thus, it is an imbalance between an increase in secretion of catabolic counter-regulatory hormones (catecholamines, glucagon, cortisol, etc.) and a reduction in an anabolic hormone-like insulin secretion (98), which might have contributed to increased osmotic fragility of erythrocytes and consequent hemolysis. The resulting changes in hyperinsulin secretion - insulin sensitivity - euglycemia were brought about after antivenom administration to envenomed animals. This might have contributed to the reversal of osmotic fragility changes of RBC.
PULMONARY OEDEMA IN SCORPION ENVENOMING. Cardiogenic and non-cardiogenic factors are involved in the pathogenesis of acute pulmonary oedema following scorpion sting (3-5,12-14,30,31) (38-46) (51-55,108,116,117,146,150). Amaral et al. (3-5) reported predominantly unilateral pulmonary oedema associated with reduced left ventricular systolic function in most of their patients. Unilateral pulmonary oedema secondary to left-sided heart failure seldom occurs in the absence of previous lung injury. This may be explained by a simultaneous and localized increase in pulmonary vascular permeability induced by scorpion venom. The patchy and peripheral distribution of lung oedema indicates pulmonary oedema due to increased vascular permeability (3). An increase was reported in tracheobronchial aspirate/plasma protein concentration, light microscopic features of the lung compatible with the adult respiratory distress syndrome (ARDS), electron microscopic findings compatible with acute lung injury, and increased alvelocapillary membrane permeability in a 16-year old boy, who died with acute pulmonary oedema and shock after a Tityus serrulatus sting. (3). Pulmonary oedema with a normal wedge pressure, indicating the possibility of capillary leak syndrome has been reported (118). The clinical presentation of ARDS is essentially a constellation of symptoms and findings that would be expected to result from hypoxemia and pulmonary oedema initially. ARDS is a pulmonary manifestation of pansystemic injury and MSOF (80,99,110,118).
The chemical composition and the functional activity of surfactant are altered in ARDS (92,126). Surfactant deficiency could be the final common pathway in the pathogenesis of ARDS (92). The loss or insufficient quantity of surfactant may explain the pulmonary oedema associated with scorpion envenoming, since surfactant is preferentially formed from glucose and glycogen rather than from glycerol, and insulin is required for the formation of surfactant (74) (Figure 4).
MULTI-SYSTEM-ORGAN-FAILURE (MSOF) IN SCORPION ENVENOMING. Our earlier studies on experimental scorpion envenoming demonstrated increased lacrimation, thick ropy salivary secretion dribbling from the mouth, passing of stools sometimes stained with bile and/or blood, frequent passage of urine, ejaculation, skeletal muscle fasciculations, tetany-like contractions, laryngeal spasm, respiratory apnea, and protrusion of widely dilated pupils (96-106) (107-111) (112-116). These changes indicate general neurotoxicity of the skeletal, neuromuscular, and autonomous (parasympathetic and sympathetic) systems (99,100,139). Additionally, we have demonstrated the presence of cardiac sarcolemmal defects (95), initial transient hypertension followed by hypotension (101-105,107), reduction in the insulin levels (97,98,108,109,112,114), depletion of glycogen content of atria, ventricle, skeletal muscle, and liver (92,107-109,112), hyperglycemia (96,98,106,108,109,112,114), lipolysis, and increased FFA levels (98,103,108,109,112,114), altered erythrocyte ATPase activities and osmotic fragility changes (98,103,108,109,114), involvement of endocrine and exocrine pancreas with acute pancreatitis (111), increased clotting time, prothrombin time, thrombin time, partial thromboplastin time with kaolin, decrease in fibrinogen, an acute reduction in platelet count, absence of Factor V, VII, and VIII in envenomed animals, indicative of DIC (27,108), elevation of plasma angiotensin II levels (104), changes in thyroid hormone levels (96), reduced partial pressure of oxygen, bicarbonate, changes in pH (acidosis), increased lactic and keto acid levels (109), various ECG changes (92,95,96,98,99,100-104) (105-112,116), and an increase in glucagon and cortisol levels (98). All these changes in scorpion envenoming indicate the involvement of multiple systems and organs and their failure along with pulmonary (cardiac and non-cardiogenic) oedema, as demonstrated by earlier reports (3-5,12-14,19,30,31) (38-46) (51-55,71,93,118,125,132,137,146,150).
SCORPION ENVENOMING AND HORMONAL AND BIOCHEMICAL DISTURBANCES. The metabolic disturbances, ECG changes, and cardiovascular manifestations produced by scorpion envenoming could be due to the following:
1) Action of catecholamines, causing (a) increased myocardial oxygen consumption, (b) coronary vasoconstriction, (c) peripheral vasoconstriction, (d) increased after load, and (e) lipoloysis resulting in increased FFA.
2) Action of angiotensin, II resulting in coronary and peripheral vasoconstriction and potentiation of catecholamine-mediated effects.
3) Insulin deficiency.
4) Increased myocardial oxygen consumption by increased FFA.
5) Arrthymogenic effects due to increased catecholamines, angiotensin II, and FFA.
6) Increased FFA, producing inhibition of Na+ -K- stimulated ATPase activity and sarcolemmal defects.
7) The increased FFA, by altering the function of platelets, may increase the tendency towards intravascular thrombosis and result in DIC.
8) Increased FFA, hyperglycemia, elevated levels of counter-regulatory hormones (catecholamines, glucagon, cortisol, changes in thyroid hormones, elevated angiotensin-II, causing sympathetic stimulation) contribute to hypoinsulinemia, hyperinsulinemia, and insulin resistance, resulting in MSOF, ARDS, pulmonary oedema, and death.
ABSORPTION, DISTRIBUTION, AND ELIMINATION OF SCORPION VENOM. Nearly all distribution and pharmacokinetic studies on scorpion venom were carried out by Ismail and his colleagues (52). Following the results of this type of study in early 1980 by Ismail, many investigators at that time believed that the antivenom should be administered immediately after scorpion stings, otherwise the venom would produce irreversible lesions.
Recently, we have successfully achieved the labelling of the venom of Mesobuthus tamulus concanesis, Pocock with Tc-99m, using direct tin reduction procedure (83). Biodistribution studies were carried out in Wistar rats at different time intervals after IV administration of the labelled venom. Scintiimages were obtained after scorpion envenoming using a large field of view gamma camera to ascertain the pharmacological action of the venom in the body. Within 5 min after administration, labelled venom was found in the blood (28%), muscle (30%), bone (13%), kidney (12%), liver (11%), and other organs. The initial high blood level (28% at 5 min) declined (12% at 30 min), indicating the rapid clearance of scorpion venom from the circulatory compartment. The level of the venom in the kidney was higher than in the liver. The labelled venom was excreted through renal and hepatobiliary pathways. The maximum renal uptake of 32% at 30 min dropped to 22% at 3 h, indicating that the clearance of labelled venom from the kidney is slow. The immunoreactivity study revealed that the localization of the scorpion venom was in the kidneys rather than in other organs, such as the liver. It has been demonstrated that radioiodinated meta iodobenzylguanidine (MIBG), a new radiopharmaceutical used for the diagnostic and therapeutic applications of neural crest tumors, acts on the sympathetic nervous system. The adrenal uptake, obtained at either 5, 30, 60, 180, or 1440 min after scorpion venom injection, is comparable to the uptake of radioiodinated MIBG, thereby suggesting that scorpion venom acts through the sympathetic nervous system (83).
Scorpion venoms appear to follow an open two-compartment pharmacokinetic model with rapid distribution half-lives ranging from 4 to 7 min and relatively slow overall elimination half-lives of 4.2 to 13.4 h (52). Since scorpions generally inoculate their venom into the interstitial space and not directly into the bloodstream, an absorption step must take place before the venom enters the general circulation. This was studied pharmacokinetically using L. quinquestriatus venom as a model (52). Rapid and appreciable absorption from SQ sites took place, with about 70% of the maximum blood concentration reached within 15 min. The time needed to reach the maximum blood venom concentration was 101 + 8 min, and almost complete absorption of venom from the SQ site would occur in 7 - 8 h (52).
In another pharmacokinetic study with labelled A. crassicauda venom, it was shown that this particular scorpion venom was unique among scorpion venoms in following a tri-exponential pattern characteristic of a three-compartment open model comprising a central "blood" compartment, a rapidly equilibrating "shallow" compartment, and a slow equilibrating "deep" tissue compartment. The overall elimination half-life was 24 h, indicating a slow elimination with the long mean residence time of 33.7 h in the body and 26 h in the peripheral compartment. The long stay of the venom in the body might explain the increased risk of toxicity and the good potential for the treatment with serotherapy even hours after the scorpion sting (52).
DRUGS NOT USEFUL OR CONTRAINDICATED IN SCORPION ENVENOMING. In our view, certain drugs have no place in the treatment of scorpion stings either because they are of no use or may do more harm.
1. Cardiac glycosides are not effective in pulmonary oedema in the presence of sinus tachycardia and normal cardiac size (39,46,136). They are known inhibit Na+ -K+ ATPase activity. Scorpion venom produces cardiac sarcolemmal defects shown by alterations in Na+ - K+ ATPase and Ca++ ATPase activities (95).
2. Diuretics are contraindicated owing to their dehydrating effect, alterations in blood viscosity, and stimulation of renin-angiotensin secretion (80). In addition, diuretics are contraindicated in pulmonary oedema due to ARDS (92).
3. Atropine should not be given routinely. However, this has been a common practice because scorpion sting victims present with heavy perspiration, vomiting, and increased salivation (12,136). Atropine may intensify tachycardia and sympathetic effects of the venom after blocking the cholinergic effect (136). Atropine potentiates the hypertensive effect (39). It increases the severity of pulmonary oedema induced by scorpion toxin (30). Atropine is also a parasympatholytic drug and inhibits insulin secretion from the endocrine pancreas (35). The insulin secretion is already inhibited in scorpion envenoming (97-100,108,109,112,114). Increase in duration as well as severity of clinical signs, including myocardial injury, was observed in victims treated with atropine compared to scorpion sting victims who did not receive atropine (12,13). Atropine is indicated only in the presence of severe bradycardia, with or without hypertension (136).
4. Glucocorticoids are contraindicated because they are catabolic hormones and anti-insulin in action. They stimulate the renin-angiotensin system. In addition, they are like "asbestos suits against fire, but they do not extinguish the fire", and in the absence of a specific drug, they are likely to spread inflammation. Corticosteroids are contraindicated in non-cardiac pulmonary oedema (92), and the secretion is already increased due to scorpion envenoming (98).
5. Beta blockers do not prevent either A-V block or pulmonary oedema (30), although propranalol protects the appearance of sinus tachycardia.
6. Alpha blockers - In our hands, the administration of insulin alone (112) or insulin and alpha blockers (Tolazolin) (109) successfully reversed metabolic as well as ECG changes in experimental scorpion envenoming. Insulin administration along with alpha blocker produced little more glycogenesis and lipogenesis and reversed the rise in plasma angiotensin II levels (104,109,112) than insulin alone. However, alpha blockers are known to stimulate the gastric acid secretion, and this may, in turn, aggravate the existing sub-clinical or clinical acute pancreatitis (111) known to occur in some patients into a fully blownup fulminating acute pancreatitis in some scorpion sting victims. Abdominal pain, mild epigastric pain, was the complaint of 9 (36%) patients, and a mild elevation of serum amylase levels was found in 5 (20%) of these victims) (150). If stimulation of nerves to the pancreas inhibits insulin secretion via the release of catecholamines (via alpha adrenoceptor stimulation to the pancreas), then it is logical to convert the inhibitory response to an excitatory response by using alpha blocking drugs (35). If alpha blockers are acting by release of insulin, we can very well administer insulin instead of alpha blockade-dependent insulin release to reverse metabolic, electrocardiographic, hemodynamic changes, non-cardiogenic respiratory pulmonary oedema (ARDS-like syndrome), and many other changes induced by scorpion venom. Prazosin (alpha adrenergic antagonist) is found to enhance insulin secretion in scorpion sting victims with suppressed insulin secretion (prior to prazosin treatment) (12-14). The use of prazosin is more effective when combined with a diuretic and/or a beta blocker than when used alone (28), but diuretics and beta blockers are contraindicated in scorpion sting victims. Alpha blockers can prevent the actions caused by released catecholamines, preventing further damage. Alpha blockers can not reverse the tissue damage that has already been caused by catecholamines. Thus, insulin remains the only choice as the physiological antagonist to the actions of catecholamines.
7. Angiotensin-converting enzyme (ACE) inhibitors are suggested to be useful in scorpion envenoming with elevated angiotensin II levels (104), as reported by Karnad et al. (69). However, its use is strongly criticized by Ismail (51-55) because captopril (ACE inhibitor) inhibits the kininase II enzyme, which leads to the accumulation of bradykinin, the neurohumoral agent incriminated in the pulmonary oedema of scorpion envenoming. In addition, Rai (119) has demonstrated pulmonary oedema in envenomed animals treated with ACE inhibitors.
8. Emetine hydrochloride should not be combined with local anaesthetic agents to relieve pain at the sting site. Emetine is a protoplasmic venom and its toxic effect on the myocardium may cause hypotension, tachycardia, precordial pain, dyspnea, and ECG abnormalities. These toxic manifestations may occur with any dose level, depending on individual susceptibility to emetine. Scorpion stings produce myocarditis, and emetine injection may add a further insult to the already injured myocardium (93).
RATIONALE FOR INSULIN THERAPY IN SCORPION ENVENOMING. Scorpion envenoming causes the release of massive amounts of catecholamines (epinephrine and nor-epinephrine) (10) and other counter-regulatory hormones (glucagon and cortisol) with suppressed insulin secretion. Under these conditions, the metabolism of carbohydrate, protein, and fat is directed towards catabolism. Lipolysis results in increased FFA levels that are arrhythmogenic in nature and inhibit Na+ -K+ ATPase activity. Insulin is the only major hormone that tends to suppress the mobilization of fatty acids from adipose tissues. This effect occurs at insulin concentrations below those needed for glucose uptake in most tissues. In addition, the effect of fatty acid mobilization is immediate, even faster than the effect on plasma glucose levels. Insulin allows the incorporation of fatty acids into triglycerides in the liver and in adipose tissues. Moreover, glucose infusion along with insulin will suppress fat mobilization by favoring re-esterification.
Methods of reducing triglyceride accumulation in the myocardium depend on a decrease of elevated plasma FFA concentrations or the control of sudden increases in these concentrations. Increases in circulating glucose concentrations will, in the presence of normal insulin secretion, reduce plasma FFA levels, and might also present the incipiently ischemic myocardium with a change in arterial substrate concentration sufficient to reduce oxygen consumption in some hypoxic areas. An additional argument for raising blood sugar levels in patients with ischemia is that the ischemic myocardium is dependent on glucose for maintained function and its uptake is markedly increased when there is hypoxia and an adequate insulin presence (87).
Insulin treatment acts in two ways, the most important probably being the inhibition of glucose production by the liver and FFA by the adipose tissue, and then when the plasma concentration of FFA has fallen, by increasing peripheral utilization of glucose (138). The important implication of this analysis is that increased peripheral uptake of glucose is brought about more by the reduction of circulating FFA rather than the direct action of insulin. This is in keeping with the in vitro observations that high concentrations of insulin are required to stimulate glucose uptake into the muscle. It seems that glucose metabolism in the muscle is primarily controlled by the concentration of circulating FFA and changes in blood flow.
The low-dose insulin infusion is highly effective (138). Insulin infusion at low doses is sufficient to inhibit glucose production by the liver and fatty acid release from the adipose tissue. In diabetic dogs, the low-dose insulin infusion was found to normalize blood glucose purely by inhibiting glucose production, while higher doses had a dual effect, stimulating peripheral utilization as well, and this may also be true in humans (138). Glucose uptake into muscle tissue is associated with ionic fluxes across the cell membrane (the best recognized being that of potassium uptake). Low-dose insulin regimes to treat diabetic coma are probably associated with the need for a slower potassium replacement, perhaps because in these instances, normalization of blood glucose occurs primarily as a result of inhibition of glucose production by the liver, a process not leading to a shift of potassium from the vascular compartment. Since hypokalemia is one of the main causes of death in the management, this proves to be one of the major advantages of the low-dose regimes (138).
In vitro, the adipose tissue is more sensitive to the anti-lipolytic action of insulin than to its action in stimulating glucose metabolism, the former effect being caused by lowering the intracellular cAMP concentration, a mechanism closely similar to its action in inhibiting glucose production by the liver. In vivo, the inhibition of triglyceride hydrolysis to FFA is also extremely insulin-sensitive, maximal inhibition of FFA release with insulin doses of approximately 0.015 U kg-1 , whose hypoglycemic activity is also via inhibition of glucose production and too low to stimulate peripheral glucose uptake. Thus, low-dose insulin infusion appears to act predominantly through mechanisms involving inhibition of adenyl cyclase rather than the stimulation of glucose transport (138).
There is a dual hormonal control of glucose balance across the liver, acting as a "push-pull system. Glucagon and adrenaline mobilize glucose from glycogen by activating the enzyme phosphorylase that catalyses the breakdown of glycogen to glucose. They both do this by activating the membrane-bound adenyl cyclase and increasing the synthesis of cAMP, which in turn, transforms phosphorylase from an inactive into an active form. Insulin acts to oppose this by inhibiting the accumulation of cAMP, either by inhibiting adenyl cyclase or by increasing the rate of destruction of cAMP (by activating phosphodiesterase - the enzyme that breaks down cAMP) (138).
Insulin favors glycogen deposition and inhibits glycogen degradation. Insulin counteracts the effects of catecholemines favoring glucose uptake and inhibiting gluconeogenesis by suppressing the flow of substrates to the liver. It also inhibits lipolysis in adipose tissues, reducing the flow of FFA to the liver, thus removing a trigger for gluconeogenesis. Glycogen availability may be an important independent determinant of cardiac function. Elevated glycogen content in the heart partially protects the mechanical deterioration in anoxia.
Cardiac and liver glycogen content were found to be reduced after envenoming by the scorpion Mesobuthus tamulus concanesis, Pocock (8,9). Insulin administration resulted in a massive increase in glycogen content in the liver, skeletal, and cardiac muscles, and lipogenesis in the scorpion envenomed animals (104,112).
Insulin assists in the recovery of myocardial contractility after ischemic arrest and increases cardiac output (81). Insulin also assists glucose transport and can accelerate ATP production in ischemic areas (81). Insulin stimulates sarcolemmal Na+ -K+ ATPase activity (35), inhibits Ca++ ATPase activity, and stabilizes lysosomal membranes (35).
The Na+ -K+ pump has a key function in the exchange of substances between the cell and its surroundings, transepithelial transport, and transmission of information (135). Three isoforms of the Na+ -K+ ATPase alpha subunit have been identified. The functional implications of having three isoforms of the enzyme are still unknown. The only observation that points towards a special function is that the affinity of the alpha 2 isoform for Na+ from muscles and adipocytes is increased by insulin (135).
The activity of Na+ - K+ pump in the intact membrane is determined by a combined effect of the cytoplasmic and the extracellular Na+ to K+ concentration ratios and other factors. Insulin, epinephrine, and nor-epinephrine have a stimulating effect on the pump (135). The alterations in the cardiac sarcolemmal (95) and RBC Na+ -K+ ATPase activity and the reduction in insulin secretion (68,97,98,108,109,112,114) were shown in scorpion envenoming.
Recently, both in vivo and in vitro studies have shown that resolution of oedema from the air spaces of the lungs depended on an active sodium transport pump that removed oedema fluid, even in the face of a rising alveolar oedema protein concentration in excess of plasma protein concentration (80).
TREATMENT WITH INSULIN-GLUCOSE INFUSION. The following treatment was administered to scorpion sting victims.
LOCAL TREATMENT - Injection of local anesthetic agent, such as 2% xylocaine (without adrenaline) at the sting site to relieve pain. This was then repeated to relieve pain. Injection of tetanus toxoid was given as a prophylactic measure.
GENERAL PRINCIPLES - All patients suspected of scorpion envenoming were admitted 48 to 72 hours for:
1) maintenance of vital body functions.
2) correction of venom-induced abnormalities.
3) prevention or correction of secondary complications. All the venom-induced abnormalities were corrected by insulin-glucose infusion.
4) management of peripheral circulatory failure by intravenous fluids, since patients tend to lose considerable amounts of fluids by profuse sweating and vomiting.
INSULIN-GLUCOSE INFUSION Plain insulin 0.3 Units per gram of glucose and glucose 0.1 g/kg/h were given. This infusion was given continuously. Potassium chloride was given whenever necessary to prevent hypokalemia. The attending clinician was on the lookout for the earliest signs and symptoms of hypoglycemia so that it could be prevented and corrected.
Insulin-glucose infusion was discontinued after the clinical condition improved, as indicated by a lowering in the increased heart rate and respiratory rate, and attainment of normal central venous pressure and arterial blood pressure along with the disappearance of pulmonary oedema.
The following Protocol was observed: 1) Seven Units of additional insulin for adsorption of insulin in the IV set; 2) Simultaneously, in a separate drip dopamine and/or dobutamine (5-7 µg/kg/min) in 5% dextrose to maintain blood pressure; 3) If the patient came in the "initial transient phase of hypertension" treatment of the b1ood pressure with sublingual nifedipine (5 mg for children and 10 mg for adults); 4) Maintenance of acid-base balance, adequate fluid load, electrolyte balance, renal output, and other necessary measures; 5) Nasal oxygen whenever necessary; and 6) Anxiolytic agents if necessary. The following drugs were not given: cardiac glycosides, diuretics, atropine, corticosteroids, beta blockers, emetine hydrochloride (with local Xylocaine injection), adrenaline (with local Xylocaine injection), and ACE inhibitors.
CLINICAL TRIALS USING INSULIN-GLUCOSE INFUSION. The efficacy of insulin-glucose infusion in reversing myocarditis, hemodynamic changes, peripheral circulatory failure, and pulmonary oedema was evaluated in 25 victims of venomous scorpion stings from the Rayalaseema region in the South of India. (150). The study was based on observations in animal experiments, where insulin administration reversed metabolic and electrocardiographic changes induced by scorpion envenoming. Toxic myocarditis with peripheral circulatory failure was seen in all scorpion sting victims. Ten of these victims also had pulmonary oedema. All the patients received continuous infusion of regular crystalline insulin at the rate of 0.3 U/g of glucose and glucose at the rate of 0.1 g/kg/h with supplementary potassium as needed, as well as maintenance of fluid, electrolytes, and acid-base balance. Insulin-glucose infusion successfully reversed the cardiovascular and hemodynamic changes, and pulmonary oedema in 24 of the 25 victims. One patient, who was admitted 72 hours after scorpion sting, with myocarditis, peripheral circulatory failure, pulmonary oedema, and hemiplegia on left side recovered completely, except from neurological deficits. However, one severely envenomed victim admitted more than 72 hours after the sting died (150).
In another study, 127 scorpion sting victims were divided into two therapeutic cohorts (a cohort is a group of individuals exposed to same sort of disease and given a particular treatment) (146). The treatment of victims in for cohort I (n=105) consisted of regular crystalline insulin at the rate of 0.1-0.3 U/g of glucose and glucose at the rate of 0.1 g/kg/h with adjuvant therapy. Treatment of victims in cohort II (n=22) consisted of prazosin (0.5 mg) initially, followed by insulin-glucose infusion, as mentioned for cohort I. The adjuvant therapy for cohorts I and II consisted of maintenance of acid-base-fluid-electrolyte balance, deriphyllin or aminophiline drip, diuretics (furosemide, only once), non-steroidal anti-inflammatory drugs, dopamine drip for hypotension, and local infiltration with Xylocaine. The following were the salient features:
The ECG changes observed were hyperkalemic changes, such as tall, tented T waves, wide QRS complexes, prolonged ST segment (70 %), ST segment elevation and T inversion (36%), sinus tachycardia (54%).
Cohort I:
One of the 8 victims with myocarditis and hypertension died; 80 of the 81 victims with myocarditis and circulatory failure recovered; and 14 of the 16 victims with pulmonary oedema, myocarditis, and circulatory failure recovered (mortality 4 out of 105, 3.8%). Three of the 4 dead victims were brought to the hospital 25 to 48 hours after the stings.
Cohort II:
All the 22 victims recovered. All the victims were treated within three hours after the sting. None of them had pulmonary oedema.
ANTISCORPION VENOM (SAV) ADMINISTRATION (Figure 5). Scorpion antivenom (SAV) administration reversed ECG, cardiovascular, hemodynamic, metabolic, and hormonal changes induced by the venom in experimental animals. There was a rise in insulin levels following SAV. Hyperglycemia and the increased FFA concentrations observed after venom injection returned to euglycemia and normal FFA levels, or even less than the values observed before venom injection (68,114).
Administration of SAV prevented the toxic effects of venom. SAV administered after venom in animals neutralized the venom-induced hyperglycemia and lipolysis. SAV stopped a further rise in blood sugar and FFA levels and reversed these levels to euglycemia and lipogenesis (68,114).
If SAV did not inhibit the catecholamine-mediated toxicity, which in turn, suppressed the release of insulin secretion, we would have observed only the arrest of a further rise in hyperglycemia and FFA concentrations. The attainment of normal blood glucose level (euglycemia) and lipogenesis (reduction in FFA and a simultaneous increase in triglyceride levels) following SAV indicated that SAV effectively neutralized the inhibitory action of catecholamines on insulin secretion. This resulted in physiologically active insulin secretion.
The increased Hb, PCV, and MCHC following envenoming returned to near control values after SAV administration in animals (68,114).
An increase in circulating LDH, SGOT, CK-MB, SGPT, and alpha HBDH enzyme levels in dogs at 60 min, and a further rise 120 min following envenoming was observed. SAV administration resulted in reversal of ECG changes and reduction of these cardiac enzyme levels (116). An increase in osmotic fragility of erythrocytes (in vitro and in vivo) was observed following experimental envenomation. SAV administration reversed the osmotic fragility changes (in vivo) (68,114,116).
The return of normal blood pressure from hypertensive levels, preventing a further rise in the blood pressure, maintaining blood pressure from falling to low levels, thereby preventing the occurrence of hypotension in animals following SAV, indicated that SAV administration within 60 min is as rapid and effective as that of insulin+alpha blocker+sodium bicarbonate or insulin alone (68,97,101-107) (108-114,146,150).
Ismail et al. (51-55) showed that low doses of antivenom were unable to neutralize completely the electrocardiographic effects of the venom in experimental animals. The ineffectiveness of antivenom in preventing or abolishing cardiovascular manifestations of scorpion envenoming has been ascribed to the low titers of commercial antivenoms used (52). In Israel, 5-15 ml of antivenom (1 ml neutralizes at least 80% of 50% lethal dose in 20 g mice) was given IV to scorpion sting victims according to age (46). In Saudi Arabia, 0.5-1 ml of a commercial preparation neutralizing 50 mouse LD50/ml (activity was found equivalent to 20-40% of that stated on the label) was given IM to scorpion sting victims. (52). According to Ismail (52), the doses of antivenom shown in all these studies were very low. At least 25-50 times the Israeli doses and 10-20 times the Saudi doses were required to neutralize the effects of an average L. quinquestriatus sting (1-2 mg dry venom). We fully agree with Ismails (52) conclusions that antivenoms are ineffective in the prevention or abolition of cardiovascular manifestations in scorpion envenoming syndrome (12,46) are strongly questionable. If the stimulation of sympathetic nerves to the pancreas inhibit insulin secretion via the release of catecholamines, then it is logical to convert the inhibitory response to an excitatory response by alpha blocking drugs (35). The blocking of alpha receptors results in a marked increase of insulin response. The effectiveness of these blocking agents in correcting the hemodynamics may be partly due to the improvement of insulin secretion and not solely to myocardial effects (85). This could be the mechanism by which treatment of the systemic effects of scorpion venom with blocking agents of the autopharmacological type is effective. Administration of SAV also works, as discussed, by blocking the release of catecholamines and the consequent release of physiologically active insulin. Serotherapy, in our hands, was able to reverse the metabolic disturbances by changes in the hormonal environment.
We fully agree with Ismail (52) that if a sufficient dose of antivenom were used, adjunctive therapy is seldom required. However, we advocate the maintenance of the acid-base-fluid-electrolyte balance in the scorpion sting victims. We emphasize that it is essential to correct the acid-base balance, abnormal gaseous exchange, fluids and electrolytes, and maintenance of vital functions in all scorpion sting victims besides SAV administration.
Two hundred and three victims of scorpion envenoming and 28 control subjects were given antivenom. Antivenom is found to be effective in preventing or abolishing the various clinical manifestations of human envenoming in these clinically controlled trials (141).
We have highlighted the role of insulin as a therapeutic agent in scorpion envenoming either directly or indirectly after SAV administration. As relative or absolute insulin deficiency may be involved in the metabolism of the myocardium and be partly responsible for the development of arrhythmias and heart failure (85), more carefully controlled studies including hemodynamic and metabolic measurements are needed on the effects of insulin in scorpion envenoming.
Family I. BUTHIDAE
Subfamily I. BUTHINAE
Genus 1. Lychas, C. Koch
Sub-genus I. Distotrichus -sg. nov. Species
1. Lychas (Distotrichus) nigristernis, Pocock
2. Lychas (Distotrichus) gravelyi, Henderson Sub-Genus ii. Alterotrichus sg. nov.
3. Lychas (Alterotrichus) mucronatus, Fabricius
4. Lychas (Alterotrichus) rugosus, Pocock
5. Lychas (Alterotrichus) hendersoni, Pocock
6. Lychas (Endotrichus) tricarinatus, Simon
7. Lychas (Endotrichus) laevifrons, Pocock
8. Lychas (Endotrichus) scaber, Pocock
9. Lychas (Endotrichus) albimanus, Henderson
10. Lychas (Endotrichus) biharensis sp. nov.
11. Lychas (Endotrichus) kamshetensis sp. nov. Genus 2. Hemibuthus, Pocock
12. Hemibuthus crassimanus, Pocock Genus 3. Orthochirus, Karsch
13. Orthochirus flavescens, Pocock
14. Orthochirus pallidus, Pocock
15. Orthochirus bicolor, Pocock
16. Orthochirus krishnai sp. Nov.
17. Orthochirus melanurus, Kessler Genus 4. Cahrmus, Karsch
18. Charmus indicus, Hirst
19. Charmus singhagadensis sp. nov. Genus 5. Stenochirus, Karsch
20. Stenochirus politus, Pocock
21. Stenochirus sarasinorum, Karsch Genus 6. Buthotus vachon
22. Buthotus alticola punjabensis, Birula Genus 7. Compsobuthus, Vachon
23. Compsobuthus acutecarinatus regosulus, Pocock Genus 8. Vachonus g. nov.
24. Vachonus rajasthanicus sp. nov.
25. Vachonus atrostrlatus, Pocock Genus 9. Mesobuthus, Vachon
26. Mesobuthus tamulus concanesis, Pocock
27. Mesobuthus tamulus sindicus, Pocock
28. Mesobuthus tamulus gujaratensis, Pocock
29. Mesobuthus tamulus gangeticus, Pocock
30. Mesobuthus tamulus tamulus ,Fabricius
31. Mesobuthus hendersoni, Pocock
32. Mesobuthus ruglscults, Pocock
33. Mesobuthus pachyurus, Pocock Genus 10. Androctonus, Hempre. And Ehrenb.
34. Androctonus australis finltimus, Pocock Genus 11. Odontobuthus,Vachon
35. Odontobuthus doriac odonturus, Pocock
Subfamily 2. CENTRURINAE
Genus 12. Isometrus, Hempre. and Ehrenb.
36. Isometrus Raddyanus) vittatus, Pocock
37. Isometrus (Raddyanus) righidulus, Pocock
38. Isometrus (Raddyanus) brachycentrus, Pocock
39. Isometrus (Raddyanus) thrustoni, Pocock
40. Isometrus (Raddyanus) isadensis sp. nov.
41. Isometrus (Raddyanus) europaeus, Linn.
42. Isometrus (Raddy anus) assamensis, Oates
43. Isometrus (Raddyanus) acanthurus, (Pocock)
44. Isometrus (Raddyanus) corbetl sp. nov.
45. Isometrus (Closotrichus) sankeriensis ap. nov.
Family II. CHAERILIDAE
Genus 13. Chaerilus, Simon
46. Chaerilus tricostatus, Pocock
47. Chaerilus cey lonensis, Pocock
48. Chaerilus pictus, Pocock
49. Chaerilus insignis, Pocock
50. Chaerilus gemmifer, Pocock
51. Chaerilus gemmifer, Pocock
51. Chaerilus ganosus, Popock
52. Chaerilus truncatus, Kasch
53. Chaerilus anthracinus, Pocock
54. Chaerilus anthracinus rufescens, Pocock
Family III. VAEJOVIDAE
Subfamily 3. SCORPIOPSINAE
Genus 14. Scorpiops, Peters
Sub-genus (I). Neoscorpiops, Vachon
55. Scorpiops (Neoscorpiops) deccanensis, Tikedar and Bstawade
56. Scorpiops (Neoscorpiops) satarensis, Pocock
57. Scorpiops (Neoscorpiops) tenuicauda, Pocock
Sub-genus (ii). Scorpiops, Vachon
58. Scorpiops (Scorpiops) hardwickei, Gervais
59. Scorpiops (Scorpiops) insculptus, Pocock
60. Scorpiops (Scorpiops) affinis, Kraepelin
61. Scorpiops (Scorpiops) crassimanus Pocock
62. Scorpiops (Scorpiops) montanus, Karsch
63. Scorpiops (Scorpiops) rohtangensis, Mani
64. Scorpiops (Scorpiops) leptochirus, Pocock
65. Scorpiops(Scorpiops) petersi, Pocock Sub-genus (iii). Euscorpiops, Vachon
66. Scorpiops (Euscorpiops) bhutanesis sp. nov.
67. Scorpiops (Euscorpiops) asthenurus, Pocock
68. Scorpiops (Euscorpiops) longimanus, Pocock
69. Scorpiops (Euscorpiops) binghamii, Pocock
Family IV. ISCHNURIDAE
Genus 15. Iomachus, Pocock
70. Iomachus punctulatus, Pocock
71. lomachus nitidus, Pocock
72. Iomachus laeviceps laeviceps, Pocock
73. Iomachus laeviceps malabarensis, Pocock
Genus 16. Hormurus, Thorell
74. Hormurus australasiae, Fabr.
75. Hormurus nigripes, Pocock
Genus 17. Chiromachetes, Pocock
76. Chiromoachetes fergusoni, Pocock
Family V. SCORPIONIDAE
Subfamily 4. SCORPIONINAE
Genus 18. Heterometrus Hemprich & Ehrenberg.
Sub-genus (I). Heterometrus, Hemprich & Ehenberg
77. Hetermoetrus (Heterometrus) longimanus longimanus, Herbst
78. Heterometrus (Heterometrus) keralaensis sp. nov.
79. Heterometrus (Heterometrus) malapuramensis sp. nov.
80. Heterometrus (Heterometrus) bengalensis, Koch Sub-genus (ii). Srilankametrus, Couzijn
81. Heterometrus (Srilankametrus) latimanus, Pocock
82. Heterometrus (Srilankametrus) gravimanus, Pocock
83. Heterometrus (Srilankametrus) serratus, Pocock
Sub-genus (iii). Gigantometrus, Couzijn.
84. Heterometrus (Gigantometrus) swammerdami, Simon
85. Heterometrus (Figantometrus) flavimanus, Pocock.
Sub-genus (iv). Chersonesometrus, Couzijn
86. Heterometrus (Chersonesometrus) granulomanus, Couzijn
87. Heterometrus (Chersonesometrus) tristis, Henderson
88. Heterometrus (Chersonesometrus) fastigiousus, Couzijn
89. Heterometrus (Chersonesometrus) wroughtoni, Pocock
90. Heterometrus (Chersonesometrus) fulvipes, Koch
91. Heterometrus (Chersonesometrus) liurus, Pocock
92. Heterometrus (Chersonesometrus) xanthopus (Pocock)
93. Heterometrus (Chersonesometrus) barberi, Pocock
94. Heterometrus (Chersonesometrus) scaber, Thorell
95. Heterometrus (Chersonesometrus) collinus, Pocock
96. Heterometrus (Chersonesometrus) madraspatensis, Pocock
97. Heterometrus (Chersonesometrus) kanaraensis, Pocock
98. Heterometrus (Chersonesometrus) pelekomanus, Couzijn
99. Heterometrus (Chersonesometrus) phipsoni, Pocock
REFERENCES
01 ACHYUTHAN KE., AGARWAL OP., RAMACHANDRAN LK. Enzymes in the venoms of two species of Indian scorpions. Indian J. Biochem. 1982, 19, 356-8.
02 ALEGESAN R., RAGHAVAN S., BALAMBA R. HARANATH K., SUBRAMANIAN N., THIRUVENGADAM. Transient right Gundle Branch block following scorpion stings. J. Indian Med. Assoc., 1977, 69, 113-4.
03 AMARAL CFS., REZENDE NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon, 1997, 35, 997-8.
04 AMARAL CFS., REZENDE NA., FREIRE-MAIA L. Acute pulmonary oedema after Tityus serrulatus scorpion sting in children.Am. J. Cardiol., 1993, 71, 242-5.
05 AMARAL CFS., BARBOSA AJA., LEITE VHR., TAFURI WL., REZENDE NA. Scorpion sting induced pulmonary oedema: Evidence of increased alveolocapillary membrane permeability. Toxicon, 1994, 32, 999-1003.
06 ANSARI MY. Gangrene after scorpion sting. Br. Med. J., 1948, 2, 388.
07 AVOGARO A., CREPALDI C., PIARULI F., MILAN D., CALABRO A., MACDONALD I., CREPALDI G., SCOGNAMIGILO R., TIENGO A. The hemodynamic abnormalities in short-term insulin deficiency: the role of prostaglandin inhibition. Diabetes, 1996, 45, 602-09.
08 BALASUBRAMANYAM P., RADHA KRISHNA MURTHY K. Abnormal cardiovascular and electrocardiographic profile and cardiac glycogen content in rabbits injected with scorpion venom. Indian. J. Physiol. Pharmacol., 1981, 25, 351-5.
09 BALASUBRAMANIAM P., RADHA KRISHNA MURTHY K. Liver glycogen depletion in acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian Heart J., 1984, 36, 1014
10 BASU A., GOMES A., DASGUPTA SC., LAHIRI SC. Pharmacology of scorpion (Lychas laevifrons Pocock) Venom with special reference to its neuromuscular activity. Indian J. Exp. Biol., 1989, 27, 1028-31.
11 BASU A, GOMES A, DASGUPTA SC, LAHIRI SC. Histamine, 5 HT & Hyaluronidase in the venom of the scorpion Lychas laevifrons Pocock. Indian J. Med. Res., 1990, 92, 371-3.
12 BAWASKAR HS., BAWASKAR PH. Treatment of cardiovascular manifestations of human scorpion envenoming. Is serotherapy essential? J. Trop. Med. Hyg., 1991, 94, 156-8.
13 BAWASKAR HS., BAWASKAR PH. Role of atropine in management of cardiovascular manifestations of scorpion envenoming in humans. J. Trop. Med. Hyg., 1992, 95, 30-5.
14 BAWASKAR HS., BAWASKAR PH. Scorpion sting. J. Assoc. Physicians India, 1998, 46, 388-92.
15 BHASKARA REDDY D., SUVARNA KUMARI G. Fatal cases of scorpion sting. Autopsy study of 7 cases. Clinician, 1972, 36, 94-6.
16 BICHILE LS., KULOOR PL., SUZANNE R.GUPTA A., NANIVADEKAR S A. Fulminating pulmonary oedema following myocarditis due to scorpion sting. Indian Pract., 1984, 37, 283-5.
17 BISARYA BN., VASVADA JP., ASWIN B., BYSARYA BN., VASADA JP., GHATT A., NAIR PN., SHARMA VK. Hemiplegia and myocarditis following scorpion bite. Indian Heart J., 1977, 29, 97-100.
18 BOSE HK., SARKAR AK., BAHNERGEE S., Electrocardiographic observations in cases of peripheral circulatory failure. Indian Heart J. 1966, 18, 31-6.
19 BUCARETCHI F., BARACAT EC., NOGUEIRA, RJ., CHAVES A., ZAMBRONE FA., FONSECA MR. TOURINHO FS. A comparative study of severe scorpion envenomation in children caused by Tityus bahiensis and Tityus serrulatus. Rev. Inst. Med. Trop. São Paulo, 1995, 37, 331-6.
20 CHATWAL GS., HABERMANN E. Neurotoxins, protease inhibitors and histamine releasers in the venom of the Indian red scorpion (Buthus tamulus). Isolation and partial characterization. Toxicon, 1981, 19, 807-23.
21 CHOUDHARY L., GANGULY DK. Some cardiovascular effects of crude scorpion venom. Indian J. Med. Res., 1978, 68, 515-8.
22 CLAUSEN T. Regulation of active Na+ - K+ transport in skeletal muscle. Physiol. Rev., 1986, 66, 542-80.
23 CRONKITE EP. Blood and lymph. In: BEST & TAYLORS physiological basis of medical practice. 9. ed. Baltimore:Willian & Wilkins, 1973, 4-57.
24 DACIE JV., LEWIS SM. Practical haematology. 6. ed. New York: Churchill Livingstone, 1984. 453p.
25 DEFRONZO RA. Lilly lecture 1987: the triumvirate: b-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes, 1988, 37, 668-87.
26 DESHPANDE SB., RAO KS. Scorpion venom enhances the refractoriness of peripheral axons of frog sciatic nerve in vitro. In: ASIA PACIFIC CONGRESS ON ANIMALS, PLANT AND MICROBIAL TOXINS, 2, Varanasi, 1990. Varanasi, 1990. p.36.
27 DEVI ES., REDDY CN., DEVI SL., SUBRAHMANYAM YR., BHATT HV., SUVARNAKUMARI G., MURTHY DP., REDDY CR. Defibrination syndrome due to scorpion poisoning. Br. Med. J., 1970, 1, 345-7.
28 DOUGLAS WW. Polypetides, angiotensin, plasma kinins and others. In: GOODMAN & GILMANS: the pharmacological basis of therapeutics. 7.ed. New York: MacMillan, 1985.
29 EL-AMIN ED. Issues in management of scorpion stings in children. Toxicon, 1992, 30, 111-5.
30 FREIRE-MAIA L., CAMPOS JA. Response to the letter to the Editor by Gueron and Ovsyshcher on the treatment of the cardiovascular manifestations of scorpion envenomation. Toxicon, 1987, 25, 125-30.
31 FREIRE-MAIA L., DE MATOS IM. Heparin or PAF antagonist (BN-52021) prevents the acute pulmonary oedema induced by Tityus serrulatus scorpion venom in the rat. Toxicon, 1993, 31, 1207-10.
32 GAJALAKSMI C. Role of lytic cocktail and atropine in neutralizing scorpion venom effects. Indian J. Med. Res., 1978, 67, 1038-44.
33 GAJALAKSMI C. Coagulation studies following scorpion venom injection in animals. IndianJ. Med. Res., 1982, 76, 337-41.
34 GAJALAKSMI C., RAMASWAMI N., THIAGARAJAN C. Certain observations in electrocardiogram and enzyme variations in dogs following scorpion venom injection. Indian J. Physiol. Pharmacol., 1978, 22, 397-400.
35 GANONG WG. Review of medical physiology. 13. ed. New York: Appleton and Lange, 1987. p. 283.
36 GOLDSTEIN RE., ABUMRAD NN., WASSERMAN DH., CHERRINGTON AD. Effects of an acute increase in epinephrine and cortisol on carbohydrate metabolism during insulin deficiency. Diabetes, 1995, 44, 672-81.
37 GOYFFON M., VACHON M., BROGLION N. Epidemiological clinical characteristics of the scorpion envenomation in Tunisia. Toxicon, 1982, 20, 337-44.
38 GUERON M., ILIAS R. Non-cardiogenic pulmonary oedema after scorpion envenomation: a true entity? Toxicon, 1996, 34, 393-5.
39 GUERON M., OVSYSHCHER I. What is the treatment for the cardiovascular manifestations of scorpion envenomation? Toxicon, 1987, 25, 121-4.
40 GUERON M., OVSYSHCHER I. Cardiovascular effects of scorpion venom. In: TU AT. Ed. Handbook of natural toxins. New York: M. Dekker, 1983:639-57.
41 GUERON M., YAROM R. Cardiovascular manifestations of severe scorpion sting. Chest, 1970, 57, 156-62.
42 GUERON M., STERN J., COHEN W. Severe myocardial damage and heart failure in scorpion sting. Am. J. Cardiol., 1967, 19, 719-25
43 GUERON M., ADOLPH R., GRUFF L., GRUP O., GABEL M., FOWLER NO. Hemodymamics and myocardial consequences of scorpion venom. Am. J. Cardiol., 1980, 45, 979-86.
44 GUERON M., MARQULIS G., SOFER S. Echoardiographic and radionuclide angiographic observations following scorpion envenomation by Leiurus quinquestriatus.Toxicon, 1990; 28, 1005-9.
45 GUERON M., ILIAS R., SHAHAK E., SOFER S. Renin and aldosterons levels following envenomation by Leiurus quinquestriatus. Toxicon, 1992, 30, 765-7.
46 GUERON M., ILIAS R., SOFER S. The management of scorpion envenoming syndrome. Toxicon, 1993, 31, 1071-6.
47 HANDARGAL NH., MALLERAJA GOUDA K., RAMNATH T. A clinical study of one hundred cases of scorpion sting. J. Assoc. Physisians India, 1989; 34: 37.
48 HANNELE Y.-J. Glucose toxicity. Endocrine Rev., 1992, 13, 415-31.
49 HARVEY AL., ANDERSON AJ., BRAGE MEM., BRAGAL MFM., MARSHALL DL., ROWAN EG., VATANPOUR H., CASTANEDA O., KARLSSON E. Toxins affecting neuronal in channels. Rec. Adv. Toxinol. Res., 1992, 1, 59-70.
50 HERING SE., JURCA M., VICHI FL., AZEVEDO-MARQUES MM., CUPO P. Reversible cardiomyopathy, in patients with severe scorpion envenoming by Tityus serrulatus: evolution of enzymatic, electrocadiographic and echocardiographic alterations. Ann. Trop. Paediatr., 1993, 13, 173-82.
51 ISMAIL M. Serotherapy of the scorpion envenoming syndrome is irrationally convicted without trial. Toxicon, 1993, 31, 1077-83.
52 ISMAIL M. The scorpion envenoming syndrome. Toxicon, 1995, 33, 825-58.
53 ISMAIL M., ABD-ELSALAM MA.. Are the toxicological effects of scorpion envenomation related to tissue venom concentration? Toxicon, 1988, 26, 233-56.
54 ISMAIL M., ABD-ELSALAM MA., MORAD AM. Do changes in body temperature following envenomation by the scorpion Leiurus quinquestriatus influence the course of toxicity? Toxicon, 1990, 28, 1265-84.
55 ISMAIL M., FATANI AJY., DABEAS TT. Experimental treatment protocols for scorpion envenomation: a review of common therapies and on effect of kallikrein - kinin inhibitors. Toxicon, 1992, 30, 1257 - 79.
56 IZZO JR JL. Insulin resistance: is it truly the link? Am. J. Med., 1991, 90, suppl. 2a, 265.
57 JAIN SR., CHHABRA ML., SHAH P.,SEPAHA GC. Myocardial injury after scorpion stings. Indian J. Med. Sci., 1970, 24, 645-6.
58 JAINDANI PG., BICHILE LS., KANTHA S., HASE NK. Intrauterine foetal death following scorpion sting. Indian Pract., 1990, 43, 845-6.
59 JAMMIHAL JH., SRINIVAS HV. Hemiplegia following scorpion sting. Indian Ped., 1973, 10, 337-8.
60 KAHN NN., SINHA AK. Down regulation of alpha2 adrenergic receptor numbers in platelets by insulin. Biochim. Biophys. Acta, 1992, 1134, 292-6.
61 KAHN NN., MUELLER HS., SINHA AK. Impaired prostaglandin E1/I2 receptor activity of human blood platelets in acute ischemic heart disease. Circ. Res., 1990, 66, 932-40.
62 KAHN NN., MUELLER HS., SINHA AK. Restoration by insulin of impaired prostaglandin E1/I2 receptor activity of platelets in acute ischemic heart disease. Circ. Res., 1991, 68, 245-54.
63 KAHN NN., NAJEEB MA., ISHAQ M., RAHIM A., SINHA AK. Normalization of impaired response of platelets to prostaglandin E1/I2 and synthesis of prostacyclin insulin in unstable angina pectoris and in acute myocardial infarction. Am. J. Cardiol., 1992, 70, 582-6.
64 KAHN NN., BAUMAN WA., HATCHER VB., SINHA AK. Inhibition of platelet aggregation and the stimulation of prostacyclin synthesis by insulin in humans. Am. J. Physiol., 1993, 6 pt.2, H 2160-7.
65 KAHN NN., BAUMAN WA., SINHA AK. Transient decrease of binding of insulin to platelets in acute ischemic heart disease. Am. J. Med. Sci., 1994, 307, 21-6.
66 KAHN NN., BAUMAN WA., SINHA AK. Loss of high-affinity prostacyclin receptors in platelets and the lack of prostaglandin in subjects with spinal cord injury. Proc. Natl. Acad. Sci., USA., 1996, 93, 245-9.
67 KAHN NN., BAUMAN WA., SINHA AK. Insulin-induced release of plasminogen activator from human blood platelets. Am. J. Physiol., 1995, 268, H117-24.
68 KANKONKAR RC., RADHA KRISHNA MURTHY K., ZARE AM., MALATHI A., BALASUBRAMANIANI P., YEOLECAR ME. Reversal of cardiovascular and haemodynamic disturbances by scorpion antivenin administration in myocarditis due to envenomation by Indian red scorpion (Buthidae family) venom. Rec. Adv. Toxinol. Res., 1992, 2, 61-9.
69 KARNAD DR., DEO AM., APTE N., LOHE AS., THATTE S., TILVE GH. Captopril for correcting diuretic induced hypotension in pulmonary oedema after scorpion sting. Br. Med. J., 1989, 298, 1430-1.
70 KOTHARI UR., SHAH SS., DOSHI HV. Myocarditis from scorpion stings. A clinical and electrocardiographic study of 50 cases. Indian Heart J., 1976, 28, 88-92.
71 KUMAR ER., HAMDANI AA., SHINY NB. Scorpion venom cardiomyopathy. Am. Heart J., 1992, 123, 725-9.
72 LAHIRI SC., NAG CHAUDHARY AK. Action of scorpion (Heterometrus bengalensis C. L. Koch ) venom on smooth muscle. Indian J. Exp. Biol., 1982, 20, 545-8.
73 LAHIRI SC., NAG CHAUDHARY AK. Release of kinin by the scorpion (Heterometrus bengalensis CL Koch ) venom. Indian J. Exp. Biol., 1983, 21, 198-202.
74 LAMBERT MG., VAN GOLDE, BATANBURG JJ., ROBERTSON B. The pulmonary surfactant system: biochemical aspects and functional significance. Physiol. Rev., 1988, 68, 374-455.
75 LARAGH JH., SEALY JE. The renin-aldosterone axis for blood pressure, electrolyte homeostasis and diagnosis of high bood pressure. In: Textbook of Endorinology. 6.ed. Philadelphia: W.B. Saunders, 1981: 273-98.
76 LATH GK., BHATTACHARYA AK. Hemiplegia following scorpion stings. J. Indian Med. Assoc., 1969, 53, 148-9.
77 MANGAL BD., GUPTA BK., MOHANTY C. Scorpion stings in children: efficacy of lytic cocktail therapy. Indian Pract., 1991, 44, 197-200.
78 MARTIN-EAUCLEIRE MF., DELBRE ML., CEARD B, RIBEIRO A., SOGGARD M., SVENSSON B., DINIZ C., SMITH LA., ROCHART H., BOUIS PE. Genetics of scorpion toxins. Rec. Adv. Toxinol. Res., 1992, 2, 196-209.
79 MATHUR A., VERMA G., GEGLOT RP., UJJWAL JS. Non-cardiac pulmonary oedema in scorpion bite. J. Assoc. Physicians India, 1993, 41, 398.
80 MATTHY RA., MATTHY MA. Pulmonary oedema: cardiogenic and non-cardiogenic. In:___. Chest medicine: essentials of pulmonary and critical care medicine. 2.ed. Baltimore: Williams & Wilkins, 1990:439-52
81 MULLER JE., MOCHIZUKI S., KOSTER JK., COLLINS JR JJ., COHN LH., NEERY JR. Insulin therapy for depressed myocardial contractility after prolonged ischemia. Am. J. Cardiol., 1978, 41, 1215-21.
82 MURALEEDHARAN MV., JAYAKUMAR RV. Hyperinsulinemia: an innocent bystander. J. Assoc. Physicians India, 1997, 45, suppl 1, 30-1.
83 MURUGESAN S., RADHAKRISHNA MURTHY K., NORONHA OPD., SAMUEL AM. 99m Tc-scorpion venom: labelling, biodistribution and scintiimaging. J. Venom. Anim. Toxins, 1999, 5, 35-46.
84 NAYLER WG. The ionic basis of contractibily, relaxation and cardiac failure. In: Oliver MF. Ed. Modern trends in cardiology. London: Butterworths, 1975: 154-81.
85 NIKKILA EA. Insulin and the cardiovascular system. In: OLIVER MF. Ed. Modern trends in cardiology. 3.ed. London: Butterworths, 1975, 89-114.
86 OLEFAKY JM., KOLTERMAN DG. Mechanisms of insulin resistance in obesity and non-insulin-dependent (X Type II) diabetes. Am. J. Med., 1981, 70, 151-68.
87 OLIVER MF. The vulnerable ischaemic myocardium and its metabolism. In: OLIVER MF. Ed.: Modern trends in cardiology. 3.ed. London: Butterworths, 1975, 280-91.
88 OREILLY RA. Anticoagulant, antithrombotic, and thrombolytic drugs. In: GOODMAN and GILMANs: the pharmacological basis of therapeutics. 6.ed. New York: MacMillan, 1980: 1347-66.
89 PANDE SV., MEAD JF. Inhibition of enzyme activities by free fatty acids. J. Biol. Chem., 1968, 243, 6180-6.
90 PARPART AK., LORENG PB., PARPART ER., GREGG JR., CHASE AM. The osmotic resistance (fragility) of human red cells. J. Clin. Invest., 1947, 26, 636-4.
91 PARTHASARATHY PR., VENKAIAH B. Histopathological study on the effect of venom from the scorpion Heterometrus fulvipas. Indian J. Pathol. Microbiol., 1986, 29, 155-8.
92 PETTY TL. Acute respiratory distress syndrome. Dis. Month, 1990, 6, 1-58.
93 POON-KING T. Treatment of scorpion sting. Br. Med. J., 1963, 1, 1016.
94 PURSHOWTHAM RAO G., PREMALATA BK., VENKATAKRISHNA-BHAT H., HARANATH PS. Cardiac and behavioural effects of scorpion venom in experimental animals. Indian Pediatr., 1969, 6, 95-9.
95 RADHA KRISHNA MURTHY K. Investigations of cardiac sarcolemmal ATPase activity in rabbits with acute myocarditis produced by scorpion (Buthus tamulus) venom. Jpn. Heart J.,1982, 23, 835-42.
96 RADHA KRISHNA MURTHY K., ABBAS ZARE M. Effect of Indian red scorpion (Mesobuthus tamulus concanesis, Pocock) venom on thyoxine and triiodothyronine in experimental acute myocarditis and its reversal by species specific antivenom. Indian J. Exp. Biol., 1998, 36, 16-21.
97 RADHA KRISHNA MURTHY K., ANITA AG. Reduced insulin secretion in acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian Heart J., 1986, 38, 467-9.
98 RADHA KRISHNA MURTHY K., HAGHNAZARI L. Blood levels of glucagon, cortisol and insulin following scorpion (Mesobuthus tamulus concanesis, Pocock) envenoming in dogs. J. Venom. Anim. Toxins, 1999, 5, 47-55.
99 RADHA KRISHNA MURTHY K., HASE NK. Scorpion envenoming and role of insulin. Toxicon, 1994, 32, 1041-4.
100 RADHA KRISHNA MURTHY K., HASE NK. Scorpion envenoming and role of insulin. Med. Update, 1995, May, 61-8.
101 RADHA KRISHNA MURTHY K., HOSSEIN Z. Increased osmotic fragility of red cells after incubation at 37ºC for 24 hours in dogs with acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian J. Exp. Biol., 1986, 38, 206-10.
102 RADHA KRISHNA MURTHY K., HOSSEIN Z. Increased osmotic fragility of red cells in dogs with acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian J. Physiol. Pharmacol., 1986, 30, 215-9.
103 RADHA KRISHNA MURTHY K., MEDH JD. Increase in serum free fatty acids. phospholipids and reduction in total cholesterol in acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian Heart J., 1986, 38, 369-72.
104 RADHA KRISHNA MURTHY K., VAKIL AE. Elevation of plasma angiotensin levels in dogs by Indian red-scorpion (Buthus tamulus) venom and its reversal by administration of insulin and tolazoline. Indian J. Med. Res., 1988, 88, 376-79.
105 RADHA KRISHNA MURTHY K., YEOLEKAR ME. Electrocardiographic changes in acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian Heart J., 1986, 38, 206-10.
106 RADHA KRISHNA MURTHY K., BILLIMORIA FR., KHOPKAR M., DAVE KN. Acute hyperglycemia and hyperkalemia in acute myocarditis produced by scorpion (Buthus tamulus) venom injection in dogs. Indian Heart J., 1986, 38, 71-4.
107 RADHA KRISHNA MURTHY K., BALASUBRAMANIAM P., YEOLEKAR ME. Hyperglycemia and reduction in glycogen content of atria and liver in dogs with acute myocarditis produced by scorpion (Buthus tamulus) venom. J. Assoc. Physicians India, 1987, 35, 189-90.
108 RADHA KRISHNA MURTHY K., HOSSEIN Z., MEDH JD, KUDALKAR JÁ., YEOLEKAR ME., PANDIT SP., KHOPKAR M., DAVE KN., BILLIMORIA FR. Disseminated intravascular coagulation & disturbances in carbohydrate and fat metabolism in acute myocarditis produced by Indian red scorpion (Buthus tamulus) venom. Indian J. Med. Res., 1988, 87, 318-25.
109 RADHA KRISHNA MURTHY K., VAKIL AE., YEOLEKAR ME., VAKIL YE. Reversal of metabolic and electrocardiographic changes induced by Indian red scorpion (Buthus tamulus) venom by administration of insulin, alpha blocker and sodium bicarbonate. Indian J. Med. Res., 1988, 88, 450-57.
110 RADHA KRISHNA MURTHY K., ANITA AG., DAVE BN., BILLIMORIA FR. Erythrocyte Na+ - K+ ATPase activity inhibition and increase in red cell fragility in experimental myocarditis produced by Indian red scorpion venom. Indian J. Med. Res. 1988, 88, 536-40.
111 RADHA KRISHNA MURTHY K., MEDH JD., DAVE BN., VAKIL YE., BILLIMORIA FR. Acute pancreatitis and reduction of H+ ion concentration in gastric secretions in experimental acute myocarditis produced by Indian red scorpion (Buthus tamulus) venom. Indian J. Exp. Biol., 1989, 27, 242-4.
112 RADHA KRISHNA MURTHY K., VAKIL AE., YEOLEKAR ME. Insulin administration reverses the metabolic and electrocardiographic changes induced by Indian red scorpion (Buthus tamulus) venom in the experimental dogs. Indian Heart J., 1990, 48, 35-42.
113 RADHA KRISHNA MURTY K., SHENOI R., VAIDYANATHAN P., KELKAR K, SHARMA N., NEETA B., RAO S., MEHTA MN. Insulin reverses haemodynamic changes and pulmonary oedema in children stung by Indian red scorpion Mesobuthus tamulus concanesis, Pocock. Ann. Trop. Med. Parasitol., 1991, 85, 651-57.
114 RADHA KRISHNA MURTHY K., VAKIL AE., YOLEKAR ME., VAKIL YE. Reversal of metabolic and electrocardiographic changes by scorpion antivenin administration in experimental myocarditis induced by Indian red scorpion (Buthidae family) venom. Rec. Adv. Toxinol. Res., 1992, 2, 70-83.
115 RADHA KRISHNA MURTHY K., BHAMBURE NM., ABHYANKAR NY., PHADKE AY., SOMAN SS, SHAH AC, DESHMUKH SN. Insulin administration in respiratory distress syndrome following septic shock. Indian Pract., 1992, 45, 641-4.
116 RADHA KRISHNA MURTHY K., ABBAS ZARE M., HAGHNAZARI L. The use of serotheraphy to reserve ECG and cardiac enzyme changes caused by scorpion Mesobuthus tamulus concanesis, Pocock envenoming. J. Venom. Anim. Toxins, 1999, 5, 154-71.
117 RADMANESH M. Clinical study of Hemiscorpion lepturus in Iran. J. Trop. Med. Hyg., 1990, 93, 327-32.
118 RAHAV G., WEISS T. Scorpion sting induced pulmonary oedema. Scintigraphic evidence of cardiac dysfunction. Chest, 1980, 97, 1478-80.
119 RAI OP. Cardiopulmonary changes following scorpion (Buthus tamulus) envenomation. Varanas: Banaras Hindu University, 1993. 79p. (Thesis Doctor of Medicine)
120 RAJARJESWARI G., SIVAPRAKASAM S., VISWANATHAN J. Morbidity and mortality pattern in scorpion stings. J. Indian Med. Assoc., 1979, 73, 123-5.
121 RAMANAIAH M., PARTHASARATHY PR., VENKAIAH B. Purification and properties of phospholipase A2 from the venom of scorpion Heterometrus fulvipes. Biochem. Int., 1990, 20, 931-40.
122 REAVEN GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes, 1988, 37, 1595-607.
123 REDDY CRRM., SUVARNA KUMARI G., DEVI CS., REDDY CN. Pathology of scorpion venom poisoning. J. Trop. Med. Hyg., 1972, 75, 98-100.
124 REZENDE NAD., DIAS MB., CAMPOLINA D., CHAVEZ-OLORTEGUI C., DINIZ C., AMARAL CFS. Efficacy of antivenom therapy for neutralising venom antigen in patients stung by Tityus serrulatus scorpion. Am. J. Trop. Med. Hyg.,1995, 52, 277-80.
125 RIMSZA ME., ZIMMERMAN DR., BERGESEN PS. The scorpion envenomation. Pediatrics, 1980, 66, 298-301.
126 RINALDO JE. The adult respiratory distress syndrome. Curr. Pulmonol., 1994, 15, 137-56.
127 SANTHANAKRISHAN BR., SUNDERVALLI N., BALAGOPALARAJU V. Artificial liberation with lytic cocktail in management of peripheral failure due to scorpion sting. Indian Pediatr., 1972, 9, 23-5.
128 SANTHANAKRISHNAN BR., RANGANATHAN B., ANANTHSUBRAMANIAM P., RAJU VB. Cardiovascular manifestations of scorpion stings in children. Indian Pediatr., 1977, 14, 353-6.
129 SCHAMROTH C. The pathogenesis of ventricular arrhythmias. YU PN., GOODWIN JF. Ed. Phildelphia: Lea & Febiger, 1974: 75p.
130 SERRANO J., MATEO CM., CARO JF. Insulin resistance: cellular and molecular mechanisms. Rec. Adv. Endocrinol. Metabol., 1992, 4, 167-83.
131 SHAH SS., DOSHI HV., KULKARNI PS. Myocarditis from scorpion sting. Indian Heart J., 1972, 24, 52-5.
132 SHIVARAJ A., NAMOSHI AG., GAO MS. L.V. function in scorpion sting. Indian Heart J., 1981, 33, 150.
133 SINGH DS., SAMARIA JK. A clinical study of 36 patients of scorpion bite. J. Assoc. Physisians India, 1991, 39, 851.
134 SINGH DS., BISHT DB., SUKUMAR G., MURALIDHAR K. Scorpion stings in adult south Indians at pondicherry. J. Indian Med. Assoc., 1979, 72, 234-7.
135 SKOU JC. The Na+ - K+ pump. News Physiol. Sci., 1992, 7, 95-100.
136 SOFER S., GUERON M. Cardiovascular aspects of scorpion envenomation. Rec. Adv. Toxinol. Res., 1992, 2, 40-9.
137 SOFER S., SHAHAK E., SLONIM A., GUERON M. Myocardial injury without heart failure following envenomation by scorpion Leiurus quinquestriatus in children Toxicon, 1991; 29, 382-5.
138 SONKSEN PH., WEST TET. Carbohydrate metabolism and diabetes mellitus. Rec. Adv. Endocrinol. Metabol., 1978, 1, 160-87.
139 STAHNKE HL. The genus Centruroides (Buthidae) and its venom. In: BETTINI S. Ed. Handbook of experimental pharmacology: arthropod venoms. Berlin: Springer, 1978, 48, 277-307.
140 SUSAN P., PORTERFIELD. Endocrine pancreas. In.: Endocrine physiology. ST. Louis: Mosby, 1996, 83-104.
141 TAHERBHAI M. A report on clinical field trials of anti-scorpion-venom serum conducted in three districts of Maharashtra namely Thane, Raigad and Ratnagiri. Maharasktra, 1992. (A report submitted to Government of Maharashtra).
142 TIKEDER BK., BASTAWADE DB. The fauna of India: scorpions, Scorpionida, Arachnida. Calcutta: Zoological Survey of India, 1983, 3: 1-587.
143 TIWARI AK., DESHPANDE SB. Toxicity of scorpion (Buthus tamulus) venom in mammals is influenced by the age and species. Toxicon, 1993, 31, 1619-22.
144 TIWARI AK., GUPTA GB., GUPTA SR., MISHRA SN., PRADAHAN PK. Fatal stroke following scorpion bite. J. Assoc. Physicians India, 1988, 36, 225-6.
145 TROVATI M., MOSSOCCO P., ANFOSSI G. Insulin influences the renin: angiotensin-aldosteron on system in humans. Metabolism, 1989, 38, 501-3.
146 USHA KIRAN P. A retrospective study on treatment of red scorpion sting. Vijayawada: The Andhra Pradesh Health University,1996. 109p. (M.D. Thesis).
147 VANAJA G., APARNA JR., VIJAYAKUMARI K. Effects of scorpion Heterometrus fulvipes venom on goat tissues and its detoxification through chemical dissociation Indian Zool., 1990, 14, 165-8.
148 VIK HARALD MO., OLE DM. Influence of free fatty acids on myocardial oxygen consumption and ischemic injury. Am. J. Cardiol., 1981, 48, 361-67.
149 WANNAMETHEE SG., PARRY IJ., SHAPER AG. Haematocrit and risk of NIDDM. Diabetes, 1966, 45, 576-9.
150 YUGANDHAR B. Clinical study of scorpion sting with special reference to insulin-glucose treatment. Vijayawada: Andhra Pradesh University of Health Sciences, 1995. (M.D. thesis).
Received 24 March 1998
Accepted 06 October 1998
- 01 ACHYUTHAN KE., AGARWAL OP., RAMACHANDRAN LK. Enzymes in the venoms of two species of Indian scorpions. Indian J. Biochem. 1982, 19, 356-8.
- 02 ALEGESAN R., RAGHAVAN S., BALAMBA’ R. HARANATH K., SUBRAMANIAN N., THIRUVENGADAM. Transient right Gundle Branch block following scorpion stings. J. Indian Med. Assoc., 1977, 69, 113-4.
- 03 AMARAL CFS., REZENDE NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon, 1997, 35, 997-8.
- 04 AMARAL CFS., REZENDE NA., FREIRE-MAIA L. Acute pulmonary oedema after Tityus serrulatus scorpion sting in children.Am. J. Cardiol., 1993, 71, 242-5.
- 05 AMARAL CFS., BARBOSA AJA., LEITE VHR., TAFURI WL., REZENDE NA. Scorpion sting induced pulmonary oedema: Evidence of increased alveolocapillary membrane permeability. Toxicon, 1994, 32, 999-1003.
- 06 ANSARI MY. Gangrene after scorpion sting. Br. Med. J., 1948, 2, 388.
- 07 AVOGARO A., CREPALDI C., PIARULI F., MILAN D., CALABRO A., MACDONALD I., CREPALDI G., SCOGNAMIGILO R., TIENGO A. The hemodynamic abnormalities in short-term insulin deficiency: the role of prostaglandin inhibition. Diabetes, 1996, 45, 602-09.
- 08 BALASUBRAMANYAM P., RADHA KRISHNA MURTHY K. Abnormal cardiovascular and electrocardiographic profile and cardiac glycogen content in rabbits injected with scorpion venom. Indian. J. Physiol. Pharmacol., 1981, 25, 351-5.
- 09 BALASUBRAMANIAM P., RADHA KRISHNA MURTHY K. Liver glycogen depletion in acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian Heart J., 1984, 36, 101–4
-
1010 BASU A., GOMES A., DASGUPTA SC., LAHIRI SC. Pharmacology of scorpion (Lychas laevifrons Pocock) Venom with special reference to its neuromuscular activity. Indian J. Exp. Biol., 1989, 27, 1028-31.
- 11 BASU A, GOMES A, DASGUPTA SC, LAHIRI SC. Histamine, 5 HT & Hyaluronidase in the venom of the scorpion Lychas laevifrons Pocock. Indian J. Med. Res., 1990, 92, 371-3.
- 12 BAWASKAR HS., BAWASKAR PH. Treatment of cardiovascular manifestations of human scorpion envenoming. Is serotherapy essential? J. Trop. Med. Hyg., 1991, 94, 156-8.
- 13 BAWASKAR HS., BAWASKAR PH. Role of atropine in management of cardiovascular manifestations of scorpion envenoming in humans. J. Trop. Med. Hyg., 1992, 95, 30-5.
- 14 BAWASKAR HS., BAWASKAR PH. Scorpion sting. J. Assoc. Physicians India, 1998, 46, 388-92.
- 15 BHASKARA REDDY D., SUVARNA KUMARI G. Fatal cases of scorpion sting. Autopsy study of 7 cases. Clinician, 1972, 36, 94-6.
- 16 BICHILE LS., KULOOR PL., SUZANNE R.GUPTA A., NANIVADEKAR S A. Fulminating pulmonary oedema following myocarditis due to scorpion sting. Indian Pract., 1984, 37, 283-5.
- 17 BISARYA BN., VASVADA JP., ASWIN B., BYSARYA BN., VASADA JP., GHATT A., NAIR PN., SHARMA VK. Hemiplegia and myocarditis following scorpion bite. Indian Heart J., 1977, 29, 97-100.
-
1818 BOSE HK., SARKAR AK., BAHNERGEE S., Electrocardiographic observations in cases of peripheral circulatory failure. Indian Heart J. 1966, 18, 31-6.
- 19 BUCARETCHI F., BARACAT EC., NOGUEIRA, RJ., CHAVES A., ZAMBRONE FA., FONSECA MR. TOURINHO FS. A comparative study of severe scorpion envenomation in children caused by Tityus bahiensis and Tityus serrulatus. Rev. Inst. Med. Trop. São Paulo, 1995, 37, 331-6.
- 20 CHATWAL GS., HABERMANN E. Neurotoxins, protease inhibitors and histamine releasers in the venom of the Indian red scorpion (Buthus tamulus). Isolation and partial characterization. Toxicon, 1981, 19, 807-23.
- 21 CHOUDHARY L., GANGULY DK. Some cardiovascular effects of crude scorpion venom. Indian J. Med. Res., 1978, 68, 515-8.
- 22 CLAUSEN T. Regulation of active Na+ - K+ transport in skeletal muscle. Physiol. Rev., 1986, 66, 542-80.
- 23 CRONKITE EP. Blood and lymph. In: BEST & TAYLOR’S physiological basis of medical practice 9. ed. Baltimore:Willian & Wilkins, 1973, 4-57.
- 24 DACIE JV., LEWIS SM. Practical haematology. 6. ed. New York: Churchill Livingstone, 1984. 453p.
- 25 DEFRONZO RA. Lilly lecture 1987: the triumvirate: b-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes, 1988, 37, 668-87.
- 26 DESHPANDE SB., RAO KS. Scorpion venom enhances the refractoriness of peripheral axons of frog sciatic nerve in vitro In: ASIA PACIFIC CONGRESS ON ANIMALS, PLANT AND MICROBIAL TOXINS, 2, Varanasi, 1990. Varanasi, 1990. p.36.
- 27 DEVI ES., REDDY CN., DEVI SL., SUBRAHMANYAM YR., BHATT HV., SUVARNAKUMARI G., MURTHY DP., REDDY CR. Defibrination syndrome due to scorpion poisoning. Br. Med. J., 1970, 1, 345-7.
- 28 DOUGLAS WW. Polypetides, angiotensin, plasma kinins and others. In: GOODMAN & GILMAN’S: the pharmacological basis of therapeutics. 7.ed. New York: MacMillan, 1985
- 29 EL-AMIN ED. Issues in management of scorpion stings in children. Toxicon, 1992, 30, 111-5.
- 30 FREIRE-MAIA L., CAMPOS JA. Response to the letter to the Editor by Gueron and Ovsyshcher on the treatment of the cardiovascular manifestations of scorpion envenomation. Toxicon, 1987, 25, 125-30.
- 31 FREIRE-MAIA L., DE MATOS IM. Heparin or PAF antagonist (BN-52021) prevents the acute pulmonary oedema induced by Tityus serrulatus scorpion venom in the rat. Toxicon, 1993, 31, 1207-10.
- 32 GAJALAKSMI C. Role of lytic cocktail and atropine in neutralizing scorpion venom effects. Indian J. Med. Res., 1978, 67, 1038-44.
-
3333 GAJALAKSMI C. Coagulation studies following scorpion venom injection in animals. IndianJ. Med. Res., 1982, 76, 337-41.
- 34 GAJALAKSMI C., RAMASWAMI N., THIAGARAJAN C. Certain observations in electrocardiogram and enzyme variations in dogs following scorpion venom injection. Indian J. Physiol. Pharmacol., 1978, 22, 397-400.
- 35 GANONG WG. Review of medical physiology. 13. ed. New York: Appleton and Lange, 1987. p. 283.
- 36 GOLDSTEIN RE., ABUMRAD NN., WASSERMAN DH., CHERRINGTON AD. Effects of an acute increase in epinephrine and cortisol on carbohydrate metabolism during insulin deficiency. Diabetes, 1995, 44, 672-81.
- 37 GOYFFON M., VACHON M., BROGLION N. Epidemiological clinical characteristics of the scorpion envenomation in Tunisia. Toxicon, 1982, 20, 337-44.
-
3838 GUERON M., ILIAS R. Non-cardiogenic pulmonary oedema after scorpion envenomation: a true entity? Toxicon, 1996, 34, 393-5.
- 39 GUERON M., OVSYSHCHER I. What is the treatment for the cardiovascular manifestations of scorpion envenomation? Toxicon, 1987, 25, 121-4.
- 40 GUERON M., OVSYSHCHER I. Cardiovascular effects of scorpion venom. In: TU AT. Ed. Handbook of natural toxins. New York: M. Dekker, 1983:639-57.
-
4141 GUERON M., YAROM R. Cardiovascular manifestations of severe scorpion sting. Chest, 1970, 57, 156-62.
- 42 GUERON M., STERN J., COHEN W. Severe myocardial damage and heart failure in scorpion sting. Am. J. Cardiol., 1967, 19, 719-25
- 43 GUERON M., ADOLPH R., GRUFF L., GRUP O., GABEL M., FOWLER NO. Hemodymamics and myocardial consequences of scorpion venom. Am. J. Cardiol., 1980, 45, 979-86.
- 44 GUERON M., MARQULIS G., SOFER S. Echoardiographic and radionuclide angiographic observations following scorpion envenomation by Leiurus quinquestriatusToxicon, 1990; 28, 1005-9.
- 45 GUERON M., ILIAS R., SHAHAK E., SOFER S. Renin and aldosterons levels following envenomation by Leiurus quinquestriatus. Toxicon, 1992, 30, 765-7.
-
4646 GUERON M., ILIAS R., SOFER S. The management of scorpion envenoming syndrome. Toxicon, 1993, 31, 1071-6.
- 47 HANDARGAL NH., MALLERAJA GOUDA K., RAMNATH T. A clinical study of one hundred cases of scorpion sting. J. Assoc. Physisians India, 1989; 34: 37.
- 48 HANNELE Y.-J. Glucose toxicity. Endocrine Rev., 1992, 13, 415-31.
- 49 HARVEY AL., ANDERSON AJ., BRAGE MEM., BRAGAL MFM., MARSHALL DL., ROWAN EG., VATANPOUR H., CASTANEDA O., KARLSSON E. Toxins affecting neuronal in channels. Rec. Adv. Toxinol. Res., 1992, 1, 59-70.
- 50 HERING SE., JURCA M., VICHI FL., AZEVEDO-MARQUES MM., CUPO P. Reversible cardiomyopathy, in patients with severe scorpion envenoming by Tityus serrulatus: evolution of enzymatic, electrocadiographic and echocardiographic alterations. Ann. Trop. Paediatr., 1993, 13, 173-82.
- 51 ISMAIL M. Serotherapy of the scorpion envenoming syndrome is irrationally convicted without trial. Toxicon, 1993, 31, 1077-83.
- 52 ISMAIL M. The scorpion envenoming syndrome. Toxicon, 1995, 33, 825-58.
- 53 ISMAIL M., ABD-ELSALAM MA.. Are the toxicological effects of scorpion envenomation related to tissue venom concentration? Toxicon, 1988, 26, 233-56.
- 54 ISMAIL M., ABD-ELSALAM MA., MORAD AM. Do changes in body temperature following envenomation by the scorpion Leiurus quinquestriatus influence the course of toxicity? Toxicon, 1990, 28, 1265-84.
- 55 ISMAIL M., FATANI AJY., DABEAS TT. Experimental treatment protocols for scorpion envenomation: a review of common therapies and on effect of kallikrein - kinin inhibitors. Toxicon, 1992, 30, 1257 - 79.
- 56 IZZO JR JL. Insulin resistance: is it truly the link? Am. J. Med., 1991, 90, suppl. 2a, 265.
- 57 JAIN SR., CHHABRA ML., SHAH P.,SEPAHA GC. Myocardial injury after scorpion stings. Indian J. Med. Sci., 1970, 24, 645-6.
- 58 JAINDANI PG., BICHILE LS., KANTHA S., HASE NK. Intrauterine foetal death following scorpion sting. Indian Pract., 1990, 43, 845-6.
-
5959 JAMMIHAL JH., SRINIVAS HV. Hemiplegia following scorpion sting. Indian Ped., 1973, 10, 337-8.
- 60 KAHN NN., SINHA AK. Down regulation of alpha2 adrenergic receptor numbers in platelets by insulin. Biochim. Biophys. Acta, 1992, 1134, 292-6.
-
6161 KAHN NN., MUELLER HS., SINHA AK. Impaired prostaglandin E1/I2 receptor activity of human blood platelets in acute ischemic heart disease. Circ. Res., 1990, 66, 932-40.
- 62 KAHN NN., MUELLER HS., SINHA AK. Restoration by insulin of impaired prostaglandin E1/I2 receptor activity of platelets in acute ischemic heart disease. Circ. Res., 1991, 68, 245-54.
- 63 KAHN NN., NAJEEB MA., ISHAQ M., RAHIM A., SINHA AK. Normalization of impaired response of platelets to prostaglandin E1/I2 and synthesis of prostacyclin insulin in unstable angina pectoris and in acute myocardial infarction. Am. J. Cardiol., 1992, 70, 582-6.
- 64 KAHN NN., BAUMAN WA., HATCHER VB., SINHA AK. Inhibition of platelet aggregation and the stimulation of prostacyclin synthesis by insulin in humans. Am. J. Physiol., 1993, 6 pt.2, H 2160-7.
-
6565 KAHN NN., BAUMAN WA., SINHA AK. Transient decrease of binding of insulin to platelets in acute ischemic heart disease. Am. J. Med. Sci., 1994, 307, 21-6.
-
6666 KAHN NN., BAUMAN WA., SINHA AK. Loss of high-affinity prostacyclin receptors in platelets and the lack of prostaglandin in subjects with spinal cord injury. Proc. Natl. Acad. Sci., USA., 1996, 93, 245-9.
- 67 KAHN NN., BAUMAN WA., SINHA AK. Insulin-induced release of plasminogen activator from human blood platelets. Am. J. Physiol., 1995, 268, H117-24.
- 68 KANKONKAR RC., RADHA KRISHNA MURTHY K., ZARE AM., MALATHI A., BALASUBRAMANIANI P., YEOLECAR ME. Reversal of cardiovascular and haemodynamic disturbances by scorpion antivenin administration in myocarditis due to envenomation by Indian red scorpion (Buthidae family) venom. Rec. Adv. Toxinol. Res., 1992, 2, 61-9.
- 69 KARNAD DR., DEO AM., APTE N., LOHE AS., THATTE S., TILVE GH. Captopril for correcting diuretic induced hypotension in pulmonary oedema after scorpion sting. Br. Med. J., 1989, 298, 1430-1.
- 70 KOTHARI UR., SHAH SS., DOSHI HV. Myocarditis from scorpion stings. A clinical and electrocardiographic study of 50 cases. Indian Heart J., 1976, 28, 88-92.
- 71 KUMAR ER., HAMDANI AA., SHINY NB. Scorpion venom cardiomyopathy. Am. Heart J., 1992, 123, 725-9.
- 72 LAHIRI SC., NAG CHAUDHARY AK. Action of scorpion (Heterometrus bengalensis C. L. Koch ) venom on smooth muscle. Indian J. Exp. Biol., 1982, 20, 545-8.
- 73 LAHIRI SC., NAG CHAUDHARY AK. Release of kinin by the scorpion (Heterometrus bengalensis CL Koch ) venom. Indian J. Exp. Biol., 1983, 21, 198-202.
-
7474 LAMBERT MG., VAN GOLDE, BATANBURG JJ., ROBERTSON B. The pulmonary surfactant system: biochemical aspects and functional significance. Physiol. Rev., 1988, 68, 374-455.
- 75 LARAGH JH., SEALY JE. The renin-aldosterone axis for blood pressure, electrolyte homeostasis and diagnosis of high bood pressure. In: Textbook of Endorinology 6.ed. Philadelphia: W.B. Saunders, 1981: 273-98.
- 76 LATH GK., BHATTACHARYA AK. Hemiplegia following scorpion stings. J. Indian Med. Assoc., 1969, 53, 148-9.
- 77 MANGAL BD., GUPTA BK., MOHANTY C. Scorpion stings in children: efficacy of lytic cocktail therapy. Indian Pract., 1991, 44, 197-200.
- 78 MARTIN-EAUCLEIRE MF., DELBRE ML., CEARD B, RIBEIRO A., SOGGARD M., SVENSSON B., DINIZ C., SMITH LA., ROCHART H., BOUIS PE. Genetics of scorpion toxins. Rec. Adv. Toxinol. Res., 1992, 2, 196-209.
- 79 MATHUR A., VERMA G., GEGLOT RP., UJJWAL JS. Non-cardiac pulmonary oedema in scorpion bite. J. Assoc. Physicians India, 1993, 41, 398.
- 80 MATTHY RA., MATTHY MA. Pulmonary oedema: cardiogenic and non-cardiogenic. In:___. Chest medicine: essentials of pulmonary and critical care medicine. 2.ed. Baltimore: Williams & Wilkins, 1990:439-52
-
8181 MULLER JE., MOCHIZUKI S., KOSTER JK., COLLINS JR JJ., COHN LH., NEERY JR. Insulin therapy for depressed myocardial contractility after prolonged ischemia. Am. J. Cardiol., 1978, 41, 1215-21.
- 82 MURALEEDHARAN MV., JAYAKUMAR RV. Hyperinsulinemia: an innocent bystander. J. Assoc. Physicians India, 1997, 45, suppl 1, 30-1.
- 83 MURUGESAN S., RADHAKRISHNA MURTHY K., NORONHA OPD., SAMUEL AM. 99m Tc-scorpion venom: labelling, biodistribution and scintiimaging. J. Venom. Anim. Toxins, 1999, 5, 35-46.
- 84 NAYLER WG. The ionic basis of contractibily, relaxation and cardiac failure. In: Oliver MF. Ed. Modern trends in cardiology. London: Butterworths, 1975: 154-81.
- 85 NIKKILA EA. Insulin and the cardiovascular system. In: OLIVER MF. Ed. Modern trends in cardiology. 3.ed. London: Butterworths, 1975, 89-114.
- 86 OLEFAKY JM., KOLTERMAN DG. Mechanisms of insulin resistance in obesity and non-insulin-dependent (X Type II) diabetes. Am. J. Med., 1981, 70, 151-68.
- 87 OLIVER MF. The vulnerable ischaemic myocardium and its metabolism. In: OLIVER MF. Ed.: Modern trends in cardiology. 3.ed. London: Butterworths, 1975, 280-91.
- 88 O’REILLY RA. Anticoagulant, antithrombotic, and thrombolytic drugs. In: GOODMAN and GILMAN’s: the pharmacological basis of therapeutics 6.ed. New York: MacMillan, 1980: 1347-66.
- 89 PANDE SV., MEAD JF. Inhibition of enzyme activities by free fatty acids. J. Biol. Chem., 1968, 243, 6180-6.
-
9090 PARPART AK., LORENG PB., PARPART ER., GREGG JR., CHASE AM. The osmotic resistance (fragility) of human red cells. J. Clin. Invest., 1947, 26, 636-4.
- 91 PARTHASARATHY PR., VENKAIAH B. Histopathological study on the effect of venom from the scorpion Heterometrus fulvipas Indian J. Pathol. Microbiol., 1986, 29, 155-8.
- 92 PETTY TL. Acute respiratory distress syndrome. Dis. Month, 1990, 6, 1-58.
-
9393 POON-KING T. Treatment of scorpion sting. Br. Med. J., 1963, 1, 1016.
- 94 PURSHOWTHAM RAO G., PREMALATA BK., VENKATAKRISHNA-BHAT H., HARANATH PS. Cardiac and behavioural effects of scorpion venom in experimental animals. Indian Pediatr., 1969, 6, 95-9.
- 95 RADHA KRISHNA MURTHY K. Investigations of cardiac sarcolemmal ATPase activity in rabbits with acute myocarditis produced by scorpion (Buthus tamulus) venom. Jpn. Heart J.,1982, 23, 835-42.
- 96 RADHA KRISHNA MURTHY K., ABBAS ZARE M. Effect of Indian red scorpion (Mesobuthus tamulus concanesis, Pocock) venom on thyoxine and triiodothyronine in experimental acute myocarditis and its reversal by species specific antivenom. Indian J. Exp. Biol., 1998, 36, 16-21.
- 97 RADHA KRISHNA MURTHY K., ANITA AG. Reduced insulin secretion in acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian Heart J., 1986, 38, 467-9.
- 98 RADHA KRISHNA MURTHY K., HAGHNAZARI L. Blood levels of glucagon, cortisol and insulin following scorpion (Mesobuthus tamulus concanesis, Pocock) envenoming in dogs. J. Venom. Anim. Toxins, 1999, 5, 47-55.
- 99 RADHA KRISHNA MURTHY K., HASE NK. Scorpion envenoming and role of insulin. Toxicon, 1994, 32, 1041-4.
-
100100 RADHA KRISHNA MURTHY K., HASE NK. Scorpion envenoming and role of insulin. Med. Update, 1995, May, 61-8.
- 101 RADHA KRISHNA MURTHY K., HOSSEIN Z. Increased osmotic fragility of red cells after incubation at 37ºC for 24 hours in dogs with acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian J. Exp. Biol., 1986, 38, 206-10.
- 102 RADHA KRISHNA MURTHY K., HOSSEIN Z. Increased osmotic fragility of red cells in dogs with acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian J. Physiol. Pharmacol., 1986, 30, 215-9.
- 103 RADHA KRISHNA MURTHY K., MEDH JD. Increase in serum free fatty acids. phospholipids and reduction in total cholesterol in acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian Heart J., 1986, 38, 369-72.
- 104 RADHA KRISHNA MURTHY K., VAKIL AE. Elevation of plasma angiotensin levels in dogs by Indian red-scorpion (Buthus tamulus) venom and its reversal by administration of insulin and tolazoline. Indian J. Med. Res., 1988, 88, 376-79.
- 105 RADHA KRISHNA MURTHY K., YEOLEKAR ME. Electrocardiographic changes in acute myocarditis produced by scorpion (Buthus tamulus) venom. Indian Heart J., 1986, 38, 206-10.
- 106 RADHA KRISHNA MURTHY K., BILLIMORIA FR., KHOPKAR M., DAVE KN. Acute hyperglycemia and hyperkalemia in acute myocarditis produced by scorpion (Buthus tamulus) venom injection in dogs. Indian Heart J., 1986, 38, 71-4.
-
107107 RADHA KRISHNA MURTHY K., BALASUBRAMANIAM P., YEOLEKAR ME. Hyperglycemia and reduction in glycogen content of atria and liver in dogs with acute myocarditis produced by scorpion (Buthus tamulus) venom. J. Assoc. Physicians India, 1987, 35, 189-90.
- 108 RADHA KRISHNA MURTHY K., HOSSEIN Z., MEDH JD, KUDALKAR JÁ., YEOLEKAR ME., PANDIT SP., KHOPKAR M., DAVE KN., BILLIMORIA FR. Disseminated intravascular coagulation & disturbances in carbohydrate and fat metabolism in acute myocarditis produced by Indian red scorpion (Buthus tamulus) venom. Indian J. Med. Res., 1988, 87, 318-25.
- 109 RADHA KRISHNA MURTHY K., VAKIL AE., YEOLEKAR ME., VAKIL YE. Reversal of metabolic and electrocardiographic changes induced by Indian red scorpion (Buthus tamulus) venom by administration of insulin, alpha blocker and sodium bicarbonate. Indian J. Med. Res., 1988, 88, 450-57.
- 110 RADHA KRISHNA MURTHY K., ANITA AG., DAVE BN., BILLIMORIA FR. Erythrocyte Na+ - K+ ATPase activity inhibition and increase in red cell fragility in experimental myocarditis produced by Indian red scorpion venom. Indian J. Med. Res. 1988, 88, 536-40.
- 111 RADHA KRISHNA MURTHY K., MEDH JD., DAVE BN., VAKIL YE., BILLIMORIA FR. Acute pancreatitis and reduction of H+ ion concentration in gastric secretions in experimental acute myocarditis produced by Indian red scorpion (Buthus tamulus) venom. Indian J. Exp. Biol., 1989, 27, 242-4.
- 112 RADHA KRISHNA MURTHY K., VAKIL AE., YEOLEKAR ME. Insulin administration reverses the metabolic and electrocardiographic changes induced by Indian red scorpion (Buthus tamulus) venom in the experimental dogs. Indian Heart J., 1990, 48, 35-42.
-
114114 RADHA KRISHNA MURTHY K., VAKIL AE., YOLEKAR ME., VAKIL YE. Reversal of metabolic and electrocardiographic changes by scorpion antivenin administration in experimental myocarditis induced by Indian red scorpion (Buthidae family) venom. Rec. Adv. Toxinol. Res., 1992, 2, 70-83.
- 115 RADHA KRISHNA MURTHY K., BHAMBURE NM., ABHYANKAR NY., PHADKE AY., SOMAN SS, SHAH AC, DESHMUKH SN. Insulin administration in respiratory distress syndrome following septic shock. Indian Pract., 1992, 45, 641-4.
- 116 RADHA KRISHNA MURTHY K., ABBAS ZARE M., HAGHNAZARI L. The use of serotheraphy to reserve ECG and cardiac enzyme changes caused by scorpion Mesobuthus tamulus concanesis, Pocock envenoming. J. Venom. Anim. Toxins, 1999, 5, 154-71.
- 117 RADMANESH M. Clinical study of Hemiscorpion lepturus in Iran. J. Trop. Med. Hyg., 1990, 93, 327-32.
- 118 RAHAV G., WEISS T. Scorpion sting induced pulmonary oedema. Scintigraphic evidence of cardiac dysfunction. Chest, 1980, 97, 1478-80.
- 119 RAI OP. Cardiopulmonary changes following scorpion (Buthus tamulus) envenomation. Varanas: Banaras Hindu University, 1993 79p. (Thesis – Doctor of Medicine)
- 120 RAJARJESWARI G., SIVAPRAKASAM S., VISWANATHAN J. Morbidity and mortality pattern in scorpion stings. J. Indian Med. Assoc., 1979, 73, 123-5.
- 121 RAMANAIAH M., PARTHASARATHY PR., VENKAIAH B. Purification and properties of phospholipase A2 from the venom of scorpion Heterometrus fulvipes Biochem. Int., 1990, 20, 931-40.
- 122 REAVEN GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes, 1988, 37, 1595-607.
- 123 REDDY CRRM., SUVARNA KUMARI G., DEVI CS., REDDY CN. Pathology of scorpion venom poisoning. J. Trop. Med. Hyg., 1972, 75, 98-100.
- 124 REZENDE NAD., DIAS MB., CAMPOLINA D., CHAVEZ-OLORTEGUI C., DINIZ C., AMARAL CFS. Efficacy of antivenom therapy for neutralising venom antigen in patients stung by Tityus serrulatus scorpion. Am. J. Trop. Med. Hyg.,1995, 52, 277-80.
- 125 RIMSZA ME., ZIMMERMAN DR., BERGESEN PS. The scorpion envenomation. Pediatrics, 1980, 66, 298-301.
- 126 RINALDO JE. The adult respiratory distress syndrome. Curr. Pulmonol., 1994, 15, 137-56.
- 127 SANTHANAKRISHAN BR., SUNDERVALLI N., BALAGOPALARAJU V. Artificial liberation with lytic cocktail in management of peripheral failure due to scorpion sting. Indian Pediatr., 1972, 9, 23-5.
- 128 SANTHANAKRISHNAN BR., RANGANATHAN B., ANANTHSUBRAMANIAM P., RAJU VB. Cardiovascular manifestations of scorpion stings in children. Indian Pediatr., 1977, 14, 353-6.
- 129 SCHAMROTH C. The pathogenesis of ventricular arrhythmias YU PN., GOODWIN JF. Ed. Phildelphia: Lea & Febiger, 1974: 75p.
- 130 SERRANO J., MATEO CM., CARO JF. Insulin resistance: cellular and molecular mechanisms. Rec. Adv. Endocrinol. Metabol., 1992, 4, 167-83.
- 131 SHAH SS., DOSHI HV., KULKARNI PS. Myocarditis from scorpion sting. Indian Heart J., 1972, 24, 52-5.
- 132 SHIVARAJ A., NAMOSHI AG., GAO MS. L.V. function in scorpion sting. Indian Heart J., 1981, 33, 150.
- 133 SINGH DS., SAMARIA JK. A clinical study of 36 patients of scorpion bite. J. Assoc. Physisians India, 1991, 39, 851.
- 134 SINGH DS., BISHT DB., SUKUMAR G., MURALIDHAR K. Scorpion stings in adult south Indians at pondicherry. J. Indian Med. Assoc., 1979, 72, 234-7.
- 135 SKOU JC. The Na+ - K+ pump. News Physiol. Sci., 1992, 7, 95-100.
-
136136 SOFER S., GUERON M. Cardiovascular aspects of scorpion envenomation. Rec. Adv. Toxinol. Res., 1992, 2, 40-9.
- 137 SOFER S., SHAHAK E., SLONIM A., GUERON M. Myocardial injury without heart failure following envenomation by scorpion Leiurus quinquestriatus in children Toxicon, 1991; 29, 382-5.
-
138138 SONKSEN PH., WEST TET. Carbohydrate metabolism and diabetes mellitus. Rec. Adv. Endocrinol. Metabol., 1978, 1, 160-87.
- 139 STAHNKE HL. The genus Centruroides (Buthidae) and its venom. In: BETTINI S. Ed. Handbook of experimental pharmacology: arthropod venoms. Berlin: Springer, 1978, 48, 277-307.
- 140 SUSAN P., PORTERFIELD. Endocrine pancreas. In.: Endocrine physiology ST. Louis: Mosby, 1996, 83-104.
- 141 TAHERBHAI M. A report on clinical field trials of anti-scorpion-venom serum conducted in three districts of Maharashtra namely Thane, Raigad and Ratnagiri Maharasktra, 1992. (A report submitted to Government of Maharashtra).
- 142 TIKEDER BK., BASTAWADE DB. The fauna of India: scorpions, Scorpionida, Arachnida. Calcutta: Zoological Survey of India, 1983, 3: 1-587.
- 143 TIWARI AK., DESHPANDE SB. Toxicity of scorpion (Buthus tamulus) venom in mammals is influenced by the age and species. Toxicon, 1993, 31, 1619-22.
- 144 TIWARI AK., GUPTA GB., GUPTA SR., MISHRA SN., PRADAHAN PK. Fatal stroke following scorpion bite. J. Assoc. Physicians India, 1988, 36, 225-6.
- 145 TROVATI M., MOSSOCCO P., ANFOSSI G. Insulin influences the renin: angiotensin-aldosteron on system in humans. Metabolism, 1989, 38, 501-3.
-
146146 USHA KIRAN P. A retrospective study on treatment of red scorpion sting Vijayawada: The Andhra Pradesh Health University,1996. 109p. (M.D. Thesis).
- 147 VANAJA G., APARNA JR., VIJAYAKUMARI K. Effects of scorpion Heterometrus fulvipes venom on goat tissues and its detoxification through chemical dissociation Indian Zool., 1990, 14, 165-8.
-
148148 VIK HARALD MO., OLE DM. Influence of free fatty acids on myocardial oxygen consumption and ischemic injury. Am. J. Cardiol., 1981, 48, 361-67.
-
149149 WANNAMETHEE SG., PARRY IJ., SHAPER AG. Haematocrit and risk of NIDDM. Diabetes, 1966, 45, 576-9.
- 150 YUGANDHAR B. Clinical study of scorpion sting with special reference to insulin-glucose treatment Vijayawada: Andhra Pradesh University of Health Sciences, 1995. (M.D. thesis).
 CORRESPONDENCE TO:
CORRESPONDENCE TO:Publication Dates
-
Publication in this collection
25 Feb 2000 -
Date of issue
2000
History
-
Received
24 Mar 1998 -
Accepted
06 Oct 1998