Abstract
Toad envenoming in dogs can cause death by cardiac fibrilation (CVF). Traditional therapy consists mainly of atropine and propranolol, the last one used to prevent the CVF, that is preceded by negative ventricular deflections (NVDs) in the QRS complex of the electrocardiogram. This study intended to verify, comparatively, the lidocaine, propranolol, amiodarone, and verapamil abilities to prevent CVF in experimentally envenomed dogs. Thirty-six dogs were divided into 6 groups (GL, GP, GA, GV, GST, and GSV) with n=6; the dogs were submitted to volatile anaesthesia. The animals of the groups GL, GP, GA, and GV received 0.38g of toad venom through oro-gastric catheter and were treated with the following drugs respectively: lidocaine (4mg/Kg), propranolol (0.1mg/Kg), amiodarone (8mg/Kg), and verapamil (2mg/Kg). These drugs were repeated if NVDs reappeared with cardiac frequency >150, GST was not treated and GSV was just anaesthetized. The following results were obtained: GL, NVDs present in 4 animals, 100% recuperation with 3.66 doses/animal; GP, NVDs present in 2 animals, 100% recuperation with 1.66 dose/animal, with bradycardia at the anaesthetic return; GA, NVDs present in 3 animals, 33.33% recuperation with 1.5 dose/animal; GV, NVDs present in 4 animals, 100% recuperation with 2.16 doses/animal; GST, NVD present in 6 animals, 100% death and GSV, NVDs absent, 100% recuperation. As a conclusion, the anaesthetic proceedings used, did not cause NVDs, the envenoming that was not treated was lethal, and among the antiarrhythmics drugs used, verapamil was the most efficient, as it did not cause any serious bradycardia at the anaesthetic return and did not require repeated administrations. For lidocaine, it was efficient but required various administrations; amiodarone could not prevent the death of 4 animals; propranolol was efficient in relation to NVDs control, but caused serious bradycardia at the anaesthetic return.
toad; Bufo; envenoming; intoxication; Lidocaine; Propranolol; Amiodarone; Verapamil; dogs; Atropine; cardiac fibrilation; antiarrythmic drugs
Original paper
USE OF LIDOCAINE, PROPRANOLOL, AMIODARONE, AND VERAPAMIL IN TOAD ENVENOMING (GENUS BUFO) IN DOGS
M. SAKATE1,2, P. C. LUCAS DE OLIVEIRA3 CORRESPONDENCE TO:
P. C. LUCAS DE OLIVEIRA - Rua Dirce Macuco Sandoval, 198, Presidente Prudente, São Paulo, Brazil, CEP 19.053-670.
E-mail:
lucas@unoeste.br
CORRESPONDENCE TO:
P. C. LUCAS DE OLIVEIRA - Rua Dirce Macuco Sandoval, 198, Presidente Prudente, São Paulo, Brazil, CEP 19.053-670.
E-mail:
lucas@unoeste.br
1 Department of Veterinary Clinics of the School of Veterinary Medicine and Animal Science of Botucatu - UNESP, State of São Paulo, Brazil; 2 Center for the Study of Venoms and Venomous Animals - CEVAP-UNESP, State of São Paulo, Brazil; 3 Veterinary Hospital of the School of Veterinary Medicine of Presidente Prudente - UNOESTE, State of São Paulo, Brazil.
ABSTRACT: Toad envenoming in dogs can cause death by cardiac fibrilation (CVF). Traditional therapy consists mainly of atropine and propranolol, the last one used to prevent the CVF, that is preceded by negative ventricular deflections (NVDs) in the QRS complex of the electrocardiogram. This study intended to verify, comparatively, the lidocaine, propranolol, amiodarone, and verapamil abilities to prevent CVF in experimentally envenomed dogs. Thirty-six dogs were divided into 6 groups (GL, GP, GA, GV, GST, and GSV) with n=6; the dogs were submitted to volatile anaesthesia. The animals of the groups GL, GP, GA, and GV received 0.38g of toad venom through oro-gastric catheter and were treated with the following drugs respectively: lidocaine (4mg/Kg), propranolol (0.1mg/Kg), amiodarone (8mg/Kg), and verapamil (2mg/Kg). These drugs were repeated if NVDs reappeared with cardiac frequency >150, GST was not treated and GSV was just anaesthetized. The following results were obtained: GL, NVDs present in 4 animals, 100% recuperation with 3.66 doses/animal; GP, NVDs present in 2 animals, 100% recuperation with 1.66 dose/animal, with bradycardia at the anaesthetic return; GA, NVDs present in 3 animals, 33.33% recuperation with 1.5 dose/animal; GV, NVDs present in 4 animals, 100% recuperation with 2.16 doses/animal; GST, NVD present in 6 animals, 100% death and GSV, NVDs absent, 100% recuperation. As a conclusion, the anaesthetic proceedings used, did not cause NVDs, the envenoming that was not treated was lethal, and among the antiarrhythmics drugs used, verapamil was the most efficient, as it did not cause any serious bradycardia at the anaesthetic return and did not require repeated administrations. For lidocaine, it was efficient but required various administrations; amiodarone could not prevent the death of 4 animals; propranolol was efficient in relation to NVDs control, but caused serious bradycardia at the anaesthetic return.
KEY WORDS: toad, Bufo, envenoming, intoxication, Lidocaine, Propranolol, Amiodarone, Verapamil, dogs, Atropine, cardiac fibrilation, antiarrythmic drugs.
INTRODUCTION
Toads despite not having an inoculation apparel for their venom are venomous animals because they possess on the surface of their skin glands that produce a high toxicity venom (28). Among them, there are bilateral glands, in post-orbital position, visible with nude eyes and in diamond shape (5), known as paratoyds.
The dogs can be envenomed by biting or ingesting the toad that secretes venom in the oral mucous of the predator. The effects of the toad venom are, mainly, of cardiotoxic nature resemble the digitalic envenoming (2,4,6,15,16,23,25,27,30,32).
In 1935, a child died in Hawaii after having ingested a toad caught by its father in a sugar cane field (25), what also denotes the importance of this envenoming for human medicine. Toad venoms are an important source of information for biopharmacology. The deep knowledge of these venoms can contribute to the synthesis of active substances, with possible uses in the health area (22).
The chemical composition of the toad venom (Bufo spp) is very complex and varied among the species belonging to this genus (5). Nevertheless, this composition can be divided into the following: basic compounds; adrenaline and noradrenaline, agonists of the sympathetic autonomous nervous system (11), and Bufotenin, dihidrobufotenin and bufotyonin, that possess hallucinogenic effects by action in the central nervous system (6,22,32); and derived steroids, cholesterol, ergosterol, and sistosterol that constitute the neutral considered fraction of the poison (32), and Bufodienolids and bufotoxins that possess action similar to the digitalics (1,4,7,8,9).
The effects of venom appear almost immediately after envenoming. These can limit to local irritation or can cause systemic signs that eventually culminate with the death of the dog (15,16,20,23,24). This diversity of symptoms is due to some variables as: the toad species, regional variation, probably for environmental influences, and vomit occurrence and sialorrhea, as both serve as an elimination way of part of the venom (3).
The electrocardiographic alterations observed consist in gradual deterioration of the normal patterns with progressive appearing of negative ventricular deflections. Consisting of a exclusively ventricular rhythm with negative and deep QRS complexes. Eventually, if the toad venom intoxicated dog is not treated, it can result in chaotic ventricular rhythm, denominated ventricular fibrillation, given by the simultaneous discharging of many ectopic ventricular focuses, and death (25,26,28).
There are lots of divergences in the treatment choice for toad poisoning (26). However, the following therapeutics is the most accepted: propranolol (non-selective adrenergic blocking), 5 mg/kg IV, with repetition in twenty minutes if necessary to control the heart arrhythmia; atropine (muscarinic blocking), 0,04 mg/kg IV, to reduce the respiratory tract secretions and the sialorrhea (26), and sodic pentobarbital (barbituric of short duration), 30 mg/kg IV, to enable the orotracheal intubation and the washing of the oral cavity with jet of water, intending to reduce the amount of venom present in this mucous. Besides, pentobarbital has anticonvulsant action (25,26,27).
Actually, many dogs and even persons die by toad venom intoxication (25), although treated as described by Palumbo and Perry (26). So it shows necessary to test other drugs to control the ventricular fibrilation, the main cause of death in this kind of poisoning.
The objectives of this study were to observe the lethality of samples of toad venom obtained for use in the experiment, to study the clinical and electrocardiographic signs developed by animals anaesthetized and envenomed by toad venom, and to analyze comparatively the effectiveness of lidocaine, propranolol, amiodarone, and verapamil in the prevention of the negative ventricular deflections development, as well as other arrhythmia types and of the obit, due to the toad venom intoxication in dogs.
MATERIALS AND METHODS
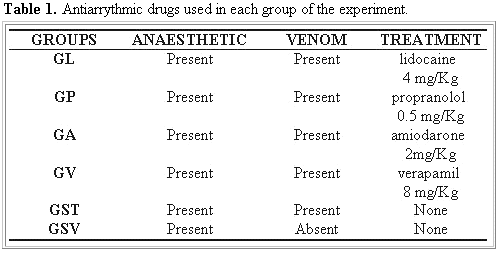
Animals: Thirty-six canines were used, without defined race, males and females, with age between one and six years old, established by the characteristics of the teething according to Kirk and Bistner (14), of medium load, weighing between 7 and 14 Kg of alive weight and good corporal state, according to the parameters of McCurnin and Poffenbarger (18). The animals were divided in six groups (Table 1). The dogs, coming from the Universidade do Oeste Paulista (UNOESTE) Biotery Kennel of Presidente Prudente, were kept in individual cages, in the Veterinary Hospital of UNOESTE, receiving water and ration ad libitum up to 24 hours before the experiment.
Venom extraction: venom was obtained from 36 toads (genus Bufo) near Botucatu, at Faxinal District, São Paulo State. The extraction was done by manual compression of the paratoyd glands of these animals. Obtaining 13.68g of a homogeneous venom pool in order to eliminate variations in the toxicity of each donor's secretion toad. After venom extraction, the toads were returned to their original habitat. The pool of venom was formed from the paratoyd secretion of different toad species (B. paracnemis, B. ictericus, and B. crucifer).
Venom storage: venom pool was homogenized, with glass stick and divided into 36 portions of 0.38g, corresponding of a median secretion of one toad. This amount probably is the same quantity that the dog makes contact in natural envenoming. Each portion was put in 20 ml flasks, previously sterilized in autoclave, and stored at -5°C.
Pre-anaesthetic evaluation: each dog was submitted to meticulous clinical exam, according to McCurnin and Poffenbarger (18), and accomplished by electrocardiographic exam, in agreement with Tilley (29). Those that demonstrated any kind of clinical or electrocardiographic alteration were discarded.
These drugs were chosen as being the principle representing of one of the four antiarrythmic groups which each one belongs as described by Hoffman and Lefkowitz (11).
Anaesthetic induction: the animals were anaesthetized with drugs of little interference in the cardiovascular activities, so that cardiocirculatory were exclusively attributed to the toad poison. Thus, after fast of 24 hours for solids and of 12 hours for liquids, the dogs were submitted to the following anaesthetic protocol (17):
- pre-anaesthetic medication, 1 mg/Kg of clorpromazine IV;
- after 15 minutes, anaesthetic induction with 12.5 mg/Kg IV of sodic tiopental and anaesthetic maintenance with 3% isofluorane.
Dog envenoming: venom aliquot of 0.38g was re-suspended in 10 ml of water, shaken manually, and administrated to each animal of the groups GL, GP, GA, GV, and GST by orogastric cannulae. The animals of the group control GSV were also anaesthetized and they just received 10 ml of water by orogastric cannulae.
Treatment: the animals of the groups GL, GP, GA, and GV were submitted to different antiarrythmic therapeutic options (Table 1), administered immediately after the intoxication by toad venom by intravenous route in doses mentioned by Ettinger (10).
The medication was reapplied as many times as the electrocardiographic registration demonstrated the development of negative ventricular deflections with heart frequency superior to 150 beatings/minute.
Clinical records: each animal was kept anaesthetized for a period of two hours after the administration of the antiarrythmic drug. The following parameters were evaluated every 10 minutes during the anaesthetic evolution: rectal temperature, pulse, respiratory frequency, presence or not of sialorrhea, coloration of the oral mucous, irritation of the oral mucous, evacuation and urination, lung oedema and, mainly, cardiac frequency and rhythm, evaluated using an electrocardiograph (Cardtest®, model EK51), calibrated to the speed of 50 mm/s and sensibility of 1mm/mv. The animals were kept linked to the electrocardiograph with constant observation of the cardiac frequency. The records were turned on every 10 minutes or whenever the cardiac frequency surpassed 150 beatings per minute. This procedure was done until complete anaesthetic return, evidenced by the return of the conscience and of the sensibility to deep and superficial pain, as described by Massone (17). Later, the dogs remained isolated under observation. The results were logged in individual record for tabulation, analysis, and statistical tests.
Statistical Analysis: For comparison among groups, about the variables maximum and minimum rectal temperature, maximum and minimum respiratory frequency, maximum and minimum frequency of pulse and maximum and minimum cardiac frequency, it was used variance analysis, for a delineation entirely randomized with the multiple comparisons of the Tukey method at 5% level of significance (31). For comparison among groups, about the number of times that the animals presented cardiac frequency superior to 150 beatings per minute, as well as for the number of times that the animals presented arrhythmia of negative ventricular deflections (DVN) types and sinoatrial bradycardia (BS), Kruskal Wallis's non parametric test was used.
RESULTS AND DISCUSSION
In pilot study, toad venom was deposited in the oral cavity of the dogs and, during the whole time of accompaniment under general anaesthesia, the development of negative ventricular deflections was not observed (DVNs) by the electrocardiographic exam. Even so, minutes after the anaesthetic return, when then the dog swallowed the poison, such electrocardiographic signs appeared. Therefore, the option was the administration of the venom through orogastric cannulae and, in that way the animals were intoxicated inside the period of electrocardiographic monitoring of 2 hours after the introduction of the venom. Thus, it could be observed that the toad venom was not well absorbed by the oral mucous, thwarting the statements of Knowles (16), Miranda (21) and Monti and Cardello (22) that commend the penetration of the venom trough the superior gastrointestinal tract mucous.
The effects of the toad venom were, mainly, of cardiotoxic nature by a digoxin-like effect, corroborating the statements of Knowles (15,16), Zelnik (32), Chen and Kovarikova (6), Bedford (2), Palumbo et al. (25), Russel (27), Oehme et al. (23), Toledo (30), Brownlee et al. (4), and Hoffman and Bigger (12).
The results obtained by Palumbo et al. (25) could not be reproduced in this experiment. The dose of 1 mg/Kg of propranolol used by them was shown to be inductor of intense bradycardia taking animals of pilot experiment to obit by assistoly. Thus, the therapeutic dose adopted was (0.1 mg/Kg) as recommended by Michael (19). In this case, the obit did not happen, even so bradycardia was patent sign of propranolol use.
It was observed that in the animals of all groups the rectal temperatures suffered intense fall along the moments, and alterations in the respiratory frequency were constantly observed. These reactions are normal due to the anaesthetic influences (17).
The variations of the pulse accompanied those referring to the cardiac frequency.
More intense salivation was observed in the animals treated with verapamil, the urination incidence was larger in the groups that received the venom and there were not differences about the defecation frequency, however, these results could not be explained in this experiment.
The presence of pale mucous always happened when the obit of the dog was imminent or before serious hemodynamic unbalances.
Although a discreet lung oedema has been found in the necropsy of the animals that died, clinical signs of such alteration were not evidenced in any moment.
The cardiac frequency suffered intense variation along the experiment. The increase of this parameter, in the groups which the animals were envenomed (GL to GST), preceded the appearing of the negative ventricular deflections (NVDs) that in some animals also preceded the ventricular fibrilation and death.
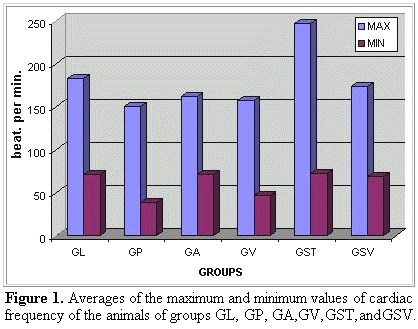
It could be observed that lidocaine was the less effective drug in maintaining the cardiac frequency inside normal values, it means, smaller or equal to the observed in the group GST (anaesthetized and poisoned control). About the comparison of the averages of the values of minimum cardiac frequency, the groups GP and GV differed significantly from the groups GL, GA, GST, and GSV (Figure 1).
It could be observed that propranolol (GP) and verapamil (GV) were drugs more bradycardia inductor than lidocaine (GL) or amiodarone (GA). Even so, such bradycardia was more worrying in the propranolol use (GP) that in the verapamil use (GV) (Figure 2).
Several types of alterations of the cardiac rhythm were observed along the experiment. The main arrhythmia observed was the negative ventricular deflection (NVD), characteristic of the intoxication by toad poisoning in dogs (Figure 4), agreeing with the data of Palumbo et al. (25), Russel (27), Palumbo and Perry (26) and Bicudo et al. (3). Other arrhythmias observed were: sinoatrial tachycardia (ST); sinoatrial bradycardia (SB); ventricular tachycardia (VT); ventricular fibrillation (VF); ventricular arrhythmia (VA) and atrioventricular block (AVB). The last one demonstrated in all the possible presentation degrees, it means, AVB of 1°, 2°, and 3° degrees. Each group was characterized by the presence of one or more types of arrhythmia (Figure 3 and Figure 4).
It could be stated that the therapy with lidocaine (GL) demands larger number of doses and more intense monitoring so that the dog intoxicated by toad venom can be kept alive, being taken larger risk of evolution control loss of the cardiac rhythm and death (Figure 5).
None of the animals belonging to the group GSV died, demonstrating that the anaesthetic procedure used did not cause the death (Table 2). Meanwhile, the animals of the group GST, all died, proving that, in the conditions of this experiment, the intoxication for toad poison is 100% lethal (Table 2). The lidocaine (GL) and the verapamil (GV) demonstrated to be the most effective drugs in the prevention of the obit in dogs intoxicated by toad venom, in spite of the literature do not do mention to the these drugs in the treatment of the referred envenoming. In the GP group, one dog died during the anaesthetic return presenting intense bradycardia followed by heart stop, however, without presenting negative ventricular deflections (NVDs). Thus, the property of the propranolol of taking to the bradycardia was considered responsible by this obit. In relation to the amiodarone, the results showed that this drug was ineffective, allowing the obit of 4 from the 6 animals of this group even under normal or little increased cardiac frequency. Thus, the amiodarone should not be recommended in that envenoming.
The necropsies of the animals that died during or after the experiment always revealed petechias in the gastric and duodenal mucous, probably, current of the direct irritative action of the toad poison on these mucous. It was also observed discreet lung oedema and hemorrhages, of the suffusion type, in the lung parenchyma in adjacent areas of the lung hilus area. Both secondary to the hemodynamic alterations due to the cardiac failure. This, for its time, takes to the congestion and venous hypertension that shows itself, above all, in the small circulation for its smallest complacence, incurring in the hemorrhage and oedema above mentioned. The causa mortis of those animals was attributed to the heart failure. This corroborates the statements of Humphreys (13) who, besides the macroscopic signs mentioned above, indicated the discovery of the toad or of parts of it, inside the gastrointestinal tract of the dog, as the only incontestable evidence of death by intoxication for toad poison.
The atropine absence, in the treatment of the dogs envenomed by toad venom in this experiment, did not harm the recovery of the animals, questioning its need for the success of the treatment. This fact thwarts the reports of Palumbo and Perry (26), who recommended the atropine as being an important coadjutant of propranolol in the treatment of the intoxication for toad poison in dogs. It fits to comment that the excessive salivation is a physiologic mechanism of elimination of part of the poison, and it can contribute in the reduction of the seriousness of the poisoning and, in that way, the atropine use would be harmful to the intoxicated animal.
CONCLUSIONS
By means of the conditions in which this experiment was accomplished, it can be concluded that:
1 - The experimental intoxication, by 0.38g of the toad venom through orogastric cannulae, of anaesthetized dogs and weighing between 7 and 14 kg of alive weight, induces the development of negative ventricular deflections (NVDs) in the electrocardiographic exam and, when not treated, it is lethal. The intoxication, in these conditions, also induces the urination.
2 - Lidocaine is not effective in avoiding the development of NVDs. It is necessary to reapply this drug, as a rule, 3.66 times in each animal intoxicated by toad poison. It avoids the obit in 100% of the cases.
3 - Propranolol, when used in dogs intoxicated by toad poison, does not avoid the development of NVDs. It is necessary to reapply this drug as a rule, 1.66 time in each intoxicated animal. It avoids death in 83% of the cases (five dogs), however, the cause of the only death was attributed to the bradycardia provoked by propranolol.
4 - Amiodarone does not avoid the development of NVDs. It is necessary to reapply this drug, as a rule, 1.5 times in each envenomed animal. It did not avoid the obit of 66% of the dogs of the experiment (four animals), two of which died presenting NVDs in the electrocardiographic exam and the others died by disturbances of the cardiac rhythm characterized as ventricular arrhythmia. Therefore, the amiodarone use in dogs intoxicated by toad poison is not indicated.
5 - Verapamil does not avoid the development of NVDs. Justifying the reapplying of this drug, as a rule, 2.16 times in each intoxicated animal. However, it avoids the obit in 100% of the cases.
6 - Arrhythmia more frequently observed in the dogs intoxicated by toad venom that received antiarrhythmic drugs are, in decreasing order of incidence, for lidocaine, negative ventricular deflections, sinoatrial bradycardia, sinoatrial tachycardia and atrioventricular block; for propranolol, sinoatrial bradycardia, negative ventricular deflections, atrioventricular block and ventricular arrhythmia; for amiodarone, negative ventricular deflections, ventricular tachycardia and ventricular fibrillation and for verapamil, sinoatrial bradycardia, negative ventricular deflections, ventricular tachycardia and sinoatrial tachycardia.
7 - Therefore, among the drugs used in the experiment, lidocaine although effective requests several applications; propranolol induces serious bradycardia that can lead the dog to death; amiodarone was not shown effective and verapamil is effective, requests less administrations than lidocaine and it does not induce such serious bradycardia as the one of propranolol. Thus, verapamil is the choice drug for the treatment of dogs intoxicated by toad poison.
Received 14 December 1999
Accepted 21 February 2000
- 01 BALARZ T., HANIG JP., HERMAN EH. Toxic responses of cardiovascular system. In: CASARETT LJ., DOULL J. Toxicology: the basic science of poisons. 3.ed. New York: MacMillan, 1986. p.402.
- 02 BEDFORD PGC. Toad venom toxicity and its clinical occurrence in small animals in the United Kingdom. Vet. Rec., 1974, 94, 613-4.
- 03 BICUDO PL., AMORIM RM., LAUFER R. Protocolo de envenenamento experimental de cães por veneno de sapo (Bufo marinus): aspectos clínicos e eletrocardiográficos. Botucatu: Faculdade de MedicinaVeterinária e Zootecnia UNESP, 1995. 14p.
- 04 BROWNLEE AA., JOHNSON P., MILLS IH. Actions of bufalin and cinobufalin, two bufadienolides respectively more active and less active than ouabain, on ouabain binding and 6Rb uptake by human erythrocytes. Clin. Sci., 1990, 78, 169-74.
- 05 CHEN KK., CHEN AL. Notes on the poisonous secretions of twelve species of toads. Pharmacol. Exp. Ther., 1933, 47, 281-93.
- 06 CHEN KK., KOVARIKOVA A. Pharmacology and toxicology of toad venom. Pharma Sci., 1967, 56, 1535-41.
- 07 DUELLMAN WE., TRUEB L. Introduction to the Amphibian. In: ___. Biology of amphibians. New York: MacCraw-Hill, 1986: 1-9.
- 08 DUELLMAN WE., TRUEB L. Enemies and defenses. In: ___. Biology of amphibians. New York: MacCraw-Hill, 1986: 252-60.
- 09 DUELLMAN, W.E., TRUEB, L. Integumentery, sensory, and visceral systems. In: ___. Biology of the amphibians. New York: Macgraw-Hill, 1986: 367-73.
-
10ETTINGER SJ. Tratado de medicina interna veterinária. 3.ed. São Paulo: Manole, 1992: 1104-52.
-
11HOFFMAN BF., LEFKOWITZ RJ. Catecolaminas e drogas simpatomiméticas. In: GOODMAN LS., GILMAN A. As bases farmacológicas da terapêutica. 8.ed. Rio de Janeiro: Guanabara Koogan, 1991: 123-44.
-
12HOFFMAN BF., BIGGER JR JT. Digitálicos e glicosídeos cardíacos associados. In: GOODMAN L.S., GILMAN A. As bases farmacológicas da terapêutica. 8.ed. Rio de Janeiro: Guanabara Koogan, 1991: 536-52.
-
13HUMPHREYS DJ. Veterinary toxicology. 3.ed. London: Balliere Tindall, 1988: 315-6.
-
14KIRK RW., BISTNER SI. Manual de procedimentos e tratamento de emergência em medicina veterinária. 3.ed. São Paulo : Manole, 1987: 330-2.
-
15KNOWLES RP. The poison toad and the canine. Vet. Med. Small Anim. Clin., 1964, 39-42.
-
16KNOWLES RP. Toad poisoning in dogs. J. Am. Vet. Med. Assoc., 1968, 153, 1202.
-
17MASSONE, F. Anestesiologia veterinária-farmacologia e técnicas. 2.ed. Rio de Janeiro: Guanabara Koogan, 1994. 252 p.
-
18MCCURNIN DM., POFFENBARGER EM. Small animal physical diagnosis and clinical procedures. Philadelphia: W. B. Saunders, 1991. 222 p.
-
19MICHAEL M. Toxicologia. In: ETTINGE J. Tratado de medicina interna veterinária. 3.ed. São Paulo: Manole, 1992, 1: 496-7.
-
20MICUDA J. Toad poisoning. In: HOSKINS HP. Canine medicine. Santa Barbara: American Veterinary Publications, 1968: 260-1.
-
21MIRANDA IM. Principais lagartos, anfíbios e animais aquáticos de interesse médico. In: SOERENSEN B. Animais peçonhentos. 2.ed. Rio de Janeiro: Atheneu, 1990: 85-6.
-
22MONTI R., CARDELO L. Bioquímica do veneno de anfíbios. In: BARRAVIERA B. Venenos animais: uma visão integrada. Rio de Janeiro: EPUC, 1994, 225-32.
-
23OEHME FW., BROWN JF., FOWLER ME. Toxins of animal origin. In: CASARETT LJ., DOULL J. Toxicology: the basic science of poisons. 2.ed. New York: MacMillan, 1980: 568-75.
-
24OTANI A., PALUMBO NE., READ G. Pharmacodynamics and treatment of mammals poisoned by Bufo marinus toxin. Am. Vet. Res., 1969, 30, 1865-72.
-
25PALUMBO NE., PERRY S., READ G. Experimental induction and treatment of toad poisoning in the dog. J. Am. Vet. Med. Assoc., 1975, 167, 1000-5.
-
26PALUMBO NE., PERRY SF. Toad poisoning. In: KIRK, R.W. Current veterinary therapy: small animal practice. 8.ed. Philadelphia: W.B. Saunders, 1983. p.160-2.
-
27RUSSEL FE. Toad poisoning. In: HOSKINS HP. Canine medicine. 4.ed. Santa Barbara : American Veterinary Publications Incorporation, 1979, 1:183-5.
-
28RUSSEL FE. Toxic effects of animal toxin. In: CASARETT LJ., DOULL J. Toxicology: the basic science of poisons. 3.ed. New York : MacMillan, 1986: 718-9.
-
29TILLEY LP. Essentials of canine and feline eletrocardiography. 3.ed. Phidelphia: Lea & Febiger, 1992, 127-384.
-
30TOLEDO RC. Breve apreciação sobre a secreção cutânea dos anfíbios. Ciênc. Cult., São Paulo, 1984, 38, 279-84.
-
31ZAR JH. Biostatistical analysis. 2 ed. Englewood Cliffs: Prentice Hall, 1984 718 p.
-
32ZELNIK R. A natureza química do veneno de sapo. Ciênc. Cult., São Paulo, 1965, 17, 10-4.
Publication Dates
-
Publication in this collection
08 Oct 2002 -
Date of issue
Dec 2001
History
-
Accepted
21 Feb 2000 -
Received
14 Dec 1999