Abstract
Aurelia aurita is a scyphozoan, abundant in the Mexican Caribbean during summer. Although usually innocuous, there is evidence of it causing harm to humans. This work investigates the biological activities of crude and fractionated extracts of A. aurita. Live specimens were collected between July and September 1999 from the Mexican Caribbean. The tentacular margin was dissected immediately and frozen at -50ºC. A nematocyst suspension was prepared, discharged, and the supernatants lyophilized. Hemolytic assay was performed with lyophilized crude extract on bovine, sheep, and human red blood cells. Erythrocyte sensitivity to the toxin was ranked in descending order: human, sheep, and bovine. Toxic activity on Artemia nauplii was evaluated using the same crude extract for different exposure periods (3, 5, and 10 hours); only 48 and 72 hour old Artemia nauplii showed 50% mortality. Partial toxin purification was completed by sequential liquid chromatography using three gels (Sephadex G-200, DEAE Sephadex A-50, and Sephadex G-100). Intramuscular neuroactivity was detected in the crab Ocypode quadrata for two partially purified fractions. These fractions were found to have molecular weight components of 66 and 45 kDa, respectively.
Aurelia aurita; sting; hemolytic activity; crustacean lethality; venom
Original paper
SOME TOXINOLOGICAL ASPECTS OF Aurelia aurita (LINNÉ) FROM THE MEXICAN CARIBBEAN
L. Segura-Puertas1 CORRESPONDENCE TO:
L. Segura-Puertas - Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Puerto Morelos, P.O. Box 1152, 77501 Cancún, Q. Roo, México.
lsegura@mar.icmyl.unam.mx
, G. Avila-Soria1, J. Sánchez-Rodríguez1, M. E. Ramos-Aguilar2, J. W. Burnett3
CORRESPONDENCE TO:
L. Segura-Puertas - Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Puerto Morelos, P.O. Box 1152, 77501 Cancún, Q. Roo, México.
lsegura@mar.icmyl.unam.mx
, G. Avila-Soria1, J. Sánchez-Rodríguez1, M. E. Ramos-Aguilar2, J. W. Burnett3
1 Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, México; 2 Centro de Neurobiología, UNAM-Campus Juriquilla, México; 3 Department of Dermatology, School of Medicine, University of Maryland, USA.
ABSTRACT: Aurelia aurita is a scyphozoan, abundant in the Mexican Caribbean during summer. Although usually innocuous, there is evidence of it causing harm to humans. This work investigates the biological activities of crude and fractionated extracts of A. aurita. Live specimens were collected between July and September 1999 from the Mexican Caribbean. The tentacular margin was dissected immediately and frozen at -50ºC. A nematocyst suspension was prepared, discharged, and the supernatants lyophilized. Hemolytic assay was performed with lyophilized crude extract on bovine, sheep, and human red blood cells. Erythrocyte sensitivity to the toxin was ranked in descending order: human, sheep, and bovine. Toxic activity on Artemia nauplii was evaluated using the same crude extract for different exposure periods (3, 5, and 10 hours); only 48 and 72 hour old Artemia nauplii showed 50% mortality. Partial toxin purification was completed by sequential liquid chromatography using three gels (Sephadex G-200, DEAE Sephadex A-50, and Sephadex G-100). Intramuscular neuroactivity was detected in the crab Ocypode quadrata for two partially purified fractions. These fractions were found to have molecular weight components of 66 and 45 kDa, respectively.
KEYWORDS: Aurelia aurita, sting, hemolytic activity, crustacean lethality, venom.
INTRODUCTION
Aurelia aurita is a scyphozoan found worldwide with a variable stinging potency. It is commonly regarded as an innocuous species, but this animal can cause significant skin lesions in humans (3). Research about the biological activity of A. aurita is scarce. Studies in Thailand revealed that A. aurita venom has hemolytic and proteolytic activity (8). Myotoxic activity was reported in A. aurita venom from Japan (5). Recently, Radwan et al. (9) compared the chemical and toxinological properties of A. aurita nematocyst venoms from the Red Sea with those from Chesapeake Bay, USA, finding the former more potent. Many stings in humans have also been reported in South Florida after the most recent El Niño season (9). In the Mexican Caribbean, Aurelia is quite abundant during summer. The purpose of this study was to evaluate the biotoxicity of crude and fractionated extracts of A. aurita from the Mexican Caribbean.
MATERIALS AND METHODS
Collection and venom preparation
Specimens of A. aurita were collected from the Mexican Caribbean during the summer of 1999. Immediately after collection, several whole specimens were frozen at -50ºC for 3 days, thawed, stirred in 3 cycles of dionized water, and centrifuged at 3500 rpm for 15 min at 4ºC for nematocyst concentration. In addition, tentacular margins were cut from several living specimens and frozen at -50ºC for 3 days before treating as above. All extracts were lyophilized and stored at -50ºC until needed.
Protein concentration
Protein concentration was determined according to Bradford (1).
Bioassays
After cutting the tentacular margins, the nematocysts adhered to the hands of one of the co-authors, a healthy young female, who touched her abdomen to evaluate the nematocyst potency. Brine shrimp lethality was determined using Artemia salina nauplii (n=30) at 3 different ages (24, 48, and 72 hours, corresponding to instars I, II, and III, respectively); they were placed in 150 ml of filtered seawater containing crude extract (0.1 mg protein/ml) for different exposure periods (3, 5, and 10 hours). This assay was performed in triplicate and results are expressed as mean ± S.D. Statistical significance of the results was determined by the Students t test.
Hemolytic activity
Hemolytic activity of crude extract from the tentacular margin was evaluated by a modified Rottini et al. method (10). Erythrocytes from human, sheep, and bovine blood were placed in Alsevers solution, pH 7.4, and incubated at 30ºC for 30 min. After centrifugation, hemolysis was measured spectrophotometrically at 415 nm. This procedure was repeated four times.
Gel chromatographic separation
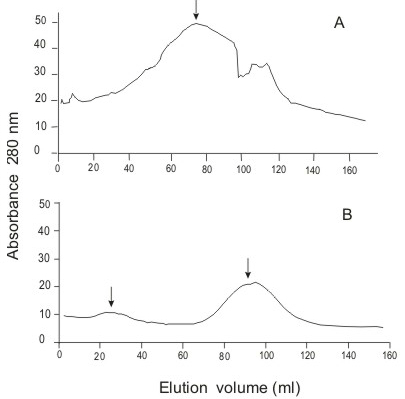
The crude extract (tentacular margin, 8.77 ml) was chromatographed on a G-200 column (60 x 2 cm) eluted with 0.05 M phosphate buffer, pH 7.2, with a flow rate adjusted to 0.8 ml/min. Fractions of 3 ml were collected. The 48-ml active fraction was selected by crab lethality screening at a volume of 0.1 ml, and then fractionated on a DEAE A-50 (22 x 1.2 cm), using a step gradient elution with 0.05, 0.1, 0.3, and 0.5 M phosphate buffers, pH 6.3, at a flow rate of 0.7 ml/min. Fractions of 5 ml were collected and again screened for crab lethality. The fractions eluted with 0.3 M phosphate buffer (432 ml) were desalted on a Sephadex G-100 (50 x 1.8 cm) column, eluted with 0.2 M acetic acid solution at a flow rate of three ml/min, and scanned at 280 nm. The protein fractions named Aa-1 (33 ml) and Aa-2 (75 ml) were concentrated by rotary-evaporation.
Neurotoxic activity
The two partially purified fractions (Aa-1 and Aa-2) were assayed using adult Ocypode quadrata crabs (10 g) with 0.6 mg protein in 0.1 ml volume. These assays were performed by injecting Aa-1 and Aa-2 into the third walking legs of the crabs, monitoring behavior and time until death.
SDS Gel electrophoresis
Sodium dodecyl sufate (SDS)-polyacrylamide gel electrophoresis was carried out as per Laemmli (6) in 10-20 % discontinuous gel at 20 volts for 12 hours in Tris-glysine buffer; the gel was stained with Coomassie blue. Molecular masses were determined by comparison to Sigma Chemical range standards: myosin, 205 kDa; ß- galactosidase, 116 kDa; phosphorylase ß, 97.4 kDa; bovine albumin, 66 kDa; egg albumin, 45 kDa; pepsin, 34.7 kDa; carbonic anhydrase, 29 kDa; trysinogen, 24 kDa; and ß-lactoglobulin, 18.4 kDa.
RESULTS
Positive stinging activity from the nematocyst preparation
Aurelia aurita from the Caribbean produced obvious human skin dermotoxicity. Immediately after contact with the nematocysts, the volunteer developed sharp local pain that lasted 30 minutes. About 3 minutes after the sting, intense itching was experienced and a sharply defined vesiculopapular, erythematous eruption appeared that lasted 10 days (Figure 1).
Nematocyst venom extract is lethal to Artemia salina nauplii
Precipitate and tentacular margin extracts of A. aurita were lethal to Artemia salina nauplii only after 5-hour exposure and only if shrimp were 48-72 hours old (instars II and III). The supernatant obtained from nematocyst centrifugation contained only a slight activity, presumably from early rupturing organelles (Figure 2).
Nematocyst venom extract has hemolytic activity
Crude extract from A. aurita tentacular margin hemolyzed different mammal cells. Human erythrocytes were the most susceptible, followed by sheep and bovine. In human erythrocytes, 50 % lysis was obtained with 0.61 mg of crude venom protein (Figure 3).
Liquid chromatographic separation of crude nematocyst extract
Only one distinct crab-lethal protein peak was found after Sephadex G-200 chromatography (Figure 4A, arrow). All lethality was then eluted by 0.3 M phosphate buffer from a DEAE A-50 column. This buffer was desalted by Sephadex G-100 gel filtration. Two protein peaks lethal to crabs could be subsequently demonstrated in the final column eluate (Figure 4B, arrow). Fraction Aa-2 contained 6 mg/ml protein.
Partial purification of A. aurita crude extract on A) Sephadex G-200 and B) Sephadex G-100.
Neurotoxic activity is present in partially purified nematocyst extracts
After intramuscular injections with 0.06 mg protein/kg in 0.1 ml/vol of fractions Aa-1 or Aa-2, adult crabs developed tetanic reactions followed by total paralysis and death within three minutes.
SDS gel electrophoresis
The molecular weights of Aa-1 and Aa-2 were 66 and 45 kDa, respectively (Figure 5).
SDS-PAGE analysis of A. aurita extracts. Samples were submitted to electrophoresis in a gradient (10-20%) polyacrylamide slab gel prepared as per Lammeli (1970). Crude extracts and pre-purified proteins were analyzed under reducing conditions with ß-mercaptoethanol. Aliquots of 30 and 15 µg were injected and the gel was stained with Coomassie blue. Lane 1 - Sigma SDS molecular weight standards (30-200 kDa); 2 - precipitate (30 µg); 3 - precipitate (15 µg); 4 - mucus (15 µg); 5 - supernatant (15 µg); 6 - Aa-2 fraction (15 µg); 7 - Aa-1 fraction (15 µg); 8 to 11 - replicates from lanes 2 to 5; 12 - Sigma SDS molecular weight standards (10-70 kDa).
DISCUSSION
It is now clear that Aurelia aurita can be venomous to man and elicit local skin eruption. However, Aurelia aurita in Mexican and eastern USA coastal waters is usually innocuous and children often play with it. The effect of the venom on man is unpredictable and limited to skin; its nematocyst venom is also toxinologically active to other lower species. The presence of hemolysins in cnidarian venom is common, and many of these animals have multiple cytolytic agents (4). The fact that low doses of Aurelia extract hemolyzed in a similar way human, bovine, and sheep erythrocytes whereas higher inocula of the same material alters human cells more dramatically, suggests that at least two active cytological principles are present. The effect of this second hemolysin becomes apparent at doses above 0.4 mg/protein/ml. Potency of Aurelia hemolysins is similar to Chrysaora venom, but in this latter, hemolysin was equipotent for both human and sheep erythrocytes (7). On the other hand, Cassiopea xamachana had hemolysin 50-100 times more potent than Aurelia, whereas in this instance, human erythrocytes were substantially more sensitive than sheep (9,11). Species selectivity of hemolysin might be explained by differences in the erythrocyte membrane of various animals. For example, sheep red cell membranes are composed of three times more sphingomyelin than human (10). This variance may be important for Aurelia and Cassiopea hemolysin and not for Chrysaora.
Significant lethal action of Aurelia extract against Artemia nauplii required a three-hour threshold time of venom contact and a >24-hour threshold degree of maturation of Artemia nauplii (Figure 2). The target of this lethal toxin had have evolved beyond a very primitive state (instar I) to be reactive to the venom. Thus, knowledge of nauplii embryogenesis could shed some light on Aurelia lethal factor pathogenesis.
In the partial purification process reported here, we found two active crab-lethal fractions. (Figure 4B). Elution location of these fractions followed a similar pattern to other nematocyst venoms separated by gel filtration chromatography (2). A large initial protein fraction, exhibiting maximal lethality at its peak, appears with a subsequent secondary protein later in the elution. The Aurelia specimens capable of causing harm to humans had a considerable lethality potency (at least 6 mg protein/kg crab) to crab. Whether there are other Aurelia medusas, innocuous to human skin but with two crab lethal factors, remains to be investigated.
Examination of SDS gels inoculated with the partially purified Aurelia crab-lethal fractions showed peptides of 66 and 45 kDa. This layer of highly toxic components is similar to that of several other jellyfish.
Aurelia sting produces skin irritation in man, and thus, cannot be considered harmless even though the vast majority of its medusas in any given locality can be handled without fear. The problem is to differentiate which of these should be avoided from those that can be handled with impunity. We as scientists have found that a difficult task and active bathers will find it impossible.
ACKNOWLEDGEMENTS
We thank Laura Celis for helping with the figures and to Amauri Mendoza for his assistance in collecting A. aurita specimens.
Received October 9, 2001
Accepted November 9, 2001
-
1BRADFORD MM. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem, 1976, 72, 248-54.
-
2BURNETT JW., GOLDNER R. Partial purification of sea nettle (Chrysaora quinquecirrha) nematocyst toxin. Proc. Soc. Exp. Biol. Med, 1970, 133, 978-81.
-
3BURNETT JW., CALTON CJ., LARSON JB. Significant envenomation by Aurelia aurita, the moon jellyfish. Toxicon, 1988, 26, 215-7.
-
4ENDEAN R., MONKS SA., CAMERON AM. Toxins from the box jellyfish Chironex fleckeri. Toxicon, 1993, 31, 397-410.
-
5KIHURA H., ANRAKU M., OHNO M., HASHIMURA S. Tetrodotoxin-unaffected depolarization of frog muscles induced by venom of jellyfish (Genus Aurelia). Jpn. J. Physiol., 1988, 38, 839-49.
-
6LAEMMELI UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature, 1970, 227, 680-5.
-
7LONG-ROWE KO., BURNETT JW. Characteristics of hyaluronidase and hemolytic activity in fishing tentacle nematocyst venom of Chrysaora quinquecirrha Toxicon, 1994, 32, 165-74.
-
8PONG-PRAYOON U., BOHLIN L., WASOWAT S. Neutralization of toxic effects of different crude jellyfish venoms by an extract of Ipomoea pes-caprue (L.) R. Br. J. Ethnopharmacol, 1991, 35, 65-9.
-
9RADWAN FFY., BURNETT JW., BLOOM DA., COLIANO T., ELDEFRAWI ME., ERDERLY H., AURELIAN L., TORRES M., HEIMER-DE LA COTERA EP. A comparison of the toxinological characteristics of two Cassiopea and Aurelia species. Toxicon, 2001, 39, 245-57.
-
10ROTTINI G., DOBRINA A., FORGIARINI O., NARDON E., AMIRANTE GA., PATRIARCA P. Identification and partial characterization of a cytologic toxin produced by Gardnerella vaginalis Infect. Immun, 1990, 58, 3751-8.
-
11TORRES M., AGUILAR MB., FALCÓN A., SÁNCHEZ L., RADWAN FFY., BURNETT JW., HEIMER-DE LA COTERA E., ARELLANO RO. Electrophysiological and hemolytic activity elicited by the venom of the jellyfish Cassiopea xamachana Toxicon, 2001, 39, 1297-307.
Publication Dates
-
Publication in this collection
16 Sept 2002 -
Date of issue
2002
History
-
Received
09 Oct 2001 -
Accepted
09 Nov 2001