Abstracts
The grasshopper Rhammatocerus conspersus (Bruner) (Orthoptera: Acrididae: Gomphocerinae) is an occasional pest in pasturelands of Rio Grande do Sul State. It is a univoltine species with an embryonic diapause. Nymphal and adult stages occur during the warmer months (November-March). Eggs were dissected periodically for characterization of embryonic external morphogenesis in 1994 and 1995. Ten embryonic stages were illustrated. Two diapausing stages were verified in R. conspersus: one at 25% and another at 50% of total embryonic development.
Insecta; grasshopper; embryo; diapause; morphology
O gafanhoto crioulo Rhammatocerus conspersus (Bruner) (Orthoptera: Acrididae: Gomphocerinae) é uma praga ocasional nas pastagens do Rio Grande do Sul. É uma espécie univoltina com diapausa embrionária. As fases ninfal e adulta ocorrem nos meses mais quentes (novembro-março). Ovos foram periodicamente dissecados em 1994 e 1995 para caracterização da morfogênese externa dos embriões. Dez estádios de desenvolvimento embrionário foram ilustrados. A diapausa em R. conspersus foi verificada em dois estádios: a 25% e a 50% do desenvolvimento embrionário total.
Insecta; gafanhoto; embrião; diapausa; morfologia
SYSTEMATICS, MORPHOLOGY AND PHYSIOLOGY
Embryonic external morphogenesis of Rhammatocerus conspersus (Bruner) (Orthoptera: Acrididae: Gomphocerinae) and determination of the diapausing embryonic stage
Morfogênese externa do embrião de Rhammatocerus conspersus (Bruner) (Orthoptera: Acrididae: Gomphocerinae) e determinação do estádio embrionário de diapausa
Cintia C. NivaI; Miriam BeckerII
ICurso de Pós Graduação em Ecologia, UFRGS, Campus do Vale, Centro de Ecologia, Porto Alegre, RS. Present address: Rua Souza Naves, 260 apto. 701, 86010-170, Londrina, PR
IIDepartamento de Zoologia, Instituto de Biociências, UFRGS, Av. Paulo Gama s/n, 90040-000, Porto Alegre, RS
ABSTRACT
The grasshopper Rhammatocerus conspersus (Bruner) (Orthoptera: Acrididae: Gomphocerinae) is an occasional pest in pasturelands of Rio Grande do Sul State. It is a univoltine species with an embryonic diapause. Nymphal and adult stages occur during the warmer months (November-March). Eggs were dissected periodically for characterization of embryonic external morphogenesis in 1994 and 1995. Ten embryonic stages were illustrated. Two diapausing stages were verified in R. conspersus: one at 25% and another at 50% of total embryonic development.
Key words: Insecta, grasshopper, embryo, diapause, morphology.
RESUMO
O gafanhoto crioulo Rhammatocerus conspersus (Bruner) (Orthoptera: Acrididae: Gomphocerinae) é uma praga ocasional nas pastagens do Rio Grande do Sul. É uma espécie univoltina com diapausa embrionária. As fases ninfal e adulta ocorrem nos meses mais quentes (novembro-março). Ovos foram periodicamente dissecados em 1994 e 1995 para caracterização da morfogênese externa dos embriões. Dez estádios de desenvolvimento embrionário foram ilustrados. A diapausa em R. conspersus foi verificada em dois estádios: a 25% e a 50% do desenvolvimento embrionário total.
Palavras-chave: Insecta, gafanhoto, embrião, diapausa, morfologia.
The grasshopper Rhammatocerus conspersus (Bruner) (Orthoptera: Acrididae: Gomphocerinae) is an occasional pest in pasturelands of Rio Grande do Sul State. High densities of this grasshopper population cause losses for cattle breeders because it competes with cattle for food source (Ferreira 1996). R. conspersus occurs between 28 to 30°S and 54 to 57°W. It is disseminated around 32 cities of Rio Grande do Sul State. The most damaged pasturelands are located in Santiago, Itaqui, Uruguaiana, São Borja and São Francisco de Assis (Caetano et al. 1990).
The diapause is a state of dormancy that synchronizes the active phases of organisms to periods favorable for their development, growth and reproduction (Tauber et al. 1986). In R. conspersus the active stages (nymphs and adults) in Rio Grande do Sul State are restricted to the warmer months of the year, from the end of October to March. During the rest of the year, the population is found buried in soil, in the egg stage. R. conspersus, therefore, may be considered a univoltine insect with the intervention of an embryonic diapause (Becker & Ferreira 1995).
The embryonic development has been commonly studied by means of the embryonic external morphogenesis (Wheeler 1893, Moore 1948, Van Horn 1966, Chapman & Whithan 1968, Micciarelli-Sbrena 1969, Wardaugh 1978). This kind of study has been useful for a better understanding of the embryonic diapause in several orthopterans (Khalifa 1957, Newmann-Visscher 1976, Dingle & Mousseau 1994).
The present study characterized the embryonic stages of R. conspersus based on external morphogenesis and determined in which moment, within the embryonic development, the diapause intervened.
Material and Methods
R. conspersus adults were collected on 2-3/ 2/ 1994 (80 females, 60 males) and 8-10/2/1995 (120 females, 100 males) in Santiago (29° 11'S 54° 53'W). Captured grasshoppers were transported to the laboratory (Department of Zoology, UFRGS, Porto Alegre, RS) and reared in cages with food and recipients filled up with soil for oviposition (Ferreira 1996). The photophase was 14 h and the temperature 28,7±7°C during all the rearing period (February to May 1994).
Eggs kept inside recipients with soil were maintained in the laboratory (25±8,5°C) from the first day after oviposition to the last week of June 1994. After this period the egg pods were taken outdoors (terrace of the Laboratory of Herpetology, Department of Zoology, UFRGS) where they remained until the end of January 1995.
Externally healthy eggs (turgid eggs with opaque golden color and micropilar region easily observed with the naked eyes) were dissected according to modifications observed on the embryos at intervals of 15 days. Almost all the examined eggs showed a stationary embryo without any sign of morphological modifications from April to July 1994. When some morphological modifications were noticed in some embryos, at the end of July, dissections proceeded weekly. And, in October, when a greater variety of modifications among the embryos were detected, dissections proceeded at intervals of two days. Dissections ceased when nymphs started to eclode. Eggs from about six pods were examined at each occasion of dissection. From these pods, two were from ovipositions dated February, two from March and two from April.
Eggs from each egg pod were individualized and washed in tap water for the examination of embryos. The eggs were then immersed in sodium chloride 0,3% for 3-5 minutes to remove the chorion (brownish protective layer of the egg) and washed again in tap water. This procedure permitted examination of the embryo through egg cuticle (Slifer 1945). The embryo could be seen with the naked eyes, as a white conic spot surrounded by a yellowish yolk. The egg was then put into some drops of Bouin's fixative solution on a plain surface. A bit of the shell was clipped from the posterior end of the egg and the anterior end slightly pressed for the embryo extrusion into the fixative. Finally the embryos were drawn. Embryos position inside the egg were illustrated from eggs without the chorion.
In 1995, the embryonic development was studied under constant temperatures (25, 30 and 35°C) and under outdoor conditions (19±11,5°C). On this occasion, the embryonic stages which had not been illustrated in 1994 were added to the previous sequence.
Results and Discussion
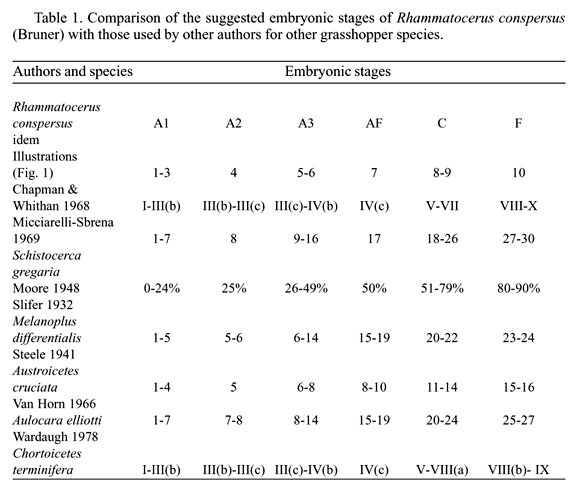
Dissections of externally healthy eggs permitted the recognition of ten different embryonic stages according to external morphogenesis of the embryos (Fig. 1). The ten embryonic stages were grouped in six types according to common morphological characteristics and following classifications of Chapman & Whithan 1968) (C&W) and Micciarelli-Sbrena (1969) (M-S), as described below:
Stage A1: embryo as a germinal disc or differentiated into protocephalon and protocormic region with or without sign of segmentation; stages I to III(b) of (C&W) and stages 1 to 7 of (M-S).
Stage A2: embryo differentiated into cephalic, thoraxic and abdominal portions; appendages observable as lobes on the superior portion and partial abdominal segmentation; embryo about 1/3 length of the egg, cephalic region pointing the posterior pole of egg; stages III(b)-III(c) of (C&W) and stage 8 of (M-S).
Stage A3: all stages after A2 and before AF; growth of the embryo, segmentation intensified and appendages developing; stages III(c) to IV(b) of (C&W) and stages 9 to 16 of (M-S).
Stage AF: embryo at the final stage of anatrepsis (AF) presenting antennae, mouthparts and legs primordium, complete abdominal segmentation; hind legs folded in N-shape; ocular region may present pigmentation; embryo occupies half of the egg with head still pointing the posterior pole of egg; stage IV(c) of (C&W) and stage 17 of (M-S).
Stage C: embryo going through katatrepsis, lengthening and broadening; embryos similar to AF with partial pigmentation of eyes; embryo strongly flexed backwards rotating in a head first movement towards the anterior pole of egg; or embryos with complete rotation, but not occupying the whole egg; stages V to VII of (C&W) and stages 18 to 26 of (M-S).
Stage F: final stage (F) of embryonic development; embryo occupying whole egg; stages V to VII of (C&W) and stages 27 to 30 of (M-S).
The embryonic stages considered above are listed in Table 1 and compared to the external morphogenesis of Austroicetes cruciata Sauss. (Steele 1941), Aulocara elliotti (Thomas) (Van Horn 1966), Chortoicetes terminifera (Walk.) (Wardaugh 1978), Melanoplus differentialis (Thomas) (Slifer 1932), Schistocerca gregaria Forsk. (Micciarelli-Sbrena 1969) and categories within the embryonic development of Moore (1948) and Chapman & Whithan (1968). Chapman & Whithan (1968) suggested that a scheme holding for all the grasshopper species considered in their study was possible, due to the basically similar embryonic development. Certainly the number of embryonic stages adopted by different authors varies depending on the objectives, criteria and methodologies used , besides the natural species specific variation of the embryo development (Van Horn 1966, Micciarelli-Sbrena 1969).
From stage I to VII (A1 to AF) (Fig. 1; Table 1) the embryo of R. conspersus is situated ventrally at the posterior pole of the egg, with the head pointing the posterior pole (1 to 7 - Fig. 1) - step of the embryonic development called anatrepsis (Wheeler 1893). During anatrepsis the embryo grew and differentiated, but never grew beyond half the egg length. Sometimes the embryo of R. conspersus, as well as observed in A. cruciata by Steele (1941), presented its tail (abdominal region) bent or folded, probably because of the resistance offered by the yolk (Steele 1941).
The stage AF (Fig. 1; Table 1), at the end of anatrepsis, corresponds to 50% of the total embryonic development (Moore 1948). It was one of the embryonic stages most frequently observed in R. conspersus, especially in 1994, pointing to the embryonic stage in which diapause occurred. In 1994, stage AF was observed with high frequency from 60 to 240 days of incubation (March - September) in outdoor conditions. In 1995, this same stage was verified from 30 to 150 days of incubation (March - July). From 150 to ca. 300 days of incubation dissections did not proceed as previously, but the few embryos observed suggested high frequencies of AF for at least the latter two or three months. The highest frequency of AF was observed at the end of the experimental period (ca. 300 days after oviposition - january 1996). This confirmed the diapause in stage AF. In the australian grasshopper C. terminifera (Wardaugh 1978) and in Camnula pellucida (Scud.) from Canada (Moore 1948), the diapause occurred in this same stage of embryonic development. The diapause also occurred in A. elliotti at this same stage of embryonic development, immediately before rotation. At this stage, the embryo of A. elliotti presented pigmented eyes although the cephalic and thoracic appendages segmentation in R. conspersus were more advanced (Van Horn 1966).
The stage AF (Fig. 1; Table 1) presented protocephalic lobes with a pronounced ocular region whose dorsal margin was pigmented with brown in 1994. On this occasion, this pigmentation was considered as the starting point of embryo rotation. However, in 1995, this pigmentation was rarely observed in AF. Wardaugh (1978) also verified the pigmentation of the ocular region at a corresponding stage of C. terminifera (Table 1). Hunter & Gregg (1984) associated the variation of the eye pigmentation of C. terminifera to the diapause intensity. Khalifa (1957) used the eye pigmentation to detect the end of anatrepsis in Euprepocnemis plorans (Charp.). The eye spots in this species were perceptible through the egg cuticle.
Stages VIII to X (C to F) (Fig. 1; Table 1) were considered to belong to katatrepsis, i.e., stage of development during which the embryo rotated towards the anterior pole of egg (blastokinesis), grew and differentiated until it was full egg size (8 to 10 - Fig. 1). The rotation was little observed in R. conspersus suggesting that this is a rapid process, as stated by Chapman & Whithan (1968). Internal differentiation (Wigglesworth 1972) and intensification of body pigmentation were believed to occur (Chapman & Whithan 1968) only at the moment the embryo occupied the whole egg. In 1995, stage IV (A2), corresponding to 25% of embryonic development (Moore 1948) (Fig. 1; Table 1), occurred in high frequencies along 150 days after oviposition in every treatment. This fact suggested that, besides the diapause at stage AF, another one occurred at stage A2 in R. conspersus.
The results strongly suggest that the embryonic development of R. conspersus presented two diapausing stages: one diapause at A2 (25% of total embryonic development) and another at AF (50% of total embryonic development) (Fig. 1; Table 1). This information is of fundamental importance for the study and comprehension of R. conspersus population dynamics.
Acknowledgements
We are grateful to Prof. MSc. Maurício Ursi Ventura, Prof. Dr. Amarildo Pasini and Flávio Yoshida for the suggestions in this manuscript.
Literature Cited
Received 15/IV/98. Accepted 21/VIII/98.
- Becker, M. & C. M. L. Ferreira. 1995. The internal reproductive organs and physiological age-grading of females of Rhammatocerus conspersus (Bruner, 1904) (Orthoptera: Acrididae: Gomphocerinae). Rev. Bras. Entomol. 39:171-181.
- Caetano, W., N. Bertoldo & M. Silva. 1990. Ocorrência, danos, e controle do gafanhoto crioulo Rhammatocerus conspersus no Rio Grande do Sul. Trigo e Soja 11: 26-27.
- Chapman, R. F. & F. Whithan. 1968. The external morphogenesis of grasshopper embryos. Proc. R. Entomol. Soc. Lond. 43:161-169.
- Dingle, H. & T. Mousseau. 1994. A. Geographic variation in embryonic development time and stage in a grasshopper. Oecologia 97: 179-185.
- Ferreira, C. M. L. 1996. Estudo da longevidade, fecundidade e fertilidade em fêmeas do gafanhoto-crioulo Rhammatocerus conspersus (Bruner, 1904) (Orthoptera: Acrididae: Gomphocerinae) em condições de laboratório. Tese de mestrado, UFRGS, 134p.
- Hunter, D. M. & P. C. Gregg. 1984. Variation in diapause potential and strength in eggs of the australian plague locust, Chortoicetes terminifera (Walker) (Orthoptera: Acrididae). J. Insect Physiol. 30: 867-870.
- Khalifa, A. 1957. The development of eggs of some egyptian species of grasshoppers, with a special reference to the incidence of diapause in the eggs of Euprepocnemis plorans Charp. (Orthoptera: Acridiidae). Bull. Soc. Entomol. 41: 299-330.
- Micciarelli-Sbrena, A. 1969. Gli stadi normali di sviluppo degli embrioni di Schistocerca gregaria Forskal (Orthoptera: Acrididae). Boll. Zool. 36: 77-95.
- Moore, H. W. 1948. Variations in fall embryological development in three grasshopper species. Can. Entomol. 80: 83-88.
- Newmann-Visscher, S. N. 1976. The embryonic diapause of Aulocara elliotti (Orthoptera, Acrididae). Cell. Tiss. Res. 174: 433-452.
- Slifer, E. H. 1932. Insect development. IV. External morphology of grasshopper embryos of known age and with a known temperature history. J. Morph. 53: 1-21.
- Slifer, E. H. 1945. Removing the shell from living grasshopper eggs. Science 102: 282.
- Steele, H. V. 1941. Some observations on the embryonic development of Austroicetes cruciata Sauss. (Acrididae) in the field. Trans. R. Soc. S. Aust. 65: 329-332.
- Tauber, M. J., C. A. Tauber & S. Masaki. 1986. Seasonal adaptations of insects. New York, Oxford University Press, 411p.
- Van Horn, S. N. 1966. Studies on the embryogenesis of Aulocara elliotti (Thomas) (Orthoptera, Acrididae). I. External morphogenesis. J. Morph. 120: 83-114.
- Wardaugh, K. G. 1978. A description of the embryonic stages of the Australian plague locust, Chortoicetes terminifera (Walk.). Acrida 7: 1-9.
- Wheeler, W. M. 1893. Contribution to insect embryology. J. Morph. 8: 141-160.
- Wigglesworth, W. M. 1972. The principles of insect physiology. 7th ed. Chapman and Hall, 827p.
Publication Dates
-
Publication in this collection
05 June 2006 -
Date of issue
Dec 1998
History
-
Received
15 Apr 1998 -
Accepted
21 Aug 1998