Abstract
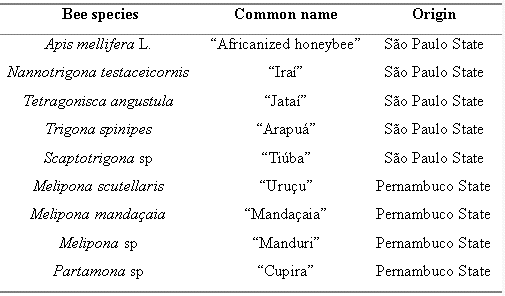
This study investigated the antibacterial activity of propolis produced by A. mellifera and Brazilian stingless bees, called "meliponíneos". Susceptibility tests to ethanolic extracts of propolis (EEP) were performed using bacterial strains (Staphylococcus aureus, Enterococcus sp, and Escherichia coli) isolated from human infections. Dilution of EEP in agar (%v/v) was used for determination of minimal inhibitory concentration (MIC). The stingless bee species (and common names) were: Nannotrigona testaceicornis ("Iraí"), Tetragonisca angustula ("Jataí"), Trigona spinipes ("Arapuá"), Scaptotrigona sp ("Tiúba"), Partamona sp ("Cupira"), Melipona scutellaris ("Uruçu"), Melipona sp ("Manduri"), and Melipona mandaçaia ("Mandaçaia"). EEP inhibitory efficiencies according to bacterial strains were: S. aureus - "Cupira" > "Manduri" = A. mellifera > "Uruçu" > "Mandaçaia" > "Iraí" > "Tiúba" > "Jataí" > "Arapuá" = Ethanol; Enterococcus sp - "Cupira" > "Manduri" > A. mellifera > "Mandaçaia" > "Uruçu" > "Tiúba" > "Jataí" > "Arapuá" = Ethanol; E. coli - "Manduri" > "Jataí" > Ethanol > A. mellifera > "Uruçu" > "Cupira" > "Iraí". Propolis produced by "Cupira" and "Manduri" bees showed higher antibacterial activity than A. mellifera.
Ethanolic extract of propolis; Apis mellifera; Brazilian stingless bees; S. aureus; E. coli; Enterococcus sp
Original paper
THE ANTIBACTERIAL ACTIVITY OF PROPOLIS PRODUCED BY Apis mellifera L. AND BRAZILIAN STINGLESS BEES
A. FERNANDES JR.1 CORRESPONDENCE TO:
A. FERNANDES JR. - Depart amento de Microbiologia e Imunologia, Instituto de Biociências de Botucatu, UNESP, Distrit o de Rubião Júnior s/n, CEP 18618-000, Botucatu, São Paulo, Brasil.
E-mail:
ary@ibb.unesp.br
, L. LEOMIL1, A.A.H. FERNANDES2, J.M. SFORCIN1
CORRESPONDENCE TO:
A. FERNANDES JR. - Depart amento de Microbiologia e Imunologia, Instituto de Biociências de Botucatu, UNESP, Distrit o de Rubião Júnior s/n, CEP 18618-000, Botucatu, São Paulo, Brasil.
E-mail:
ary@ibb.unesp.br
, L. LEOMIL1, A.A.H. FERNANDES2, J.M. SFORCIN1
1 Department of Microbiology and Immunology; 2 Department of Chemistry and Biochemistry, Institute of Biosciences of Botucatu, UNESP, State of São Paulo, Brazil.
ABSTRACT: This study investigated the antibacterial activity of propolis produced by A. mellifera and Brazilian stingless bees, called "meliponíneos". Susceptibility tests to ethanolic extracts of propolis (EEP) were performed using bacterial strains (Staphylococcus aureus, Enterococcus sp, and Escherichia coli) isolated from human infections. Dilution of EEP in agar (%v/v) was used for determination of minimal inhibitory concentration (MIC). The stingless bee species (and common names) were: Nannotrigona testaceicornis ("Iraí"), Tetragonisca angustula ("Jataí"), Trigona spinipes ("Arapuá"), Scaptotrigona sp ("Tiúba"), Partamona sp ("Cupira"), Melipona scutellaris ("Uruçu"), Melipona sp ("Manduri"), and Melipona mandaçaia ("Mandaçaia"). EEP inhibitory efficiencies according to bacterial strains were: S. aureus - "Cupira" > "Manduri" = A. mellifera > "Uruçu" > "Mandaçaia" > "Iraí" > "Tiúba" > "Jataí" > "Arapuá" = Ethanol; Enterococcus sp - "Cupira" > "Manduri" > A. mellifera > "Mandaçaia" > "Uruçu" > "Tiúba" > "Jataí" > "Arapuá" = Ethanol; E. coli - "Manduri" > "Jataí" > Ethanol > A. mellifera > "Uruçu" > "Cupira" > "Iraí". Propolis produced by "Cupira" and "Manduri" bees showed higher antibacterial activity than A. mellifera.
KEY WORDS: Ethanolic extract of propolis, Apis mellifera, Brazilian stingless bees, S. aureus, E. coli, Enterococcus sp.
INTRODUCTION
Propolis is a natural resinous hive product collected by bees from buds of different trees. Bees use it as a general sealant, draught excluder, antibiotic, and embalming substances to cover carcasses from hive invaders (16).
Because of its several biological properties, understanding its chemical composition has attracted research. Even though propolis composition is mentioned in literature, new substances have been reported, such as flavonoids, which may be responsible for antibacterial, fungicidal, and anesthetic biological properties, etc. (14).
There have been studies on the geographic origin of propolis, bee species, and chemical composition (2,3,5,6,10) (11,12,14,19,20). Plants have been proposed as sources of propolis, and chemical analyses must be performed in order to confirm this (4).
There are several reports about Brazilian propolis due to its excellent quality. Japan imports about 60 tons of propolis in natura from Brazil annually (1).
Propolis antimicrobial activity is one of most extensively investigated biological action, and some factors may influence its inhibitory capacity (extract preparation, tested microorganisms, propolis origin, bee species, etc). Several studies related to its antimicrobial activity have been performed in our laboratories (7,8,13,21). Some authors attribute the complex composition of propolis as a reason for its antimicrobial activity, and some mechanisms of action have been proposed (15,18,22,23,24).
The antibacterial activity of propolis produced by Brazilian stingless bees was studied by Levy Jr. (1997), who reported a higher efficiency of propolis produced by A. mellifera against that of some stingless bees (12). Kujumgiev et al. (1999) reported no differences in the antibacterial, antifungal, and antiviral activities of propolis from different geographic origins, including four samples from Brazilian A. mellifera and two stingless bees. They also reported no inhibitory activity against E. coli (11).
Bankova et al. (1998a) identified more than 50 compounds in Brazilian stingless bees geopropolis, mainly terpenoids and phenolics (5). Some variations were seen in chemical composition according to the bee species.
This work reports the antibacterial activity of Brazilian propolis produced by Apis mellifera and stingless bees, by determining the minimal inhibitory concentration (MIC) of propolis for bacterial strains isolated from human infections.
MATERIALS AND METHODS
Propolis
Propolis samples were collected, as follows:
Ethanolic extracts of propolis (EEP)
Propolis samples were ground and ethanolic extracts were prepared, as follows:
A. mellifera, 30 g propolis/100 mL of ethanol (95%) and 7 days at room temperature for extraction and filtration; N. testaceicornis, two parts (weight) of propolis were mixed with one volume of ethanol 95%. After 24 hours, the mixture was filtered and the liquid portion was centrifuged at 400 xg for 20 minutes and stored in amber glass at -10°C (12); Melipona sp, M. scutellaris, M. mandaçaia, and Partamona sp, 100 g propolis/100 mL of ethanol 95% and 2 days at room temperature for extraction and filtration (9); T. spinipes, T. angustula, and Scaptotrigona sp, the same methodology as for A. mellifera.
The final concentration (dry matter) (mg/mL) of each extract was: "Uruçu" (49.5), "Manduri" (57.2), "Cupira" (46.0), "Mandaçaia" (16.0), "Tiúba" (121.0), "Arapuá" (74.0), "Iraí" (insufficient volume for determination), "Jataí" (110.0), and A. mellifera (128.0). These values were obtained by ethanol evaporation placing EEP aliquots at 50°C/48 h.
Bacterial strains and susceptibility tests
Ninety-one bacterial strains isolated from human infections were tested: Staphylococcus aureus (n=30), Enterococcus sp (n=30), and Escherichia coli (n=31). After isolation and identification, the strains were kept in nutrient agar.
Determination of minimal inhibitory concentration was performed for 90% of the strains (MIC 90%) by the agar dilution method, following the National Committee of Clinical Laboratory Standard Guidelines (17).
Serial EEP concentrations were made (%v/v) in Petri dishes containing Mueller Hinton Agar to S. aureus and E. coli and Brain Heart Infusion to Enterococcus sp. Concentrations were obtained in previous assays for each EEP and bacterial species, ranging from 0.2 to 11% for "Cupira" and "Iraí" and from 0.2 to 13% for the remaining bees species. Control plates with ethanol were prepared in order to obtain a control of the solvent antimicrobial effect. Ethanol concentrations ranged from 5 to 12%.
The agar was allowed to solidify, and a standard number of test bacteria (approximately 105 colony forming units (CFU)/mL), obtained from suspensions adjusted to 0.5 Mac Farland Standard, were spot inoculated onto each plate using a multipoint inoculator (Steer's replicator) with a capacity of 32 different isolates. The MIC was defined as the lowest concentration of propolis that resulted in no bacterial growth after incubation at 37°C for 24 h. MIC 90% values were also estimated in mg/mL (Table 1).
Statistical analysis
Results were analyzed using the non-parametric test of Kruskal-Wallis for independent samples and/or treatments.
RESULTS AND DISCUSSION
MIC 90% values for bacterial strains are summarized in Table 1.
There were significant differences in MIC 90% values between stingless bee species. All propolis samples from the Northeast of Brazil (Pernambuco State) ("Uruçu", "Manduri", "Cupira", and "Mandaçaia") were more effective than the samples obtained in the Southeast (São Paulo State) ("Iraí", "Jataí", "Tiúba", and "Arapuá") against S. aureus strains. It is important to note that the propolis produced by the "Arapuá" bee gave MIC 90% values similar to those obtained with ethanol, and showing no activity against S. aureus. Similar results were obtained with propolis produced by the stingless bees ("Cupira", "Manduri", and "Uruçu") and A. mellifera.
The propolis from "Cupira" and "Manduri" bees were again the most effective against Enterococcus sp, with "Arapuá" being the least effective. The propolis from the "Uruçu" bee gave very different MIC 90% values for Gram-positive bacteria: 0.48% for S. aureus and 4.8% for Enterococcus sp.
It is interesting to note that all the propolis had higher MIC values against E. coli than against the S. aureus and Enterococcus sp strains. The propolis from the "Manduri" bee was the most effective, and that from the "Iraí" was the least effective of the stingless bee samples. Thus, one may conclude that propolis action against Gram-negative bacteria, independent of bee species, is strongly attributable to the solvent used (ethanol). This is based on the observation that the MIC 90% of ethanol for E. coli (9.0%v/v) was lower than MIC 90% values for "Uruçu", "Iraí", "Cupira", and A. mellifera. Similar results have been reported (11).
According to the statistical analysis and concentration in %v/v, the order of propolis activity was for: S. aureus - "Cupira" > "Manduri" = A. mellifera > "Uruçu" > "Mandaçaia" > "Iraí" >"Tiúba" > "Jataí" > "Arapuá"= Ethanol; Enterococcus sp - "Cupira" > "Manduri" > A. mellifera > "Mandaçaia" > "Uruçu" > "Tiúba" > "Jataí" > "Arapuá" = Ethanol; and E. coli - "Manduri" > "Jataí" > Ethanol > A. mellifera > "Uruçu" > "Cupira" > "Iraí".
Although chemical analysis of EEP was not performed, propolis composition should certainly differ between these samples and be responsible for their different antibacterial activity. This statement is supported by Bankova et al. (1998a), who reported differences in propolis chemical composition produced by three species of Brazilian stingless bees (4). Therefore, new studies on the chemical composition of EEP would be important for a better understanding of this issue.
It is also possible to report that the extract preparation may also influence these results, although all of them were ethanolic extracts. Further investigations should be performed using a single methodology to prepare the EEP for all bee species studied here, as in this paper there were differences in EEP preparation.
According to the results, it may be concluded that, in general, Gram-positive bacteria were more susceptible to EEP antibacterial action than Gram-negative bacteria. Propolis showed a different antibacterial activity by the bee species.
Received 19 July 2000
Accepted 29 August 2000
- 01 ABREU, J.A.S. Comercialização de própolis. In: CONGRESSO BRASILEIRO DE APICULTURA, 11, Teresina, 1996. Resumos e Palestras... Teresina: Confederação Brasileira de Apicultura, 1996: 203.
- 02 AGA H., SHIBUYA T., SUGIMOTO T., KURIMOTO M., NAKAJIMA S. Isolation and identification of antimicrobial compounds in Brazilian propolis. Biosci. Biotechnol. Biochem., 1994, 58, 945-6.
- 03 BANKOVA V., BOUDOUROVA-KRASTEVA G., POPOV S., SFORCIN JM., FUNARI SRC. Seasonal variations of the chemical composition of Brazilian propolis. Apidologie, 1998, 29, 361-7.
- 04 BANKOVA V., BOUDOUROVA-KRASTEVA G., SFORCIN JM., FRETE X., KUJUMGIEV A., RODELLA RM., POPOV S. Phytochemical evidence for the plant origin of Brazilian propolis from São Paulo State. Z. Naturforsch. Sect. C Biosci., 1999, 54, 401-5.
- 05 BANKOVA V., CHRISTOV R., MARCUCCI C, POPOV S. Constituents of Brazilian geopropolis. Z. Naturforsch. Sect. C Biosci., 1998, 53, 402-6.
- 06 BOUDOUROVA-KRASTEVA G., BANKOVA V., SFORCIN JM., NIKOLOVA N., POPOV S. Phenolic from Brazilian propolis. Z. Naturforsch. Sect. C Biosci., 1997, 52, 676-9.
- 07 FERNANDES JR A., LOPES CAM., SFORCIN JM., FUNARI SRC. Population analysis of susceptibility to propolis in reference strains of Staphylococcus aureus and Escherichia coli. J. Venom. Anim. Toxins, 1997, 3, 287-94.
- 08 FERNANDES JR A., SUGIZAKI M.F., FOGO ML., FUNARI SRC., LOPES CAM. In vitro activity of propolis against bacterial and yeast pathogens isolated from human infections.J. Venom. Anim. Toxins, 1995, 1, 63-9.
- 09 KERR WE. Abelhas indígenas brasileiras (meloponíneos) na polinização e na produção de mel, pólen, geoprópolis e cera. Inf. Agropec. (Belo Horizonte), 1987, 13, 15-22.
-
10KOO MH., PARK YK. Investigation of flavonoid aglycones in propolis collected by two different varieties of bees in the same region. Biosci. Biotechnol. Biochem., 1997, 61, 367-9.
-
11KUJUMGIEV A., TSVETKOVA I., SERKEDJIEVA Y., BANKOVA V., CHRISTOV R., POPOV S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin.J. Ethnopharmacol., 1999, 64, 235-40.
-
12LEVY JR NC. Estudo da atividade antimicrobiana de méis e própolis de Apis mellifera e Meliponinae brasileiros. Rio Claro: Universidade Estadual Paulista, Instituto de Biociências, 1997 116p. (Dissertação - Mestrado).
-
13LOPES MMR. Atividade antimicrobiana da própolis frente a linhagens bacterianas de Staphylococcus aureus e Escherichia coli em função do local de coleta. Botucatu: Universidade Estadual Paulista, Instituto de Biociências, 1997. 68p. (Monografia em Ciências Biológicas).
-
14MARCUCCI MC., BANKOVA VS., FERRERES F., ZAGO EB., DE CASTRO SL. Avanços recentes na investigação química e biológica de própolis brasileiras. In: CONGRESSO BRASILEIRO DE APICULTURA, 11, Teresina, 1996. Resumos e Palestras... Teresina: Confederação Brasileira de Apicultura, 1996: 205-8.
-
15MIRZOEVA OK., GRISHANIN RN., CALDER PC. Antimicrobial action of propolis and some of its components: the effects on growth membrane potential and motility of bacteria. Microbiol. Res., 1997, 152, 239-46.
-
16MORENO MIN., ISLA MI., CUDMANI NG., VATTUONE, MA., SAMPIETRO AR. Screening of antibacterial activity of Amaicha del Valle (Tucumán, Argentina) propolis. J. Ethnopharmacol., 1999, 68, 97-102.
-
17NATIONAL COMMITTEE FOR CLINICAL LABORATORY STANDARDS. Method for dilution antimicrobial susceptibility tests for bacterial that grow aerobically. Villanova, Pa.: 1997. (Approved Standard M7-A).
-
18PARK YK., KOO MH., ABREU JAS, IKEGAKI M., CURY JA., ROSALEN PL. Antimicrobial activity of propolis on oral microorganisms. Curr. Microbiol., 1998, 36, 24-8.
-
19PARK YK., KOO MH., IKEGAKI M., CONTADO JL. Comparison of the flavonoid aglycone contents of Apis mellifera propolis from various regions of Brazil. Arq. Biol. Tecnol. (Curitiba), 1997, 40, 97-106.
-
20SERRA-BONVEHI J., VENTURA-COLL F. Phenolic composition of propolis from China and from South America. Z. Naturforsch. Sect. C Biosci., 1994, 49, 712-8.
-
21SFORCIN JM., FERNANDES JR A, LOPES CAM., FUNARI SR. Efeito da sazonalidade sobre a atividade antibacteriana da própolis. In: CONGRESSO BRASILEIRO DE APICULTURA, 11, Teresina, 1996. Resumos e Palestras... Teresina: Confederação Brasileira de Apicultura, 1996: 389.
-
22SIMUTH J., TRNOVSKY J., JELOKOVÁ J. Inhibition of bacterial DNA-dependent RNA polymerases and restriction endonuclease by UV- absorbing components from propolis.Pharmazie, 1986, 41, 131-2.
-
23STREHL E., VOLPERT R., ELSTNER EF. Biochemical activities of propolis-extracts. III. Inhibition of dihydrofolate reductase. Z. Naturforsch. Sect. C Biosci., 1994, 49, 39-43.
-
24TAKAISI-KIKUNI NB., SCHILCHER H. Electron microscopic and microcolorimetric investigations of the possible mechanism of the antibacterial action of the defined propolis provenance.Planta Méd., 1994, 60, 222-7.
 CORRESPONDENCE TO:
CORRESPONDENCE TO: Publication Dates
-
Publication in this collection
08 Oct 2002 -
Date of issue
Dec 2001
History
-
Accepted
29 Aug 2000 -
Received
19 July 2000