Abstract
Bothrops jararacussu venom and its major toxin bothropstoxin-I (BthTX-I) possess myotoxic and neurotoxic properties. The efficacy of a rabbit antivenom raised against B. jararacussu venom in the neutralization of physiological, biochemical, and morphological changes induced by the venom and its major toxin BthTX-I was studied in mouse isolated phrenic nerve-diaphragm (PND) and extensor digitorum longus (EDL) preparations. The times required for 50% neuromuscular blockade in PND and EDL preparations for venom were 70+11.5 (S.E.M., n=5) min and 58+8 (n=16) (50 mu g/mL), and for BthTX-I 31+6 (n=3) min and 30+3 (n=5) min (20 mu g/mL), respectively. After 120 min incubation, creatine kinase (CK) concentrations in solution containing the EDL preparations were 3464+346 U/L after exposure to venom (50 mu g/mL, n=5) and 3422+135 U/L to BthTX-I (20mu g/mL, n=4), respectively. Rabbit antivenom dose-dependently neutralized venom and toxin-induced neuromuscular blockade in both preparations and effectively prevented venom and toxin-induced CK release from EDL. Histological analysis showed that rabbit antivenom neutralized morphological damage caused by B. jararacussu venom and BthTX-I in EDL preparations. These results indicate that rabbit antivenom effectively neutralized the biological activities of B. jararacussu venom and BthTX-I.
Bothrops venom; myotoxin; neutralization; neuromuscular junction; histological analysis; Bothrops jararacussu
Original paper
RABBIT ANTIVENOM EFFICACY AGAINST MYOTOXIC AND NEUROTOXIC ACTIVITIES OF Bothrops jararacussu VENOM AND BOTHROPSTOXIN-I
Y. Oshima-Franco1, G. B. Leite1, A. A. Valério1, S. Hyslop1, S. Andriao-Escarso3, J. R. Giglio3, J. Prado-Franceschi1, M. A. Cruz-Höfling2, L. Rodrigues-Simioni1 CORRESPONDENCE TO:
L. Rodrigues-Simioni Departamento de Farmacologia, Faculdade de Ciências Médicas Universidade Estadual de Campinas (UNICAMP), Caixa Postal 6111, 13083-970, Campinas, SP, Brasil
Phone: 55 19 3788-7482 / Fax: 55 19 3289 2968
simioni@obelix.unicamp.br
CORRESPONDENCE TO:
L. Rodrigues-Simioni Departamento de Farmacologia, Faculdade de Ciências Médicas Universidade Estadual de Campinas (UNICAMP), Caixa Postal 6111, 13083-970, Campinas, SP, Brasil
Phone: 55 19 3788-7482 / Fax: 55 19 3289 2968
simioni@obelix.unicamp.br
1 Department of Pharmacology and 2 Department of Histology and Embryology, State University of Campinas (UNICAMP), 13083-970, Campinas, SP, Brazil; 3 Department of Biochemistry, Faculty of Medicine, University of São Paulo (USP), 14049-900, Ribeirão Preto, SP, Brazil.
ABSTRACT:Bothrops jararacussu venom and its major toxin bothropstoxin-I (BthTX-I) possess myotoxic and neurotoxic properties. The efficacy of a rabbit antivenom raised against B. jararacussu venom in the neutralization of physiological, biochemical, and morphological changes induced by the venom and its major toxin BthTX-I was studied in mouse isolated phrenic nerve-diaphragm (PND) and extensor digitorum longus (EDL) preparations. The times required for 50% neuromuscular blockade in PND and EDL preparations for venom were 70+11.5 (S.E.M., n=5) min and 58+8 (n=16) (50 m g/mL), and for BthTX-I 31+6 (n=3) min and 30+3 (n=5) min (20 m g/mL), respectively. After 120 min incubation, creatine kinase (CK) concentrations in solution containing the EDL preparations were 3464+346 U/L after exposure to venom (50 m g/mL, n=5) and 3422+135 U/L to BthTX-I (20m g/mL, n=4), respectively. Rabbit antivenom dose-dependently neutralized venom and toxin-induced neuromuscular blockade in both preparations and effectively prevented venom and toxin-induced CK release from EDL. Histological analysis showed that rabbit antivenom neutralized morphological damage caused by B. jararacussu venom and BthTX-I in EDL preparations. These results indicate that rabbit antivenom effectively neutralized the biological activities of B. jararacussu venom and BthTX-I.
KEYWORDS: Bothrops venom, myotoxin, neutralization, neuromuscular junction, histological analysis, Bothrops jararacussu.
INTRODUCTION
Bothrops jararacussu is one of the largest species of crotalid snake found in southern Brazil, northern Argentina, and eastern Paraguay. Its venom produces envenoming signs and symptoms similar to other Bothrops species, including intense myonecrosis (5,10,18). Although B. jararacussu venom has no pronounced neurotoxic effects in vivo, it abolishes directly and indirectly evoked muscle contractions in mouse (7,22), chick (7), and frog (19) neuromuscular preparations. This activity has been attributed to the presence of phospholipases (6,21) and myotoxins (8,19). Bothropstoxin-I (BthTX-I) (8,19), the major myotoxin in B. jararacussu venom, is a 13.7 kDa single chain polypeptide capable of forming dimers (1). This toxin is devoid of phospholipase A2 and proteolytic activity. Antivenom administration is the most effective treatment for snakebite envenoming. Commercial bothropic antivenom is raised in horses and may be either monovalent (against Bothrops only) or polyvalent (against Crotalus and Bothrops or Bothrops and Lachesis muta).
We have previously demonstrated that commercial antivenom protected against B. jararacussu venom and BthTX-1 neuromuscular activity, but not their myotoxic action (14). In this study, we examined the efficacy of rabbit antivenom raised against B. jararacussu venom in the neutralization of myotoxic and neurotoxic activities of both the venom and BthTX-I. Neurotoxicity was assessed by their ability to cause neuromuscular blockade; myotoxicity was assessed by their ability to induce CK release and cause morphological damage, as shown by light microscopy. In both cases, neutralization was tested by preincubating the antivenom with venom or toxin, and then testing the residual biological activity.
MATERIALS AND METHODS
Venom and toxin
B. jararacussu venom was purchased from the Instituto Butantan (São Paulo, SP) and BthTX-I was purified as described by Homsi-Brandeburgo et al. (8).
Animals
Male Swiss white mice (26-32 g) were supplied by the Animal Service Unit of Campinas State University (UNICAMP). The mice were housed at 25+3° C on a light/dark cycle of 12h, with access to food and water ad libitum. Male New Zealand white rabbits (2-2.5kg) were purchased from an established breeder (Granja Criex, Mogi das Cruzes, SP) and housed individually, with access to food and water ad libitum.
Antivenom
Rabbits were immunized by successive weekly SQ inoculations with 750m g of B. jararacussu venom/kg over a two-month period. The first injection included Freunds complete adjuvant (Difco Laboratories, Detroit, MI, USA), and subsequent boosters were given with incomplete adjuvant (Nujol®, Schering-Plough). Serum antibody levels were monitored weekly by gel immunodiffusion (16) and confirmed by ELISA (4) at the end of the experiment. Rabbit antivenom protein content was 69 mg/mL, as per Peterson (17). The antivenom potency, based on its ability to neutralize venom lethality, was assessed as described by Gutiérrez and Chaves (5). Briefly, mixtures containing a constant quantity of venom (2.5 LD50) and variable quantities of antivenom in a final volume of 0.5 mL were incubated at 37° C for 30 min, and then IP injected into mice. Control mice received only venom (2.5 LD50) diluted in saline. The number of surviving mice was recorded after 48h. The LD50 value for B. jararacussu venom was based on that reported by Siles Villarroel et al. (20).
Immunoblotting
B. jararacussu venom and BthTX-I (100m g each) were run on 10% polyacrylamide minigels in SDS-PAGE (Hoefer® SE 260 Mighty Small system) at 100 V for 2h, and proteins transferred electrophoretically to nitrocellulose membranes (0.45m m; Sigma Chemical Co.) in a TE-22 minitransfer tank (Hoefer-Pharmacia) at 150-200 mA for 1.5h. Subsequently, the membranes were blocked at room temperature for 2h in a solution of 5% non-fat milk/0.05% Tween 20. After washing six times in TBS (0.01 M Tris-HCl, 0.17 M NaCl and 0.02% Tween 20, pH 7.6, the membranes were incubated overnight with rabbit antivenom diluted 1:1000 in TBS. After washing again with TBS buffer, bound antibodies were detected with rabbit anti-horse IgG (whole molecule)-peroxidase conjugate (Sigma; 1:1000 in TBS) for 2h at room temperature with shaking. At the end of this incubation, the blots were washed, developed with 4-chloro-1-naphthol (Sigma; 0.03% in 0.05 M Tris-HCl, pH 7.6 containing 0.03% H2O2), and documented.
Mouse phrenic nerve-diaphragm (PND) muscle preparation
Phrenic nerve and diaphragm (2) were obtained from mice anesthetized with chloral hydrate (300 mg/kg, IP) and sacrificed by exsanguination. The diaphragm was removed and mounted under a tension of 5g in a 5mL organ bath containing Tyrode solution (pH 7.4, 37° C) of the following composition (mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 0.49, NaH2PO4 0.42, NaHCO3 11.9 and glucose 11.1, aerated with 95%O2 and 5%CO2. Supramaximal stimuli (4x threshold, 0.1Hz, 0.2ms) delivered from a Grass S48 stimulator were applied to the nerve with bipolar electrodes. Isometric muscle tension was recorded by a force displacement transducer (Load Cell BG-10 GM) coupled to a physiograph (Gould, Model RS 3400) via a Gould universal amplifier. The preparations were allowed to stabilize for at least 20 min before adding either B. jararacussu venom (50m g/mL), or BthTX-I (20m g/mL), or a mixture of venom and toxin preincubated with the desired volume of rabbit antivenom for 30 min at 37° C. In the latter case, the final venom concentration was the same as in experiments without antivenom. In these experiments, venom:antivenom ratios were selected based on the antivenom ability to neutralize neuromuscular activity and not on antivenom potency against lethal activity as in EDL preparations (see below). Control experiments were performed using Tyrode solution with or without antivenom.
Mouse extensor digitorum longus (EDL) muscle preparation
B. jararacussu venom and BthTX-I myotoxic and neurotoxic activities were assayed in EDL preparation, which was removed by sectioning the tendon without damaging the muscle, thus avoiding spontaneous CK release. The EDL muscle was isolated and transferred to a cylindrical chamber (3mL capacity), containing aerated (95%O2 and 5%CO2) Tyrode solution (described above) in which the preparation was mounted vertically under a tension of 0.5g. A bipolar platinum ring electrode was placed around the tendon, containing the peroneal nerve trunk supplying the muscle. Supramaximal stimuli (0.1Hz, 0.2ms) delivered from a Grass S48 stimulator were applied to the nerve with bipolar electrodes. Isometric muscle tension was recorded by a force displacement transducer (Load Cell BG-10 GM) coupled to a physiograph (Gould, Model RS 3400) via a Gould universal amplifier. The preparations were washed three times at 10 min intervals to allow the tissue to stabilize before adding either venom (50m g/mL), or BthTX-I (20m g/mL), or a mixture of venom or toxin plus antivenom. In the latter, final venom concentration was the same as in experiments without antivenom. The quantity of antivenom used in these experiments was based on its potency to neutralize venom lethal effect (0.8mL of rabbit antivenom neutralizing 1mg of venom). Control experiments were performed using Tyrode solution in the absence or presence of antivenom.
Samples (100m L) of bathing solution were withdrawn from the organ bath at 0, 15, 30, 60, 90, and 120 min post-venom or toxin and replaced with an equal volume of Tyrode solution. The collected samples were stored for 2 h at 4° C and CK activity (expressed in U/L) was measured using a commercial kit (Granutest® 2.5, Diagnostica Merck, Darmstadt, Germany). At the end of the experiments, the preparations were fixed in Bouin solution for 24-48h, followed by dehydration, and embedding in paraffin. Five-m m thick sections were stained with hematoxylin-eosin (H.E.) prior to examination by light microscopy. Morphological damage was quantified by counting the number of damaged cells and expressing it as a percentage of the total number of cells in three non-overlapping and non-adjacent areas of each muscle. This procedure was used in all experiments (controls and treated preparations).
Statistical analysis
Each experimental protocol was repeated at least three times, and the results expressed as mean + S.E.M. Students t-test was used for data statistical comparison. A value of p<0.05 was considered significant.
RESULTS
Immunoblotting
Figure 1 shows rabbit antivenom reactivity with B. jararacussu venom (A) and BthTX-I (B). The latter was detected mainly in its monomeric form (MW~ 14,000).
Immunoblot showing reactivity of Bothrops jararacussu antivenom (BjAV) with B. jararacussu venom (A) and BthTX-I (MW~14,000) (B).
Neuromuscular activity and its neutralization
This was assayed in PND and EDL preparations. A single concentration of B. jararacussu venom (50m g/mL) or BthTX-I (20m g/mL) was used throughout the experiment. These concentrations were chosen because they produced neuromuscular blockade within a reasonable timescale and were based on preliminary experiments with 50m g, 100m g, and 200m g venom/mL or 10m g and 20m g BthTX-I/mL.
The times required for 50% neuromuscular blockade in PND preparations were 70+11.5 min (n=5) for venom and 31+6 min (n=3) for BthTX-I. Figure 2A shows neuromuscular blockade induced by B. jararacussu venom and BthTX-I in PND preparations. Figure 2B shows that neurotoxicity neutralization depended on the quantity of rabbit antivenom, with a 1:1 venom:antivenom ratio providing no significant protection (n=3), and a 1:3 ratio providing effective neutralization (n=4; p<0.05 from 30 min onwards). Figure 2C shows neutralization of BthTX-I neuromuscular effects in PND preparations at 1:5 (n=3; p<0.05 from 40 min onwards) and 1:10 (n=3; p<0.05 from 40 min onwards) venom:antivenom ratios. There was no significant difference in neutralization afforded by these two ratios. Antivenom alone had no effect on basal neuromuscular response.
Phrenic nerve-diaphragm preparations. (A) Neuromuscular blockade of B. jararacussu venom (50m g/mL) and BthTX-I (20m g/mL) compared to BjAV. The antivenom alone caused no neuromuscular blockade. (B) Neutralization by BjAV of B. jararacussu venom neuromuscular action. At 1:3 venom:antivenom ratio, the antivenom protected against venom effects. (C) Neutralization by BjAV of BthTX-I neuromuscular action. A higher BjAV concentration was required to neutralize the neuromuscular action of the toxin than the venom. The points are mean + S.E.M. of the three experiments. BjAV, B. jararacussu antivenom.
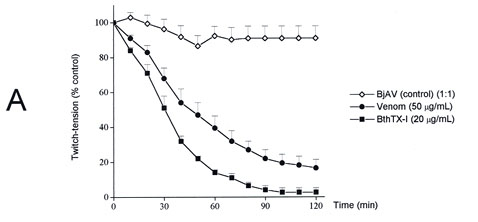
The times required for 50% neuromuscular blockade in EDL preparation were 58+8 min (n=16) for venom and 30+3 min (n=5) for toxin. Figure 3A shows neuromuscular blockade induced by B. jararacussu venom and BthTX-I in EDL preparations compared to rabbit antivenom alone. In EDL preparations, a fixed venom or toxin:antivenom ratio of 1:0.8 was used to neutralize B. jararacussu venom and BthTX-I. This antivenom concentration protected against venom lethal effect in mice and prevented neuromuscular blockade produced by both venom and toxin (Figure 3B and 3C; p<0.05).
Extensor digitorum longus preparations. (A) The effect of B. jararacussu venom (50m g/mL) and BthTX-I (20m g/mL) on indirectly evoked twitch tension compared to BjAV. The antivenom alone caused no neuromuscular blockade. (B) and (C) showed BjAV neutralizing capacity against neuromuscular action of the venom and toxin, respectively. The proportion of antivenom used was based on its neutralizing potency on venom lethal effect (0.8mL BjAV neutralizes 1mg of B. jararacussu venom). The points are mean + S.E.M. of 3-5 experiments. BjAV, B. jararacussu antivenom.
Myotoxicity and its neutralization
B. jararacussu venom (50m g/mL) and BthTX-I (20m g/mL) caused intense CK release from EDL muscle. The responses after 120 min were similar in both cases, 3464+346U/L for venom and 3422+135U/L for BthTX-I. Measurements of CK levels beyond 120 min in BthTX-I-treated preparations were not made because at 120 min, the neuromuscular blockade had already reached its maximum. Antivenom prevented a dramatic rise in CK release caused by venom and toxin (Figure 4A and 4B; p<0.01). Antivenom was significantly (p<0.05) more effective in neutralizing CK release by venom than toxin.
(A) CK release (U/L) from mouse extensor digitorum longus (EDL) muscle during 120 min incubation with B. jararacussu (50m g/mL) venom or venom + BjAV mixture. (B) CK release from EDL by BthTX-I (20m g/mL) and neutralization by antivenom. * p<0.01 compared to venom (B) or BthTX-I (C) alone. The points and bars are mean + S.E.M. of 3-6 experiments.
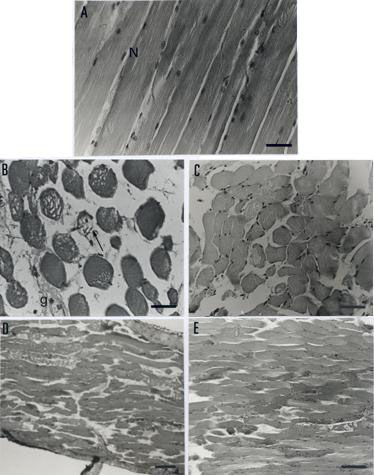
The normal histological appearance of mouse EDL muscle consisted of peripheral nuclei and well-organized fibers with regular transversal striations (Figure 5A). Incubation for 120 min with B. jararacussu venom (50m g/mL) resulted in intense myonecrosis (69+7% fibers with lesions) characterized by atrophy and aplasy of the muscle fibers. Figure 5B shows an area with fiber rarefaction and a hyaline aspect, myofibril lysis, and consequent fiber diameter deformation. Venom produced no significant alterations in the number of affected fibers in the presence of rabbit antivenom compared to antivenom alone (20+5% vs. 20+4% of fibers affected); these were both less than the damage caused by venom alone (Figure 5C; p<0.05). Rabbit antivenom protection against B. jararacussu venom myotoxic action was better compared with previous data with commercial antivenom (14). Preparations treated with venom + commercial antivenom showed an "all-or-nothing" effect, whereas preparations treated with venom + rabbit antivenom showed better protection of fibers and fewer ghost cells.
A. Normal histological appearance (control) of mouse extensor digitorum longus (EDL) muscle (longitudinal section). Note the presence of peripheral nuclei (N) and regular transversal striations. Bar = 30 mm. B. EDL muscle (transversal section) incubated with venom (50mg/ml) for 120 min. Note intense myonecrosis characterized by atrophy of muscle fibers, membrane alterations (arrow), and "ghost" cells (g) where nuclei occur extracellularl y. Bar = 30 mm. C. Ability of BjAV to prevent venom-induced myonecrosis. Most fibers were protected when compared to venom alone. Note that there were fewer empty areas and fewer "ghost" cells, indicating effective venom neutralization by the antivenom. Bar = 10 mm. D. EDL muscle (longitudinal section) incubated with BthTX-I (20mg/mL) for 120 min. The myonecrosis pattern was similar to that produced by venom. Bar = 10 mm. E. Protection by BjAV against BthTX-I myotoxic action. Destruction of some fibers is seen even in the presence of antivenom, but to a lesser extent than with toxin alone. Bar = 10 mm.
The pattern of myonecrosis observed after 120 min incubation with BthTX-I (20m g/mL, 63+7% of fibers with lesions; Figure 5D) was similar to that observed with venom. Myofibril fragmentation led to disruption of fiber outline, and presence of condensed and multi-shaped myofibril agglutinations. These changes were accompanied by darkening of the nuclei and loss of fiber striations. Rabbit antivenom effectively preserved fiber morphology in the presence of BthTX-I, with only 14+6% fibers being affected. This value was not significantly different from that with antivenom alone (20+4%). In both cases, the number of affected fibers was significantly lower (p<0.01) than in the absence of antivenom (Figure 5E; p<0.01).
DISCUSSION
Bites by B. jararacussu frequently produce severe local and systemic envenoming, including necrosis (sometimes requiring amputation), shock, spontaneous systemic bleeding, renal failure, and death (12). In addition to intense myonecrosis (18) and ultrastructural alterations in skeletal muscle (19), B. jararacussu venom inhibits muscle twitch tension in mouse isolated PND preparations (7). These effects are reproduced by BthTX-I, the main myotoxin accounting for about 15% of crude venom (8).
The efficacy of commercial bothropic antivenom for bites by B. jararacussu has been reported to be less than adequate, and it has been suggested that both bothropic and crotalic antivenoms should be given in combination for envenoming caused by this species (12). We have previously shown the low efficacy of commercial bothropic antivenom against B. jararacussu venom and BthTX-I myotoxic effects, although this antivenom protected against neurotoxic activity in both cases (14).
In this study, we examined whether rabbit antivenom against B. jararacussu venom could similarly neutralize neurotoxic and myotoxic effects of venom and BthTX-I. PND and EDL preparations were equally sensitive to venom and BthTX-I. These results differed from those obtained with C. d. terrificus venom and its main toxin, crotoxin, in which the two preparations differed in sensitivity to these agonists (13). This variation in sensitivity to venoms and toxins may partly reflect differences in action mechanisms, such as presynaptic (C. d. terrificus) vs. postsynaptic (B. jararacussu) sites. Rabbit antivenom neutralized B. jararacussu venom and BthTX-I neurotoxic effects.
Myotoxicity caused by B. jararacussu venom and BthTX-I was evaluated biochemically and histologically in EDL preparations. Quantification of an intracellular enzyme such as CK, which is released into the bathing solution after cell injury, has been used to study myotoxicity (11,13). Since the measurement of CK release was not possible in PND preparations because of the need to cut the diaphragm muscle (which would lead to the spontaneous CK release and high basal values), this activity was only measured in EDL preparations. As a quantitative biochemical marker of skeletal muscle damage, CK release provided an additional parameter to confirm histological results; there was a direct correlation between CK levels and pathological alterations. Rabbit antivenom fully prevented CK release by venom and BthTX-I, but was more effective against venom. Histological analysis showed that this antivenom was more effective against the myotoxic action of the venom and BthTX-I than was commercial bothropic antivenom (14). The lower myotoxicity efficacy of this commercial bothropic antivenom on venom and BthTX-I may reflect the lower proportion of venom (12.5%) present in the bothropic mixture used for immunization (3).
Antibodies to bothropic (9) and crotalic (13) myotoxins are useful tools for the study of neutralization of venom-induced muscle damage. Antivenom to crotoxin fully neutralized neurotoxic and myotoxic activities of BthTX-I (15). As shown here, rabbit antivenom also neutralized B. jararacussu venom and BthTX-I myotoxic action, and fully prevented neuromuscular effects. However, dissociation between these two phenomena was not as clear when heparin was used instead of antivenom (15). Comparison of the results obtained with commercial bothropic antivenom (14) and rabbit antivenom described here showed that the latter was qualitatively and quantitatively better.
ACKNOWLEDGEMENTS
The authors thank Marta Beatriz Leonardo for excellent technical assistance, and Gustavo H. Silva for the photographic work. This research was supported by FAPESP, CAPES, CNPq and FAEP/UNICAMP.
Received July 19, 2001
Accepted September 3, 2001
-
1Arni RK., Ward RJ., Cintra ACO., Giglio JR. Crystallization and preliminary diffraction data of bothropstoxin I isolated from the venom of Bothrops jararacussu Toxicon, 1995, 33, 383-6.
-
2bülbring E. Observation on the isolated phrenic nerve diaphragm preparation of the rat. Br. J. Pharmacol, 1946, 1, 38-61.
-
3CAMEY U., VERLARDE DT., SANCHEZ EF. Pharmacological characterization of bothropic antigen and of commercial serum produced at FUNED. In: Reunião Científica do Instituto Butantan, 2000, São Paulo. Resumos... São Paulo: Fundação Instituto Butantan, 2000:119.
-
4Chávez-Olórtegui C., Silva Lopes C., Drumond Cordeiro F., Granier C., Diniz CR. An enzyme-linked immunosorbent assay (ELISA) that discriminates between Bothrops atrox and Lachesis muta muta venoms. Toxicon, 1993, 31, 417-42.
-
5Gutiérrez JM., Chaves V. Proteolytic, hemorrhagic and myonecrotic effect of the venoms of Costa Rica snakes from the genera Bothrops, Crotalus and Lachesis. Toxicon, 1980, 18, 315-21.
-
6Gutiérrez JM., Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon, 1995, 33, 1405-24.
-
7Heluany NF., Homsi-Brandeburgo MI., Giglio JR., Prado-Franceschi J., Rodrigues-Simioni L. Effects induced by bothropstoxin, a component from Bothrops jararacussu snake venom, on mouse and chick muscle preparations. Toxicon, 1992, 30, 1203-10.
-
8Homsi-Brandeburgo MI., Queiroz LS., Santo-Neto H., Rodrigues-Simioni L., Giglio JR. Fractionation of Bothrops jararacussu snake venom: partial chemical characterization and biological activity of bothropstoxin. Toxicon, 1988, 26, 615-27.
-
9Lomonte B., Gutiérrez JM., Moreno E., Cerdas L. Antibody neutralization of a myotoxin from the venom of B. asper (terciopelo). Toxicon, 1987, 25, 443-9.
-
10Mebs D., Ehrenfeld M., Samejima Y. Local necrotizing effect of snake venoms on skin and muscle: relationship to serum creatine kinase. Toxicon, 1983, 21, 393-404.
-
11Melo PA., suarez-kurtz g. Release of sarcoplasmic enzymes from skeletal muscle by Bothrops jararacussu venom: antagonism by heparin and by serum of South American marsupials. Toxicon, 1988, 26, 87-95.
-
12Milani Junior R., Jorge MT., de Campos FP., Martins FP., Bousso A., Cardoso JL., Ribeiro LA., Fan HW., França FO., Sano-Martins IS., Cardoso D., Ide Fernandez C., Fernandes JC., Aldred VL., Sandoval MP., Puorto G., Theakston RD., Warrell DA. Snake bites by the jararacuçu (Bothrops jararacussu): clinicopathological studies of 29 proven cases in São Paulo State, Brazil. Q. J. Med, 1997, 90, 323-34.
-
13Oshima-Franco Y., Hyslop S., Prado-Franceschi J., Cruz-Höfling MA., Rodrigues-Simioni L. Neutralizing capacity of antisera raised in horses and rabbits against Crotalus durissus terrificus (South American rattlesnake) venom and its main toxin, crotoxin. Toxicon, 1999, 37, 1341-57.
-
14OSHIMA-FRANCO Y., HYSLOP S., CINTRA ACO., GIGLIO JR., CRUZ-HÖFLING MA., RODRIGUES-SIMIONI L. Neutralizing capacity of commercial bothropic antivenom against Bothrops jararacussu venom and bothropstoxin-I. Muscle Nerve, 2000, 23, 1832-9.
-
15OSHIMA-FRANCO Y., LEITE GB., SILVA GH., CARDOSO DF., HYSLOP S., GIGLIO JR., CRUZ-HÖFLING MA., RODRIGUES-SIMIONI L. Neutralization of the pharmacological effects of bothropstoxin-I from Bothrops jararacussu (jararacuçu) venom by crotoxin antiserum and heparin. Toxicon, 2001, 39, 1477-85.
-
16OUCHTERLONY O. Antigen-antibody reactions in gels. Acta Pathol. Microbiol. Scand, 1946, 26, 507-15.
-
17PETERSON GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem, 1977, 83, 357-63.
-
18Queiroz LS., Santo-Neto H., Assakura MT., Reichl AP., Mandelbaum FR. Pathological changes in muscle caused by haemorrhagic and proteolytic factors from Bothrops jararaca snake venom. Toxicon, 1985, 23, 341-5.
-
19Rodrigues-Simioni L., Borgese N., Ceccarelli B. The effects of Bothrops jararacussu venom and its components on frog nerve-muscle preparation. Neuroscience, 1983, 10, 475-89.
-
20SILES VILLARROEL M., ROLIM ROSA R., ZELANTE F., FURLANETTO RS. Padronização da avaliação da potência de antivenenos botrópicos, em camundongos. Mem. Inst. Butantan, 1978/1979, 42/43, 325-36.
-
21Vidal JC., Stoppani AO. Isolation and purification of two phospholipases A from Bothrops venoms. Arch. Biochem. Biophys, 1971, 145, 543-56.
-
22Vital Brazil O. Ação neuromuscular da peçonha de Micrurus Hospital, 1965, 68, 183-224.
Publication Dates
-
Publication in this collection
16 Sept 2002 -
Date of issue
2002
History
-
Accepted
03 Sept 2001 -
Received
19 July 2001