Abstracts
Nowadays alopecia areata is considered an autoimmune disease with genetic substrate. The clinical, histopathological, therapeutic and possible physiopathological mechanisms are discussed. The therapeutic approach, especially for the severe forms is considered with emphasis on the methods based on immunomodulation by drugs, diphenylcyclopropenone and squaric acid dibutylester and its possible mechanisms of action are discussed.
Alopecia; Alopecia areata; Review literature
Trata-se de artigo de revisão em que são analisados os aspectos clínicos, histopatológicos, etiopatogênicos e a terapêutica atual da alopecia areata. Enfatiza-se a posição nosológica atual da alopecia areata como doença auto-imune que se desenvolve em substrato genético e discutem-se os possíveis mecanismos fisiopatológicos da enfermidade, bem como os tratamentos atuais, particularmente para as formas mais graves da doença, compreendendo terapêuticas imunomoduladoras tópicas com dibutilester do ácido esquárico e difenciprona e seus possíveis mecanismos de ação.
Alopecia; Alopecia em áreas; Literatura de revisão
REVIEW ARTICLE
Alopecia areata: a revision and update* * Work done at Dermatology Service Hospital das Clínicas, University of São Paulo Medical School.
Evandro A. Rivitti
Titular Professor of Dermatology University of São Paulo Medical School
Correspondence Correspondence to Evandro Ararigboia Rivitti Rua Cincinato Braga, 59 / 1º Andar - Cj. 1 F2 São Paulo SP 01333-011 Tel: (11) 3285-2653
ABSTRACT
Nowadays alopecia areata is considered an autoimmune disease with genetic substrate. The clinical, histopathological, therapeutic and possible physiopathological mechanisms are discussed. The therapeutic approach, especially for the severe forms is considered with emphasis on the methods based on immunomodulation by drugs, diphenylcyclopropenone and squaric acid dibutylester and its possible mechanisms of action are discussed.
Keywords: Alopecia; Alopecia areata; Review literature.
INTRODUCTION
Alopecia areata (AA) is a chronic disease of the pilar follicles and nails, its etiology is unknown, but probably multifactorial with evident autoimmune and genetic components. It determines the loss of scalp and body hair by interruption of their synthesis, though without destruction or atrophy of the follicles and consequently can be reversible.
Historical aspects
The first clinical description of alopecia areata is attributed to Celsus (14 to 37 B.C.),1 and the designation alopecia areata is by Sauvages.2 Hebra demonstrated the incorrectness of the hypothesis of fungal etiology as proposed by Willan and Gruby (1843). Later, Von Baresprung proposed the neurotrophic theory, and Jacquet elaborated the dystrophic theory, considering the disease to be caused by infectious focuses, particularly dental, a hypothesis today that has been totally discarded. Nowadays, alopecia areata is interpreted as an autoimmune disease with a genetic substrate.
Epidemiological aspects
The statistical data registered in the literature are variable. The disease can begin at any time of life, but with a peak incidence between 20 and 50 years of age,3-5 and other articles affirm 60% of the patients present the first episode of the disease before 20 years of age.6,7 In material from the Hospital das Clínicas (teaching hospital of the University of São Paulo Medical School-FMUSP) Pimentel verified that 70% of the cases occurred between 10 and 25 years.8 Both sexes are equally affected,4,5 although at the Hospital das Clínicas it has been observed that with relation to the severe forms, 63% occur in men and 36% in women.8
Alopecia areata accounts for 2% of the first dermatological consultations in the United Kingdom and United States.4 The prevalence of AA in the United States, between 1971 and 1974, varied from 0.1% to 0.2% of the population.9 Some works have estimated that about 1.7% of the population presents at least one episode of AA during their life.10
Clinical aspects
In general, the patients report an important loss of hair and abrupt presence of an alopecic area or areas. The characteristic lesion of AA is a flat alopecic plaque with normal skin coloration involving the scalp or any other pilar region of the body. In acute phases, the lesions can be slightly erythematous and edematous, and appear in the periphery of the plaques of exclamation mark hair, presenting a thinner and less pigmented appearance at the point of emergence from the scalp and with greater thickness in the distal extremity (Figure 1).
Besides the above, these hairs demonstrate deposition of melanin pigment in the distal extremity (Widy's sign) and, although not absolutely pathognomonic, strongly suggest the diagnosis when present. The plaques of alopecia areata are usually asymptomatic, although several patients may complain about paresthetic sensations with discreet pruritus, pain or sensation of local ardor. Various symptomatological aspects can aid in the diagnosis of AA:
positive gentle traction test - in the acute phases of the disease, the hair is plucked easily by gentle traction, whether from the periphery of the plaques in localized forms, or from several areas of the scalp in diffuse forms. In the most chronic phases, this test is negative, since the hair is not plucked as easily as in the acute phases;
presence of cadaverous hair - these are hairs in which there occurs a fracture of the shaft inside the pilar follicle, producing blackened points inside the follicular ostia that resemble the comedos;
development of white fluff about half a centimeter in length along the alopecic area.
As the lesions course to more chronic phases, the presence of those signs is no longer detected and a mild follicular hyperkeratosis can appear in the alopecic area. Finally, the surface of the alopecic areas can become slightly atrophic, but never with a cicatricial aspect.
In agreement with number of lesions, extension of involvement and topography of hair loss, alopecia areata is clinically classified into several types:
1. Classic forms
A - Alopecia areata in single or unifocal plaque
In this form there is a single, round or oval, smooth alopecic plaque (Figure 2), in which the skin coloration is normal, with hair of a normal appearance in the periphery of the plaque that is easily plucked by traction (demonstrating activity of the process) typical exclamation mark hair can be present.
B - Alopecia areata in multiple or multifocal plaques
In this form typical alopecic plaques occur that affect the scalp or other pilar areas (Figure 3).
C - Ophiasic alopecia areata
In this presentation, the hair loss occurs along the line of temporo-occipital implantation, giving rise to an extensive alopecic area, in a band that reaches the inferior margins of the scalp (Figure 4).
D - Alopecia totalis
There is total loss of terminal hair of the scalp without affecting other body hair, there can also be ungual involvement (Figure 5).
E - Alopecia universalis
There is total loss of body hair, involving the scalp, eyelashes, eyebrows, beard and mustache, armpits and genital areas. In general, it occurs in association with a variety of ungual lesions.
Besides these forms that are considered classic, there are atypical presentations of alopecia areata:
2. Atypical forms
A - Sisaifo type alopecia areata (inverse ophiasis)
In this form, the hair loss involves the entire scalp except for the lower margins, along the line of temporo-occipital implantation. It is the inverse clinical image of the ophiasis form (Figure 6).
B - Reticular alopecia areata
In this form, multiple alopecic plaques occur separated by narrow bands of preserved hair, conferring a reticulated aspect to the picture (Figure 7).
C - Diffuse alopecia areata
In this form, the hair loss is acute and widespread. It can be the initial form, mainly among children and adolescents, or can develop from plaque forms. Most of these cases develop into the more serious alopecia totalis or universalis forms. It is the most difficult form to diagnose, demanding a differential diagnosis with acute telogen deffluvium, androgenetic alopecia and also alopecia syphilitica. Thus necessitating complementary exams in general and even histopathological exam by biopsy (Figure 8).
Extrafollicular involvement in alopecia areata
Extrafollicular involvement can be observed in alopecia areata, particularly in its more severe forms, comprising ungual alterations, ocular alterations, and reports of a possible relationship with salmon patch on nape of the neck.
Ungual alterations
Particularly in the most serious forms of alopecia areata, there can occur several types of ungual alterations the most frequent form of onychopathy being the presence of cupuliform depressions that can be so intense they produce true trachyonychia. There can also occur longitudinal and transverse furrows, koilonychia, onycholysis, onychomadesis, onychorrexis, pachyonychia, punctate leukonychia or transverse and red lunula.9-15 The ungual alterations are more frequent in children (12%) in relation to adults (3.3%).14 The prevalence of ungual alterations is greater in the more severe forms: alopecia universalis 15.4%; alopecia totalis 3.7%; and alopecia areata in plaque 2.25%.12 The ungual alterations can precede, accompany or succeed the alopecia lesions.
Ophthalmologic alterations
Various ocular alterations have been reported in alopecia areata, apparently related to dysfunctions of the pigment epithelium of the retina.16 The presence has been described of clusters of crystals (hyaline excretions in the choroid), focal hypopigmentation of the retina,17 opacities of the crystalline lens, posterior subcapsular cataracts, decrease in the visual acuity,18 Horner's syndrome, papillary ectopia, heterochromia and atrophy of the iris,19 miosis and palpebral ptosis.20
Salmon patch on the nape
Some works have attempted to correlate the presence of flat hemangioma with severity of the alopecia areata (Figure 9). Flat hemangioma on the nape was registered in 95.8% of the cases of areata universalis, in 86.7% of the cases of areata totalis and in 55.5% of the cases of ophiasic areata. These data showing a higher frequency of flat hemangioma on the nape in the most severe forms lead to the hypothesis that the presence of this lesion is an indication of a less favorable prognosis, though this correlation has yet to be proven in function of the high frequency of salmon patch in the nape of the general population,21,22 thus further studies are necessary with a greater number of cases and statistical significance.
DISCUSSION
The discussion regarding the etiopathogenesis and treatment of alopecia areata is considered below.
Etiopathogenesis of alopecia areata
Alopecia areata is a multifactorial disease with autoimmune components, which although seen in genetically predisposed individuals, the real causes have yet to be determined and various factors should be considered:
1. Genetic factors
These are important in the genesis of alopecia areata, as demonstrated by the high frequency of positive family history in the patients, varying from 10 to 42% in the series studied.23,24 Positivity in the family history is higher in individuals with early onset of the alopecia, reaching 37% among patients in whom the process began before 30 years of age and 7.1% when the disease begins after 30 years of age.24 Analysis of the material from Hospital das Clinicas (FMUSP) indicates familial occurrence of 4.1%.8 Also demonstrating the importance of genetic factors is the occurrence of AA in twins with concordance of 55% when identical.25-27 Likewise indicating the participation of genetic factors, is the higher incidence of alopecia areata in individuals with Down's syndrome (8.8%) in relation to the general population, signaling a possible participation of genic alteration in chromosome 21 as a causal factor for alopecia areata.28
The possible correlations between alopecia areata and histocompatibility antigens have been studied. The correlations with HLA class I have not been established, however it has been demonstrated that there is a correlation between susceptibility and severity with class II HLA. There is a significant association between alopecia areata and various class II HLA, HLA DR4, DR5 and DQ3, while HLA DR5 is related to the more severe forms with early onset.29-37
The HLA-DQB1*03 alleles appear to represent susceptibility markers for all forms of alopecia areata, whereas alleles HLA DR B1*0401, HLADQB1 *0301 seem to be markers for the more severe forms: areata totalis and areata universalis.29,35-38
Another indication for participation of genetic factors in alopecia areata is the frequent association with atopy, a condition known to be hereditary. There are correlations between the presence of atopy and the severity of alopecia areata, in that the condition is frequently present in more serious forms of alopecia areata.23,24 In the material from Hospital das Clinicas (FMUSP) the presence of atopy was observed in 42.8% of the alopecia areata patients against 26.6% of the paired controls.8
Therefore, innumerous studies have demonstrated an important participation of genetic factors in the genesis of alopecia areata, which is possibly a polygenic disease with participation of related genes both in terms of susceptibility and disease severity.
2. Immunological factors
There is considerable evidence for the participation of immunological mechanisms in the pathogenesis of alopecia areata: the association with autoimmune diseases, the presence of circulating antibodies of several types and the presence of immunologically active cells in the inflammatory infiltrations that constitute the histopathological expression of alopecia areata.
A - Association with autoimmune diseases
There are countless reports of an association between alopecia areata and autoimmune diseases, mainly autoimmune diseases of the thyroid and vitiligo,23,24 but also pernicious anemia,39 lupus erythematosus,40 myasthenia gravis,41 rheumatoid arthritis, polymyalgia rheumatica, ulcerative colitis,24 diabetes,42 and candidiasis-endochrinopathy syndrome.43
The incidence of thyroid disease among patients with alopecia areata varies from 8 to 11.8%, against 2% in the normal population.23,24 Some studies have shown that the incidence of vitiligo in alopecia areata patients is four times greater in relation to the normal population.23,39
B - Autoantibodies
The presence of autoantibodies in alopecia areata patients is quite frequent, particularly thyroid antimicrosomal antibodies.39 Anti-gastric parietal cell antibodies are also frequently detected.39,44,45 These antibodies probably represent a phenomenon secondary to the initial immunological phenomenon involving the pilar follicles.
The presence of autoantibodies against follicular structures has been described initially by detection with immunofluorescence and, more recently, by Western blot analysis. Some authors have detected class IgM and C3 antibodies in the follicular structures by direct immunofluorescence.46,47 In material from Hospital das Clinicas (FMUSP), these deposits were found in 10.3% of patients.8 Various studies, however, have demonstrated total negativity of those antifollicular antibodies by immunofluorescence.48,49 Using Western blot technique, some authors have reported the detection of antifollicular antibodies in 100% of the alopecia areata patients, against 44% of normal controls.50 The same authors have also demonstrated, by indirect immunofluorescence, the presence of circulating autoantibodies directed to multiple follicular structures, matrix, internal membrane and hair shaft.51 The controversial findings relative to the presence of these antibodies and their occurrence in normal controls suggests that these antibodies represent an event secondary to the inflammatory follicular phenomenon, that possibly exposes follicular antigens, with these antibodies appearing afterwards.
C - Cellular Immunity
Although there is controversy in the studies on cellular immunity, there is already uncontestable evidence for the participation of immune cellular mechanisms in the pathogenesis of alopecia areata. Studies relative to circulating lymphocytes in alopecia areata patients have shown varying results, from normal rates to even a reduction in their number. Studies relative to circulating lymphocytes in alopecia areata patients have shown varying results, from normal quantities to even a reduction in their number. Other works have shown a correlation between the reduction in T lymphocytes and severity of alopecia.44 Recently, some authors have obtained induction of alopecia areata in fragments of human scalp explants in mice with combined immunodeficiency through the injection of T lymphocytes from patients cultivated in presence of homogenates of pilar follicles and antigen-presenting cells.52 Microscopically, one can observe perifollicular infiltrates of T cells, increased expression of HLA class II and ICAM-1 type adhesion molecules. T cells cultivated without contact with follicular homogenates are not capable of producing micro or macroscopic alopecia areata lesions. This fact suggests that the patients' T cells recognize, through the antigen-presenting cells, antigenic structures of the follicle against which they react after sensitization by prior contact with follicular homogenates.
Furthermore, studies have demonstrated that CD8 positive T cells from patients previously cultivated with follicular homogenates are capable of producing alopecia areata in scalp fragments from patients transplanted into mice with combined immunodeficiency. The same experiment using CD4 T cells from patients does not produce alopecia areata lesions.52
Some cytokines IL-1 alpha, IL-1 beta and TNF alpha are potent growth inhibitors of the pilar follicle and in vitro produce follicular alterations morphologically identical to those observed in alopecia areata.53
In alopecia areata patients, abnormalities have been detected in the expression of type TH1 and IL-1 beta cytokines in lesions of alopecia areata in the scalp.54
Therefore, there is concrete evidence in relation to the participation of cellular immunity and this allows the hypothesis to be formulated that in alopecia areata patients, sensitization to follicular antigens occurs in T lymphocytes and especially those CD8 positive. The activation of lymphocytes that comprise the perifollicular infiltrate characteristic of alopecia areata leads to the release of cytokines capable of inhibiting growth of the pilar follicle, thereby interrupting hair synthesis. Apparently, follicular aggression exposes other antigens that act as immunogens and stimulate the secondary and variable production of the circulating antibodies that are sometimes detected in these patients.
D - Other etiopathogenic factors
Atopy
When alopecia areata occurs in atopic individuals, it has an earlier onset and tends to develop into the more severe forms.23,24 It is possible that the immune deregulation characteristic of atopic state contributes to intensifying the tissue-specific autoimmune phenomena.
Psychological trauma
Some studies have suggested that emotional stress contributes to the appearance of alopecia areata, given the observation that emotional trauma precedes the process55 together with the high prevalence of psychological disorders occurring in these patients.56 While, on the contrary, other studies have demonstrated that there is no participation of emotional phenomena in the development of alopecia areata.57
Scientific demonstration of the participation of psychiatric phenomena in the genesis of alopecia areata is very difficult. A possible explanation of the pathogenic mechanisms provoked by emotional conditions lies in the production of neuromediators capable of interfering in the immunity. Some studies have revealed a decrease in the expression of calcitonin gene related peptide (CGRP) and substance P in the scalp of alopecia areata patients.58,59 CGRP has an anti-inflammatory action,60 eand its decrease in alopecia areata could favor the characteristic follicular inflammatory phenomena. Substance P is capable of inducing hair growth in mice,61 eand its decrease in alopecia areata could be a contributing factor to the reduced proliferation of pilar follicles.
These are examples of possible biochemical means by which emotional phenomena could influence the genesis of alopecia areata lesions through the action of neuromediators. Furthermore, in these types of disease, in that the disease itself produces evident psychological alterations through detriment to the self-image, it is necessary to consider not only the possibility of emotional phenomena interfering in the disease, but also that the disease itself can produce important psychological alterations.
In spite of the progress regarding the etiopathogenesis of alopecia areata, the real etiology of the illness remains unknown and continues to be a matter for investigation. Currently, there are animal models that develop alopecia areata spontaneously or in which the disease can be induced. As is the case of the mouse C3H/HeJ62 and the Dundee experimental bald (DEB) rat,63 that will certainly contribute to an explanation of the illness and its pathogenic and therapeutic aspects.
Histopathological aspects
The presence of peribulbar lymphocytary inflammatory infiltrate is a histopathologic characteristic, found in most of the terminal hair in one evolutionary stage: catagen or telogen. The follicles become smaller during the course, forming miniaturized hair and are substituted by fibrous tracts. Eosinophils are also found in all the stages of alopecia areata, both in the peribulbar infiltrate and in the fibrous tracts.3,64-66
It has been demonstrated by immunohistochemistry that the cellular infiltrate is composed above all by T lymphocytes with CD4 T lymphocytes in greater number than CD8 T lymphocytes. There is an increase in the expression of HLA-DR. The CD4 and CD8 lymphocytes are in a varied proportion, from 2:1 to 8:1, and the CD4/CD8 ratio is slightly higher in the acute phase in relation to the chronic phase. Both CD4 and CD8 lymphocytes invade the follicular epithelium. There is also an increase in the expression of ICAM-1 in the dermal papillae, in the keratinocytes and in the external sheath of the hair root.65,66
Diagnosis
The diagnosis is clinical and generally simple in the common cases. It is only difficult in the diffuse chronic forms, necessitating subsidiary exams, trichogram and biopsy of the scalp.
Trichogram
It should be performed on hair removed from the border of the alopecic area and can reveal exclamation mark hair, as well as a smaller number of anagen hairs and larger number of telogen hairs than normal (90% anagen, 10% telogen).3
Biopsy
Biopsy reveals the histopathologic findings described above that enable differentiation from trichotillomania and other types of alopecia, such as the androgenetic form. It is underscored that biopsy should be done with a bistoury not a punch, in order that a significant sample of the pilar follicles is obtained. Currently, transversal sections of the material are used as these enable exam of a larger amount of pilar follicles cut at different levels. Besides the characteristic peribulbar infiltrate a significant decrease is demonstrated in the terminal hair in association with an increase in the vellus hair type, registering a proportion of 1:1, when the normal ratio is 7:1.
Differential diagnosis
The plaque forms should be differentiated from tinea of the scalp, pseudopelade of Brocq, lupus erythematosus and trichotillomania.
In the tineas of the scalp inflammatory phenomena usually occur that are not visible in alopecia areata: erythema and desquamation, besides the presence of tonsured hair. Direct mycological exam eliminates any doubt, and it is emphasized that this should always be performed for alopecic lesions in children. Pseudopelade of Brocq is fundamentally an atrophying alopecia and as such differs completely from alopecia areata. It is common in pseudopelade to observe the presence of scattered and isolated hair, amidst the atrophic plaque, that when removed reveals a gelatinous mass close to the bulbar portion (Sampaio's sign).67 In LE lesions of the scalp, besides atrophy there are inflammatory phenomena: erythema and hyperkeratosis, that can simulate desquamation. In trichotillomania, the alopecic plaques have an irregular configuration and exhibit different lengths of hair and there are no visible inflammatory phenomena.
Differential diagnosis may sometimes be necessary with localized pilar lichen planus. In this case, there are inflammatory phenomena, erythema, desquamation with evident follicular keratosis, and histopathologic exam enables the definitive diagnosis.
In the diffuse forms of alopecia areata, the differential diagnosis should be made with androgenic alopecia, acute telogen effluvium and secondary syphilis. The clinical aspects and complementary exams, such as serological reactions for syphilis, trichogram and biopsy for histopathologic exam, can define the picture.
Therapeutics
The treatment of alopecia areata is symptomatic and does not alter the prognostic for the disease, thus it is always important to consider the risk/benefit of systemic treatments together with the prognostic factors. The minimum period for evaluation of any treatment is three months.
The following therapeutic measures are used for alopecia areata in ascending order of complexity:
1. Topical rubefacient
This corresponds to the oldest forms of treatment, and its real value has never been demonstrated scientifically as there are controlled studies. One can use chloral hydrate, acetic acid, 5% cantharis dyes and other drugs today practically abandoned because they are only effective in the forms with a small number of benign plaques that present a normal course and cure within a few months.
2. Topical corticosteroids and intralesional infiltrations
These are widely used but there are almost no controlled studies on their real effectiveness against alopecia areata. Recently a double-blind study was published on children, comparing the topical treatment of alopecia areata with betamethasone dipropionate and placebo, which demonstrated that there were no differences in the therapeutic response between the two study groups.68 Considering the inflammatory substrate of alopecia areata, there is a pharmacological foundation for its use, and the more potent corticosteroids are used, particularly betamethasone dipropionate68 and clobetasol,69 but also halcinonide70 and fluocinolone.71 The topical corticosteroids can be used in association with topical minoxidil.
The intralesional infiltration of corticosteroid suspensions is the most effective treatment for the forms in which this procedure is feasible, adults with less than 50% involvement of the scalp. The preparation used most is triamcinolone acetonide at a concentration of 3 to 4 mg/ml in applications once every three or four weeks. Reversible atrophy might occur.
3. Anthraline
It is used at concentrations from 0.5% to 1% for 20 to 30 minutes after which the scalp should be washed with shampoos in order to avoid excessive irritant effects. The applications should be made initially every other day and later daily. There are side effects: darkening of light hair and irritant dermatitides that are subject to secondary infection, pruritus, folliculitis and regional adenomegaly. Works have registered an acceptable hair regrowth in a percentile that varies from 20 to 25% of the patients,72,73 however there are no placebo-controlled comparative studies.
It is possibility that anthralin acts as an immunomodulator inhibiting the cytotoxic activity and the production of IL-2 and normalizing the function of the suppressor T lymphocytes.
4. Minoxidil
It is used at 5% solutions in two daily applications, either separately or in association with anthralin or topical corticosteroids, or 0.025% and 0.05% retinoic acid. Its mechanism of action has yet to be determined, but it is known that it stimulates the follicular synthesis of DNA, and has a direct action, demonstrated in vitro, on the proliferation and differentiation of the keratinocytes, and it regulates the physiology of the hair independently from influences in the regional blood flow.74,75 Although useful, there are works that describe cosmetically acceptable results in proportions of only 20 to 45% of cases,76-79 it has proven little effective in the severe forms. When minoxidil functions, the first results are observed starting from the twelfth week of use. There are reports of better results when used in association with anthralin80 or clobetasol.81 Anthralin should be used two hours after the second application of minoxidil, and clobetasol is applied 30 minutes after each minoxidil application. Even the associated treatments are not effective in the severe forms.
The possible side effects from minoxidil are allergic and irritant contact dermatitis and hypertrichosis reversible with interruption of the treatment.
5. Topical immunotherapy
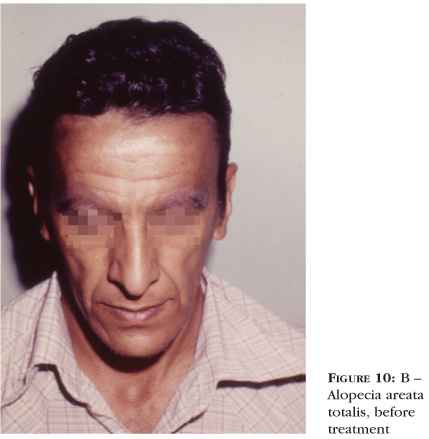
Highly sensitizing substances are used to provoke allergic contact dermatitis in the affected area and this produces an inflammatory infiltrate that substitutes the lymphocytary specific inflammatory infiltrate of alopecia areata. The substance initially employed was DNCB, nowadays discarded due to its carcinogenic potential (Figures 10A and 10B). Currently, squaric acid dibutylester and diphencyprone are used.
In relation to the possible mechanisms of action of these therapeutics two hypotheses have been formulated: the first is that the new population of immunologically active T lymphocytes, attracted by the immunogen utilized, eliminates the antigenic stimulus present in alopecia areata;82 the second hypothesis is that the generation of suppressor T lymphocytes in the treated area exercises an inhibitory effect on the autoimmune reaction associated to the follicular antigens of alopecia areata.83
6. Squaric acid dibutylester
Initially, the patient is sensitized with 2% solution and three weeks after the treatment of the affected areas begins with solutions at 0.00001%, increasing the concentration progressively up to 1%, in order to produce contact dermatitis at supportable levels. Several works have described good results in percentiles that vary from 29 to 87% of the cases treated.84-89
7. Diphencyprone
In a similar way, the patient is sensitized with 2% solutions and later the treatment of the affected areas begins with solutions at 0.001%, increasing the concentration used progressively until obtaining a mild eczematous reaction. The published works register a wide range of positive responses, of 4 to 85%.90-94 The response usually becomes apparent after 12 weeks of treatment.
The side effects from topical therapeutics with immunogens are intense eczematous reactions, including regional adenomegaly, edema, pruritus and post-inflammatory hypo- or hyperpigmentation. Topical therapeutics with immunogens are considered the best treatment today for the severe forms of alopecia areata.
8. Puva
The mechanism of action is considered to be interference in the presentation of follicular antigens to T lymphocytes by depletion of the Langerhans' cells.95 Puva therapy can be local or systemic; recurrences are frequent, sometimes demanding repeated treatments for a prolonged period with implications for carcinogenic risks.96-99
9. Systemic corticosteroids
Today, these are little used, due to the frequent recurrences after their withdrawal. They can be useful in the short term for rapidly progressive forms, in an attempt to brake that progression and other therapeutics being used in the long term.74,84 The initial doses are from 40 to 60 mg/day then decreasing by 5 mg each week.74 There are very few studies that have proposed EV pulse therapy with methylprednisolone, 250 mg twice a day during three days for those rapidly progressive forms.100
Some authors indicate, especially for children, monthly oral pulse therapy, with 300 mg of prednisolone for children up to 12 years of age and 5 mg/kg for those over 12.101
10. Cyclosporin
This has proven effective in sporadic reports, but the side effects and the high rate of recurrence render the drug an exception for therapy, to be attempted in only severe forms that are resistant to other treatment.102,103
11. Other drugs
There are reports of the use several other systemic drugs for alopecia areata. They are summarized in sporadic reports without any established scientific evidence as to their real therapeutic value. This is the case for calcium gluconate, dapsone, isoprinosine, azathioprine, tacrolimus and thymopentin and, more recently, sulfasalazine.104
CONCLUSION
Alopecia areata is a frequent disease but with rarer severe forms that provoke important psychosocial consequences to the patients. Nowadays, it is considered that alopecia areata is an autoimmune disease involving mainly the cellular immunity through the CD8 lymphocytes that act on follicular antigens. Activation of the lymphocytes of the perifollicular infiltrate specific to alopecia areata produces the release of cytokines (IL-1 alpha and beta, TNF) that inhibit the proliferation of cells in the pilar follicle, thereby interrupting the synthesis of hair without destroying the follicle. The process is aggravated by the presence of atopy and probably psychological trauma. The simple localized forms heal spontaneously or respond to simple treatment, such as corticoids either topical or injected locally by infiltration. The severe forms have a reserved prognosis and are difficult to treat, the best results are achieved topical immunotherapy techniques. q
REFERENCES
Received on March 11, 2003.
Approved by the Consultive Council and accepted for publication on October 10, 2003.
- 1. Rantuccio F, Mastrolonardo M, Conte A. Area Celsi. Osservazioni personali e revisione della letteratura. G Ital Dermatol Venereol. 1995; 130:23-35.
- 2. Dawber R. Alopecia areata. Monogr Dermatol. 1989; 2:89-102.
- 3. Moreno GA, Ferrando J. Alopecia areata. Med Cutan Ibero Latina Americana. 2000; 28:294-312.
- 4. Dawber RPR, de Berker D, Wojnarowska F. Disorders of hair. In: Champion RH, Burton JL, Burns DA, Breathnach SM, editors. Textbook of dermatology. Oxford: Blackwell Science; 1998. p. 2919-27.
- 5. Ferrando Roqueta FJ, Corral Blanco C, Lobo Satue A, Grasa Jordan MP. Estudios clínicos y de laboratorios. Estudio de los fenómenos de estrés y su relación con variables psicopatológicas, clínicas e imunológicas en pacientes com alopacia areata. Actas Dermatosifiliogr. 1996; 87:597-609.
- 6. Price V. Alopecia areata: clinical aspects. J Invest Dermatol. 1991; 96: 685.
- 7. Camacho F. Alopecia areata: clinical features. Dermatopathology. In: Camacho F, Montagna W, editors. Trichology: diseases of the pilosebaceus follicle. Madrid: Aula Medica Group; 1997. p.417-40.
- 8. Pimentel ERA. Alopecia areata. Aspectos imunológicos e tratamento pelo DNCB. [Tese]. Universidade de São Paulo; 1988.
- 9. Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey (letter). Arch Dermatol. 1992; 128:702.
- 10. Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ. Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628-33.
- 11. Shapiro J, Modani S. Alopecia areata: diagnosis and management. Int J Dermatol. 1999; 38:19-24.
- 12. Tosti A, Fanti PA, Morelli R, Bardazzi F. Trachyonichia associated with alopecia areata: a clinical and pathological study. J Am Acad Dermatol. 1991; 25:266-70.
- 13. Bergner T, Donhauser G, Ruzicka T. Red lunula in severe alopecia areata. Acta Dermato Venereol (Stockh). 1992; 72:203-5.
- 14. Tosti A, Morelli R, Bardazzi F, Peluso AM. Prevalence of nail abnormalities in children with alopecia areata. Pediatr Dermatol. 1994; 11:112-5.
- 15. Sahn EE. Alopecia areata in childhood. Semin Dermatol. 1995; 14:9-14.
- 16. Tosti A, Colombati S, De Padova MP, Guidi SG, Tosti G, Maccolini E. Retinal pigment epithelium function in AA. J Invest Dermatol. 1986; 86:553-5.
- 17. Tosti A, Colombati S, Caponeri GM, Ciliberti C, Tosti G, Basi M, et al. Ocular abnormalities occurring with alopecia areata. Dermatologica. 1985; 170:69-73.
- 18. Muller SA, Brunsting LA. Cataracts associated with dermatologic disorders. Arch Dermatol. 1963; 88:330-9.
- 19. Hordinsky MA. Alopecia areata. In: Olsen EA, editor. Disorders of hair growth. Diagnosis and treatment. New York: MacGraw-Hill, 1994. p.195-222.
- 20. Ikeda T. Produced alopecia areata based on the focal infection theory and mental theory. Dermatologica. 1967; 134:1-11.
- 21. Hatzis J, Kostakis P, Tosca A, Parissis N, Nicolis G, Varelzidis A, et al. Nuchal nevus flammeus as a skin marker of prognosis in alopecia areata. Dermatologica. 1988; 177:149-51.
- 22. Camacho F, Navas J. Nuchal nevus flammeus and alopecia areata. Dermatology 1992; 184:58.
- 23. Muller SA, Winkelmann RK. Alopecia areata: an evaluation of 736 patients. Arch Dermatol. 1963; 88:290-7.
- 24. Shellow WV, Edwards JE, Koo JY. Profile of alopecia areata: a questionnaire analysis of patient and family. Int J Dermatol. 1992; 31:186-9.
- 25. Scerri L, Pace JL. Identical twins with identical alopecia areata. J Am Acad Dermatol. 1992; 27:766-7.
- 26. Jackow C, Puffer N, Hordinsky M, Nelson J, Tarrand J, Duvic M. Alopecia areata and cytomegalovirus infection in twins: genes versus environment? J Am Acad Dermatol. 1998; 38:418-25.
- 27. Weidmann Al, Zion LS, Mamelok AE. Alopecia areata occurring simultaneously in identical twins. Arch Dermatol. 1956; 74:424-32.
- 28. Du Vivier A, Munro DD. Alopecia areata, autoimmunity and Down's syndrome. Br Med J. 1975; 1:191-4.
- 29. Colombe BW, Price VH, Khoury EL. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995; 33:757-64.
- 30. Frentz G, Thomsen K, Jakobsen BK, Svejgaard. HLA-DR4 in alopecia areata [letter]. J Am Acad Dermatol. 1986; 13:129-30.
- 31. Mikesell JF, Bergfeld WL, Braun WE. HLA-DR antigens in alopecia areata: preliminary report. Cleve Clin Q. 1986; 53:189-91.
- 32. Orecchia G, Belvedere MC, Martinetti M. Human leukocyte antigen region involvement in the genetic predisposition to alopecia areata. Dermatologica. 1987; 175:10-4.
- 33. Zhang L, Weetman AP, Friedman PS. HLA associations with alopecia areata. Tissue antigens. 1991; 38:89-91.
- 34. Duvic M, Hordinsky MK, Fiedler VC. HLA-D locus associations in alopecia areata Drw52a may confer disease resistance. Arch Dermatol. 1991; 127:64-8.
- 35. Morling N, Frentz G, Fugger L, Georgsen J, Jakobsen B, Odum N. DNA polymorphism of HLA class II genes in alopecia areata. Dis Markers. 1991; 9:35-42.
- 36. Welsh EA, Clark HH, Epstein SZ, Reveille JD, Duvic M. Human leukocyte antigen-DQB01*03 alleles are significantly associated with alopecia areata. J Invest Dermatol. 1994; 103:758-63.
- 37. Duvic M, Welsh EA, Jackow C, Papadopoulos E, Reveille JD, Amos C. Analysis of HLA-D locus alleles in alopecia areata patients and families. Arch Dermatol. 1995; 104:5S-6S.
- 38. Colombe BW, Price VH, Khoury EL, Lou CD. Class II alleles in long-standing alopecia totalis/alopecia universalis and long-standing patchy alopecia areata differentiate these two clinical groups. J Invest Dermatol. 1995; 104:4S-6S.
- 39. Friedmann PS. Alopecia areata and auto-immunity. Br J Dermatol. 1981; 105:153-7.
- 40. Werth VP, White WL, Sanchez MR, Franks AG. Incidence of alopecia areata in lupus erythematosus. Arch Dermatol. 1992; 128:368-71.
- 41. Kubota A, Komiyama A, Hasegawa O. Myasthenia gravis and alopecia areata. Neurology. 1997; 48:774-5.
- 42. Wang SJ, Shohat T, Vadheim C, Shellow W, Edwards J, Rotter JI. Increased risk for type I (insulin-dependent) diabetes in relatives of patients with alopecia areata (AA). Am J Med Genet.1994; 51:234-9.
- 43. Boni R, Tweb RM, Wuthrich B. Alopecia areata in a patient with candidiasis-endochrinopathy syndrome. Dermatology. 1995; 191:68-71.
- 44. Friedmann PS. Clinical and immunological associations of alopecia areata. Semin Dermatol. 1985; 4:9-24.
- 45. Zauli D, Veronesi S, Fusconi M, Lama L, Melino M, Tosti A. Autoantibodies in alopecia areata. Br J Dermatol. 1984; 111:247.
- 46. Bystrin IC, Orentreich N, Stewger F. Direct immunofluorescence studies in alopecia areata and male pattern alopecia. J Invest Dermatol. 1979; 73:317-20.
- 47. Sapa B, Orentreich N, Goudia A. Immunological abnormalities in patients with alopecia areata. Clin Res. 1979; 27:244.
- 48. Gollnick H, Imcke E, Orfanos CE. Immunohistochemical study on the expression of cytokeratins. Filagrin and basement membrane proteins in hair follicles of alopecia areata patients. Arch Dermatol Res. 1984;281:145.
- 49. Sato Y. Alopecia areata. Modern aspects. In: Orfanos CE, Montagna W, Stuttigen G, editors. Hair Research. New York: Springer-Verlag. 1981. p. 303-10.
- 50. Tobin DJ, Orentreich N, Fenton DA, Bystryn JC. Antibodies to hair follicles in alopecia areata. J Invest Dermatol. 1994; 102:702-4.
- 51. Tobin DJ, Hann SK, Song MS, Bystryn JC. Hair follicles structures targeted by antibodies in patients with alopecia areata. Arch Dermatol. 1997; 133:57-61.
- 52. Gilhar A, Ullmann Y, Berkutzki T, Assy B, Kalish RS. Autoimmune hair loss (alopecia areata) transferred by T-lymphocytes to human scalp explants on SCID mice. J Clin Invest. 1998; 101:62-7.
- 53. Philpott MP, Sanders DA, Bowen J, Kealy Tl. Effects of interleukines, colony-stimulating factor and tumor necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumor necrosis factor-a in alopecia areata. Br J Dermatol. 1996; 135:942-8.
- 54. Hoffmann R, Eicheler W, Huth A, Wenzel E, Happle R. Cytokines and growth factors influence hair growth in vitro: possible implications for the pathogenesis and treatment of alopecia areata. Arch Dermatol Res. 1996; 288:153-6.
- 55. Baker GHB. Psychological factors and immunity. J Psychosom Res. 1987; 31:1-10.
- 56. Colon EA, Popkin MK, Callies AL, Dessert NJ, Hordinsky MK. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychatry. 1991; 32:245-51.
- 57. van der Steen P, Boezeman J, Duller P, Happle R. Can alopecia areata be triggered by emotional stress? An uncontrolled evaluation of 178 patients with extensive hair loss. Acta Dermato Venereol (Stockh). 1992; 72:279-80.
- 58. Hordinsky M, Lorimer S, Worel S. Innervation and vasculature of the normal human and alopecia areata hair follicle: an immunohistochemical and laser scanning confocal microscope study. In: Proceedings of the First Tricontinental Meeting of Hair Research Societies. Brussels, Belgium; 1995.
- 59. Hordinsky MK, Kennedy W, Wendelschafer-Crabb G, Lewis S. Structure and function of cutaneous nerves in alopecia areata. J Invest Dermatol. 1995; 104(Suppl):28S-29S.
- 60. Raud J, Lundeberg T,Brodda-Jansen G, Theodorsson E, Hedqvist P. Potent anti-inflammatory action of calcitonin gene-related peptide. Biochem Biophys Res Commun. 1991; 180:1429-35.
- 61. Paus R, Heinzelmann T, Schultz KD, Furkert J, Fechner K, Czarnetzki BM. Hair growth induction by substance P. Lab Invest. 1994; 71:134-40.
- 62. Sundberg JP, Cordy WR, King LE. Alopecia areata in aging C3H/HeJ mice. J Invest Dermatol.1994; 102:847-56.
- 63. Michie HJ, Jahoda CAB, Oliver RF, Johnson BE. The DEBR rat: an animal model of human alopecia areata. Br J Dermatol. 1991; 125:94-100.
- 64. Ackerman AB, Guo Y, Vitale O. Clues to diagnosis in dermatopathology II. Hong Kong: Everbest Printing; 1992. p. 330-2.
- 65. Madani S, Shapiro J. Alopecia Areata Update. J Am Acad Dermatol. 2000; 42:549-66.
- 66. Ghersetich I, Campanile G, Lotti T. Alopecia areata: immunohistochemestry and ultrastructural of infiltrate and identification of adhesion molecule receptors. Int J Dermatol. 1996; 35:28-33.
- 67. Sampaio SAP, Rivitti EA. Alopecias cicatriciais. In: Dermatologia São Paulo: Artes Médicas; 2000. p. 322-3.
- 68. Maia CP & Fernandes NC. Tratamento da alopecia areata com corticóide tópico: Estudo prospectivo randomizado duplo cego em crianças. An Bras Dermatol. 2003; 78:63-71.
- 69. Fiedler VC, Alaiti S. Treatment of alopecia areata. Dermatol Clin. 1996; 14:733-8.
- 70. Montes L. Topical halcinoide in alopecia areata and alopecia totalis. J Cutan Pathol. 1977; 4:47-50.
- 71. Gill K, Baxter DL. Alopecia totalis: treatment with fluocinolone acetonide. Arch Dermatol. 1963; 87:384.
- 72. Fiedler VC. Alopecia areata. A review of therapy, efficacy, safety, and mechanism. Arch Dermatol. 1992; 128:1519-29.
- 73. Fiedler-Weiss V, Buys C. Evaluation of anthralin in the treatment of alopecia areata. Arch Dermatol. 1987; 123:1491-3.
- 74. Price V, Khoury E. Progress in dermatology. Bull Dermatol Found. 1991; 25:1.
- 75. Buhl AE. Minoxidil's action in hair follicles. J Invest Dermatol. 1991; 96(Suppl):73S-4S.
- 76. Shapiro J, Price V. Hair regrowth: therapeutic agents. Dermatol Clin. 1998; 16:341-56.
- 77. Fiedler-Weiss VC, Rumsfield JA, Buys CM, West DP, Wendrow A. Evaluation of oral minoxidil in the treatment of alopecia areata. Arch Dermatol. 1987; 123:1488-90.
- 78. Price VH. Double-blind, placebo-controlled evaluation of topical minoxidil in extensive alopecia areata. J Am Acad Dermatol. 1987; 16:730-6.
- 79. Shapiro J, Price V. Hair regrowth: therapeutic agents. Dermatol Ther. 1998; 16:341-56.
- 80. Fiedler V, Vendrow A, Szunpar G. Treatment-resistant alopecia areata: response to combination therapy with minoxidil plus anthralin. Arch Dermatol. 1990; 126:756-9.
- 81. Fiedler V. Alopecia areata: current therapy. J Invest Dermatol. 1991; 96:69S-70S.
- 82. Daman L, Rosenberg W, Drake L. Treatment of alopecia areata with dinitrochlorobenzene. Arch Dermatol. 1978; 114:1036-8.
- 83. Happle R. Antigenic competition as a therapeutic concept for alopecia areata. Arch Dermatol Res. 1980; 267:109-14.
- 84. Whiting DA. The treatment of alopecia areata. Cutis. 1987; 40:247-50.
- 85. Tosti A, Guidetti MS, Bardazzi F, Misciali C. Long-term results of topical immunotherapy in children with alopecia totalis or alopecia universalis. J Am Acad Dermatol. 1996; 35:199-201.
- 86. Micali G, Licastro-Cicero R, Nasca MR. Treatment of alopecia areata with squaric acid dibutylester. Int J Dermatol. 1996; 35:52-6.
- 87. Happle R, Kalveram KJ, Buchner U, Echternacht-Happle K, Goggelmann W, Summer KH. Contact allergy as therapeutic tool for alopecia areata: application of squaric acid dibutylester. Dermatologica. 1980; 161:289-97.
- 88. Flowers FP, Slazinski L, Fenske NA, Pullara TJ. Topical squaric acid dibutylester therapy for alopecia areata. Cutis. 1982; 30:733-6.
- 89. Gianetti A, Orecchia G. Clinical experience on the treatment of alopecia areata with squaric acid dibutylester. Dermatologica. 1983; 167:280-2.
- 90. Orecchia G, Rabbiosi G. Treatment of alopecia areata with diphencyprone. Dermatologica. 1990; 171:193-6.
- 91. Monk B. Induction of hair growth in alopecia totalis with diphencyprone sensitization. Clin Exp Dermatol. 1989; 14:154-7.
- 92. Shapiro J, Tan J, Ho V, Abbott F, Tron V. Treatment of chronic severe alopecia areata with topical diphenylcy clopropenone and 5% minoxidil: a clinical and immunopathologic evaluation. J Am Acad Dermatol. 1993; 29:729-35.
- 93. Hull SM, Cunliffe WJ. Successful treatment of alopecia areata using the contact allergen diphencyprone [letter]. Br J Dermatol. 1991; 123:212-3.
- 94. Van der Steen P, Van Baar H, Perret C. Treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991; 24:227-30.
- 95. Ree K. Reduction of Langerhans cells in human epidermis during PUVA therapy: a morphometric study. J Invest Dermatol. 1982; 78:488.
- 96. Mitchell A, Douglas M. Topical photochemotherapy for alopecia areata. J Am Acad Dermatol. 1985; 12:644-9.
- 97. Larko O, Swanbeck G. PUVA treatment of alopecia areata. Acta Dermato Venereol (Stockh). 1983; 63:546.
- 98. Lassus A, Kianto U, Johansson E. PUVA treatment for alopecia areata. Dermatologica. 1980; 151:298-304.
- 99. Claudy A, Gagnaire D. Photochemotherapy for alopecia areata. Acta Derm Venereol (Stockh).1980; 60:171-2.
- 100. Friedli A, Labarthe MP, Endelhardt E, Feldman R, Salomon D, Saurat JH. Pulse methylprednisolone therapy for severe alopecia areata: an open prospective study of 45 patients. J Am Acad Dermatol. 1998; 39:597-602.
- 101. Sharma VK, Murahdhar S. Treatment of widespread alopecia areata in young patients with monthly oral corticosteroid pulse. Pediatr Dermatol. 1998; 15:313-7.
- 102. Gupta A, Ellis C, Cooper K, et al. Oral cyclosporine for the treatment of alopecia areata: a clinical and immunohistochemical analysis. J Am Acad Dermatol 1990; 22:242-50.
- 103. Shapiro J, Lui H, Tron V, Ho V. Systemic cyclosporine and low-dose prednisone in the treatment of chronic severe alopecia areata: a clinical and immunopathologic evaluation. J Am Acad Dermatol. 1997; 36:114-7.
- 104. Ellis CN, Brown MF, Voorhees JJ. Sulfasalazine for alopecia areata. J Am Acad Dermatol. 2002; 46:541-4.
Publication Dates
-
Publication in this collection
25 Nov 2005 -
Date of issue
Feb 2005
History
-
Received
11 Mar 2003 -
Accepted
17 Oct 2003