Abstracts
Safety margin and surgical margin are terms frequently used as synonyms, although they have different meanings. Safety margin is pre-determined and is a part of the surgical planning. Surgical margin is subsequently verified by the pathologist when he or she examines the surgical piece. There is no consensus in the literature regarding the extension of the safety margin, which is based on a series of variables which are not always easy to analyze. On the other hand, the microscopically controlled surgery does not use the concept of safety margin and is the most rational treatment of skin cancer. This article discusses the determinant factors of safety margin and surgical margin, from a both clinical and laboratorial point of view, making a parallel with microscopically controlled surgeries and raising some important reflections about the relativity of the concept of safety margin.
Mohs surgery; Skin neoplasms; Pathology, surgical; Reoperation
As expressões margem de segurança e margem cirúrgica são usadas freqüentemente como sinônimas, embora tenham significados distintos. A margem de segurança é preestabelecida e faz parte do planejamento cirúrgico. A margem cirúrgica é verificada posteriormente pelo patologista ao exame da peça cirúrgica. Na literatura não existe consenso a respeito da extensão da margem de segurança, sendo ela baseada em uma série de variáveis nem sempre de fácil análise. Por outro lado a cirurgia microscopicamente controlada não utiliza o conceito de margem de segurança e se constitui na forma mais racional de tratamento do câncer cutâneo. Este artigo discorre sobre os fatores determinantes da margem de segurança e da margem cirúrgica, tanto do ponto de vista clínico como do laboratorial, traçando um paralelo com a cirurgia microscopicamente controlada e lançando algumas reflexões importantes sobre a relatividade do conceito de margem de segurança.
Cirurgia de Mohs; Neoplasias cutâneas; Patologia cirúrgica; Reoperação
REVIEW ARTICLE
Safety margin: an old and relative concept* * Work done at Hospital Biocor Belo Horizonte (MG) - Brazil.
Luis Fernando Figueiredo KopkeI; José Caldeira Ferreira BastosII; José de Souza Andrade FilhoIII; Patricia Salomé GouvêaIV
IPost-graduate in Surgical Dermatology at Munique University, LMU München - German
IIPathologist at IDAP - Instituto de Diagnóstico Anátomo Patológico Florianópolis - SC. Pathologist at Hospital de Caridade de Florianópolis (SC)
IIIPathology Professor at Universidade Federal de Minas Gerais - UFMG. Pathologist at Hospital Felicio Rocho Belo Horizonte (MG)
IVPathologist at Hospital Biocor Belo Horizonte (MG)
Correspondence Correspondence to Luis Fernando Figueiredo Kopke Rua Rio Grande do Norte, 1560 / 702 30130-131 - Belo Horizonte - MG Tel/fax: (31) 3227-9898 E-mail: luiskopke@kopkedermatologia.med.br
ABSTRACT
Safety margin and surgical margin are terms frequently used as synonyms, although they have different meanings. Safety margin is pre-determined and is a part of the surgical planning. Surgical margin is subsequently verified by the pathologist when he or she examines the surgical piece. There is no consensus in the literature regarding the extension of the safety margin, which is based on a series of variables which are not always easy to analyze. On the other hand, the microscopically controlled surgery does not use the concept of safety margin and is the most rational treatment of skin cancer. This article discusses the determinant factors of safety margin and surgical margin, from a both clinical and laboratorial point of view, making a parallel with microscopically controlled surgeries and raising some important reflections about the relativity of the concept of safety margin.
Keywords: Mohs surgery; Skin neoplasms/surgery; Pathology, surgical; Reoperation
INTRODUCTION
Even though the expressions surgical margin and safety margin are used commonly as synonyms by physicians, as though this were unanimous, this cannot be confirmed by data found in the medical literature. This work is the fruit of a long discussion on the theme between the main author and the three pathologists. As the authors are used to working with surgical cutaneous pathologies and micrographical surgery, they have long been intrigued about a few concepts regarding surgical margins, widely used by surgeons. An extense bibliographic review was thus carried out about surgical margins or safety margins, in the Medline, Lilacs and Cochrane databases, not in the sense of verifying the issue of its extension according to multiple variables, but seeking a well-grounded justification for the immutable permanence of such a concept. It was a surprise not to find any work specifically approaching the issue, namely, the circumstances under which the concepts safety or surgical margins can be applied. This review shows that they relate to a series of usually little discussed factors, which have a decisive importance in its determination, yet are only published in a vague and indirect way in the vast medical literature, as if the unanimity were so strong as to not deserve any questioning. In that sense, the authors believe that this work may even be original, given the scarcity of publications on the matter, reflecting the lack of deep discussion, mainly by surgeons and pathologists. There has not been, to the present date, a specific reasoning scheme about this topic, as approached here. In the consulted literature, another common way to refer to the topic is when discussing the surgical treatment of melanoma. It is precisely there that the issue becomes more controversial. In that sense, the work by Weyers provides a proper critical view.1
Microscopic and macroscopic safety margins and their extension
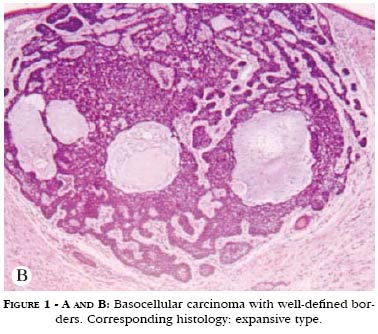
For didactic purposes only, safety margins were analyzed from both macroscopic and microscopic points of view. Macroscopic margin is defined as the piece of apparently normal tissue that the surgeon plans to resect before beginning the surgical act. To delineate it, it is necessary to recognize the borders of the tumor to be resected. In tumors the cells of which form a well-defined block, i.e., those with expansive character, the border between tumor and apparently normal skin can usually be well identified (Figures 1A and 1B). However, in case the tumor has an infiltrative-like histological architecture, its cells spread diffusely throughout the normal tissue, making it much more difficult to recognize borders (Figures 2A and 2B). This situation itself substantially alters surgical planning in relation to the safety margins to be adopted. Whereas in the first situation the adoption of a macroscopic safety margin is quite logical, in the second it is hardly justifiable. How can one delimitate something one does not see?
The second issue is that of the extension of the safety margin. When figures 1A and 1B, described above, is analyzed, as long as the macroscopically visible border of the tumor is not touched, it will be totally resected in most instances. It does not matter, from the oncological standpoint, that the scalpel passed 0.5 cm or 1 cm away from the compact block of malignant cells. The fact is that all tumoral cells were resected, and that is enough. However, from the point of view of surgical technique, the larger the safety margin, the most difficult will the reconstruction become, thus making it more difficult to avoid functional or esthetical sequelae. For instance, it is much harder to resect a neoplasm with well-defined borders located at 2mm from the palpebral border than the same neoplasm located in the center of the maxillary region. Even though some works demonstrate that macroscopic safety margins should have a certain width, a surgeon is seldom going to resect a functionally or esthetically important structure when faced with a tumor with well-defined borders that does not affect those regions. Unrestricted respect to preestablished surgical margins can be a factor causing undesired sequelae. Therefore, not always are the recommendable surgical margins strictly followed in practice.
Considering figures 2A and 2B, described above, determination of a macroscopic safety margin is seriously compromised. Since it is not possible to clinically recognize the borders of the tumor, delineation of the surgical incision is totally uncertain. It would be even harder to justify the resection of an important anatomical structure which is apparently not affected by the tumor. How could the extension of the surgical margin be measured in millimeters or centimeters, if the starting point of delineation is not known? In sum, the concept of safety margin basically depends on the tumor being well-delineated, otherwise it is not justifiable.
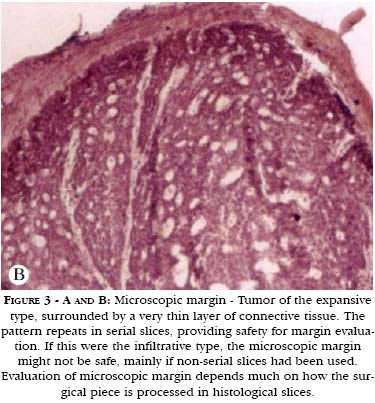
The microscopic safety margin, on the other hand, can be observed most of the time, depending on the way the surgical piece is prepared, regardless if the tumor is clinically well or ill defined. In what concerns extension, the microscopic margin can be minimal. It is enough to verify the existence of normal tissue containing the tumor. However, there is a crucial problem to be overcome. Microscopic vision is very limited. A histological slice is generally 4 to 10 micrometers thick and it likewise offers a microscopic vision of only a few micrometers. As a consequence, in order for the microscopic view to really convey the situation of the surgical margin, laboratorial techniques distinct from routine histological slicing have become necessary. (Figures 3A and 3B).
Routine histopathological examination
From the first years of medical school, it is taught that Clinics is sovereign and that all complementary exams are subordinate to the situation that yielded them. Although pathological anatomy is a medical specialty which is essential for the diagnosis of various diseases, being perhaps the most decisive exams in several of these situations, some more careful pathologists include, as a footnote to their reports, warnings like this: "every anatomopathological examination should be correlated to the clinical picture presented by the patient, without which the interpretation of this result is only relative". Even though this is irrefutable, not every physician realizes its true meaning. In particular, in the verification of surgical margins of tumors, the authors believe that there may be a misunderstanding of the meaning of the expression "free surgical margins".
The vision that a pathologist has of the microscopic surgical margin essentially depends on how the surgical piece was prepared. The first step is to know whether he or she is indeed seeing the border of the surgical piece in the microscope. From the operatory moment it is resected to the beginning of its processing in the laboratory, it is generally put in a flask containing formaldehyde and then transported into the laboratory. Depending on the situation, this may take over a day. A simple badly-done transportation, the lack of care with the specimen when putting it in the flask with formaldehyde - especially if the latter has an opening with a smaller diameter than the surgical piece - may lead to technique artifacts, such as detachment of tissue from the periphery of the piece, which are capable of falsifying the result. If the surgical piece does not arrive intact in the laboratory, the analyses of its border can be compromised, that is, simple care with the surgical specimen is important for result reliability. Obvious as this may seem, it is important to highlight that it is not the physician who handles the surgical piece right after the surgical act and transports it to the laboratory. Excluding this little detail, which is not dependent upon the excellence of the anatomopathological examination, in order to be sure about the real examination of the specimen borders it is necessary to stain its entire external surface.
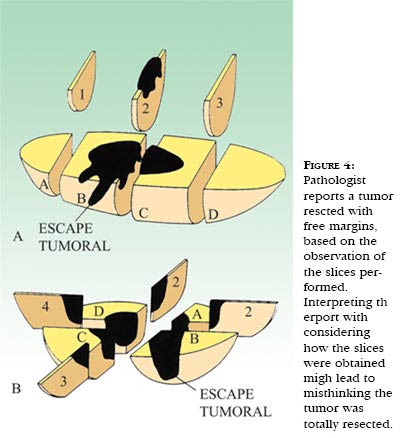
The expression "free surgical margins" signifies to the pathologist that the tumor does not touch the border of the surgical piece in the location the incision was made. And that can only be stated when the pathological anatomy laboratory was careful to stain the external area of the surgical piece. Unfortunately, many pathological anatomy reports do not describe whether the borders were indeed stained, in order to be sure that they were in fact examined. However, the meaning of "free surgical margins" is in general, for many physicians, that the tumor has been entirely resected, which can obviously be untrue (Figure 4).
Other important variables are number and incidence of incisions carried out. Figure 5 depicts some standardized forms routinely used in pathological anatomy laboratories. It is easily verifiable that the incisions do not span the totality of surgical margins, i.e., the pathologist examines slides previously prepared for the microscope, within a predefined laboratorial routine. Unless there are explicit instructions in the surgeon's request to the pathologist, surgical pieces will undergo standard routine preparation. Under certain circumstances, such as infiltrative tumors, the microscopic vision of the pathologist can be very relative in terms of the real evaluation of surgical margin, due to the type of incidence and reduced number of slices.
Analysis of microscopic margin in microscopically controlled surgery
The issue of "controlled surgical margins" is little explored by pathologists and surgeons in general. Scarcity of information about this is surprising, so much so that Rapini, in 1992, after making a review of the literature, concluded that his work was original.2 He described, compared and analyzed the different forms of surgical margin control in pathological anatomy laboratories, thus making a sort of introduction for his critical observations, later published, about micrographical surgery. These are apparently obvious facts that are easily understood, although little analyzed. Micrographical surgery is, by definition, a "microscopically controlled surgery". This expression should refer only to procedures that control de facto virtually the totality of surgical margins, as initially conceived by Frederic Mohls, creator of the technique.
Micrographical surgery is not a sampling surgery, i.e., a procedure based only in a few frozen slices. Even though the piece arrives at the laboratory properly guided by a mark or drawing, and the pathologist is careful enough to stain its external portion, sampling surgery, made with frozen slices, has a great probability of failure, for the only guarantee that a pathologist may provide is that in that place where the incision was made, tumor touched or not the border of the piece. Further information regarding the rest of the surgical margins cannot be provided because only a few slices spanning all the extension of the piece were examined. Based on this correct, albeit limited by its reduced span, information given by the pathologist, surgical decisions regarding continuing or interruption of surgical exeresis could be precipitated.
Some time ago, when reviewing these concepts, the authors found a work in the literature about "disappearance of residual malignant cells", described by Goldwyn and Kasdon.3 These authors highlighted the routine situation with which surgeons were faced when receiving a histopathological report indicating that the surgical margins of their surgeries had been compromised. They immediately submitted their patients to surgical widening, and the corresponding histopathological report did not reveal the presence of residual tumor. Goldwyn and Kasdon commented about not understanding this apparent paradox and raised some hypotheses, better studied nowadays, concerning post-surgical tumoral involution. More recent works demonstrate that this does not occur as often as commonly supposed.4 However, the existence of an easily identifiable explanation for this phenomenon is suspected.
Goldwyn and Kasdon3 propose a solution for the problem in their own work, but they prefer not to ask questions concerning the obvious. They comment on the possibility that technical problems in the preparation, slicing and setting of the surgical piece could explain this fact, but consider this unlikely. Conversely, the authors believe this may occur with a higher than expected frequency.
In the routine of many pathological anatomy laboratories, those who process the material to be examined are very often technicians or medical students. Unfortunately, not always can the pathologist dedicate time to routine work, that is, in case there is no exact information about the surgical piece topography in relation to the operated site in the patient, and the pathologist has not been properly informed and instructed in due time, the analysis of surgical margins can be harmed beyond remediation. Specimens usually undergo standard preparation, to which the laboratory is accustomed. However, this procedure might not correspond to the level required for a perfect preparation of the piece. It would necessary for the surgeon to detail very clearly his goal; otherwise a routine and standardized examination will be performed. Hence, it is not enough for the surgeon to refer his specimen to a pathologist by simply requesting "a surgical margins study". In case the latter has not been adequately warned and guided, little will he be able to do after the surgical piece has been transformed in histological slices ready to be examined under the microscope. Although they are experts and skillful in the confection of slides, not to mention being indispensable medical auxiliaries, technicians not always have a proper view to understand the importance of their jobs. Slight technical problems are likely to greatly interfere in the accuracy of microscopic control of margins, and not always does a technician really understand the difficulty of the problem.5-11
In the specific case of "disappearance of residual malignant cells" one of the alternatives below is likely to have happened:
a) False positivity of the examination of surgical piece's margins. This depends on the multiple slicing incidences, incorrect inclusion of part of the surgical material - which might have detached from the main piece in this stage of specimen preparation - and also an initial wearing of the block until the technician collects the best slice to be put in the slide. Only the latter situation, which is extremely common, could explain the presence of tumor in the margins, as a false positive result, in the case of examination of peripheral slices. In one of the cases described by Goldwin and Kasdon,3 a surgical piece originated from a shaving procedure and possibly healed the patient. However, the pathologist, having seen minimal microscopic margins, preferred to report compromised margins, especially if the tumor did not exhibit a very typical expansive pattern or if it were infiltrative. In situations like this, judging between free or compromised margins, even microscopic, depends on more than one slice. A frequent situation in micrographical surgery occurs when, using a three-dimensional micrographic method such as the Munich Method,5 perceiving the absence of visible tumor in the last stage, one understands that this happened because there was no technical safety to state that the margin had been free in the previous stage. Refer again to figures 3A and 3B. If a pathologist had seen only that narrow band of connective tissue, but the tumor were sclerodermiform, he or she would be unsafe to state the margin were free, unless numerous serial slices very close to each other had been made, still, it would be safer to overestimate. In the case of an expansive tumor, as exemplified in figures 3A and 3B, once he or she has observed serial slices, they would be more secure to report a free margin. Every technique has limitations, and, in extreme situations, performing more than one stage of micrographical surgery is preferable to having a later relapse. The same could occur in routine histopathological examinations.
b) The re-resection had not been made in the exact location of the residual tumor. In that case, the smaller the residual tumoral mass and depending on the histological type of the tumor under operation, the greater will be the difficulty to find the residual tumor again. A wide re-resection also has, in this situation, an inconvenient: by enlarging the widening area, the amount of tissue to be examined also increases significantly. Unless all surgical material is actually worn out with an absurd number of serial slices with practically no waste of material, the residual tumor will hardly be found, especially when the tumoral mass is minimal. It is like looking for a needle in a haystack. If the issue is really the verification of surgical margins, then also in the patient exeresis situation should remain unchanged until the examination result were given. In that sense, the freezing test, because of its quickness, can be more practical. However, as already mentioned, if it is done by sampling, it might not be enough. In case the reconstruction has occurred before the anamopathological examination is ready, getting back exactly to the site where there could be a residual tumor seems to be more a matter of luck than of logics, in particular, if the surgical wound was closed primarily or by use of grafting. Compensating borders for a better coaptation, as well as mobilizing surrounding tissues, and grafts, can substantially modify local topography, to the point of making it hard to locate sites supposedly involved by tumoral invasion. In case of grafting, the local architecture could be maintained, but it is one of the worst options, aesthetically speaking.
c) Finally, Goldwyn's and Kasdon's3 do not mention whether the margins were stained in the three reported cases. This fact alone, as already mentioned, can be decisive for the judgment of the compromising or not of surgical margins.
To sum it up, the specifically technical aspect of histological slices preparation should be better understood. Otherwise, the report given by the pathologist may lead to erroneous and distorted interpretations.
In microscopically controlled surgeries (micrographic surgery), both surgical exeresis and inclusion of the piece, including slicing and eventually microscopic analysis, depend on a perfect technique for handling the specimen. In this way, tissue fragments considered minimal form the microscopic standpoint can contain portions of tumor or even healthy tissue, which could modify final judgment concerning safety margins. These details attain a singular importance for micrographical surgery, to the point of justifying opinions that advocate that the surgeon, the technician handling the surgical piece and the pathologist should all be the same person.5 Clearly, this does not occur most of the time, although the micrographical surgeon is actually trained to perform these three functions at the same time. If this were not so, his or her training would be incomplete. If micrographical surgery is performed by a team, a perfect rapport among the surgeon (who should know how to supervise the entire process), the technician and the pathologist is an indispensable condition so that the rigor of the technical procedure is maintained. Only under these conditions is it possible to advance surgical excision in the exact sites that are proved to be affected by tumoral invasion. Thus, the microscopic exam becomes the controller of surgical resection.
Consequences of the misinterpretation of surgical margins
Misinterpretation of the situation of a tumor in relation to the borders of the surgical piece can lead to the emergence of misconceptions surrounding the damage capacity of skin tumors, particularly of basocellular carcinomas.
The most common cancer in humans is basocellular carcinoma. Even though there are about 300 cases described in the literature about metastases of these tumors, total incidence of this phenomenon is no higher than 0.5%.4 Nevertheless, it is not uncommon to attribute to this tumor a high local aggressiveness, when constant relapses dominate the clinical picture. Basocellular carcinoma is definitely not a cancer that may be considered as aggressive. Its cells have no autonomy, needing the presence of stroma to support themselves. These tumors have even been classified as epitheliomas instead of true carcinomas. Basocellular carcinoma happens to have several infiltrative forms. Among them, the sclerodermiform type is a typical example. It is a tumor with an initially expansive growth that can, however, acquire infiltrative chacteristics. When this occurs, clinically visible borders vanish to the point of making their identification practically impossible. This makes the indication for a controlled micrographical surgery mandatory. Otherwise, successive partial resection surgeries for a single infiltrative tumor can lead to the appearance of two or three smaller sites of residual tumor growth, separated by scar tissue or grafts. Even worse is the permanence of tumor on the floor of the surgical resection. Grafts in particular tend to bury tumoral residues, which can grow unnoticed for a long time, making it difficult to immediately recognize a relapse. Particularly in young persons, successive relapses can determine bad prognosis for the life to come.
These facts, frequently seen by professionals involved in the treatment of skin tumors, are far from being explained by tumor aggressiveness. Relapse is believed to depend mainly on tumoral residues remnant of surgical therapeutics,12 i.e., the non-utilization of microscopically controlled surgery, when its indication is imperative, is perhaps the main contributing factor for the transformation of an initially good prognosis case into one of difficult control or with severe sequelae.
Another important aspect that should be emphasized is the routine situation of early indication for radiation therapy. Indication of radiation therapy is not uncommon when a relapse occurs after anatomopathological examination of the surgical piece has revealed surgical margins free of neoplasia. The most feasible explanation for this very common phenomenon is the presence of tumoral residues not identified on routine histopathological examination, and not an exaggerated aggressiveness of the tumor, demanding adjuvant radiation therapy.
The relative concept of oncological radicalism
What is the concept of operating with oncological radicalism based on? The discussion of this article is precisely about the search for a necessary questioning of the concept of safety margin, with the previously mentioned statements. Hence, how not to challenge the expression oncological radicalism? It is possibly used as a kind of superlative safety margin. Tumors of real biological aggressiveness are not in question here, neither are those with a local-regional metastizing capacity, or those disseminated beyond lymph node chains, commonly seen in the head and neck region. Large head and neck oncological surgeries maybe were the reason why the expression oncological radicalism was forged. The bad prognosis of a regionally disseminated adenocarcinoma is generally known. Many times, not even oncological radicalism is capable of stopping the course of the disease. Radiation therapy is a valuable aid in these case. This is so because it is not possible to visualize cellular dissemination unless it appears as a macroscopic anatomic alteration. In that sense, maybe the expression oncological radicalism is justifiable, meaning block resection of the main tumor along with likely cellular dissemination in the surroundings, also resecting all lymphatic dissemination paths. These are undoubtedly truly radical surgeries.
Except for the cases of skin tumors with a loco-regional dissemination, the expression oncological radicalism becomes questionable. It does not apply, for instance, to well delimited primary nodular basocellular carcinomas. As already said, there is no reason for widened surgeries to be performed in these situations. In relapsed, infiltrative tumors, or those with clinical picture revealing difficulty for tumoral border delineation, radicalism is likewise not justifiable without proper confirmation of tumoral invasion.
As already commented, microscopic tumoral invasion alters little the macroscopy of anatomical structures neighboring the tumor. Take as an example a relapsed sclerodermiform basocellular carcinoma, situated 1 cm away from the inferior palpebral border. Clinically, one cannot visualize any functional or anatomical alteration in the lid. How could oncological radicalism justify an exeresis of one third of the lid, including tarsus, if there were no evident signs of compromising? By safety margin? In this situation, good sense would probably mandate a less radical exeresis, sparing what apparently is not affected. Blindly obeying the concept of radicalism can determine unnecessary sequelae. Once more, micrographical surgery could be an indispensable weapon to solve this dilemma. In case it is proved that the lid is compromised, the entire extension of this compromising will be revealed and extirpated, even if nothing is noticeable clinically. In case there were no sign of microscopic invasion of the lid, it would be spared, yet the tumor would be totally extirpated. Oncological radicalism, therefore, is not necessarily a synonym for microscopic radicalism, the latter being more rational when dealing with tumors of low biological aggressiveness. Microscopically controlled surgeries can be, at the same time, both radical (when resecting important anatomical structures which are supposedly not compromised) and sparing of anatomical structures located near the tumor.
Extensive or small tumors and micrographical surgery
Every extensive tumor was small one day. Without a doubt, the two extremes demand surgical acts of opposed magnitudes, but it cannot be forgotten that a 2.5 cm basocellular carcinoma located in the center of the malar region can be more easily extirpated than a 1 cm one located 2 mm away from the lacrimal caruncule - hence, size, albeit important, is not the main factor to be considered for the indication of a micrographical surgery. A tumor, apparently measuring 1 cm of diameter, but with no well-delimited borders and with an infiltrative histological type, as well as an even smaller tumor, this time at, say, 1 mm from the lacrimal carbuncule, both are indications for micrographical surgery. In these instances, if relapsed, they can become extensive.
Speaking of that, very extensive tumors, which affect anatomical structures by entirely destroying them, are not the main indications for using the method. Even if there is time and technical availability - meaning longer hospitalization and much time available for the microscopic control of margins - the possibilities of technical flaws increase proportionally. This does not mean that extensive tumors cannot be operated by means of accurate micrographical surgery. However, if the logics and precise indications of this method were largely practiced, there would doubtlessly be no need to operate extensive tumors. Neoplasms which relapsed once or twice are on their way to difficult control.
Final comments
A more substantial questioning is urged for conducts and surgical practice rules that use the concept of macroscopic safety margin as a justification for their acts. This work offers a critical view on this issue and suggests that, in what concerns skin tumors which are not yet disseminated to the point of making microscopic mapping difficult or technically unfeasible, the adoption of a microscopic margin would be more rational. q
REFERENCES
Received on December 14, 2004.
Approved by the Editorial Council and accepted for publication on May 17, 2005.
- 1. Weyers W. Excision of melanoma in historical perspective: triumph of irrationality for nearly a century. Dermatopathology: practical & conceptual 1997;3:238-46.
- 2. Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990;23:288-94.
- 3. Goldwyn RM, Kasdon EJ. The "disappearance" of residual basal cell carcinoma of the skin. Ann Plast Surg. 1978;1:286-9.
- 4. Kopke LFF, Schmidt SM. Carcinoma Basocelular. An Bras Dermatol. 2002; 77:249-85.
- 5. Kopke LFF , Konz B. As diferenças fundamentais entre as variaçőes da cirurgia micrográfica. An Bras Dermatol. 1994;69: 505-10.
- 6. Rapini RP. On the definition of Mohs surgery and how it determines appropriate surgical margins. Arch Dermatol. 1992;128:673-8.
- 7. Rapini RP. Pitfalls of Mohs micrographic surgery. J Am Acad Dermatol. 1990;22:681-6.
- 8. Dzubow LM. Chemosurgical report: recurrence (persistence) of tumor following excision by Mohs surgery. J Dermatol Surg Oncol. 1987;13:27-30.
- 9. Dzubow LM. False-negative tumor free margins following Mohs surgery. J Dermatol Surg Oncol. 1988;14:600-2.
- 10. Peterson DA, Davies JD, McLaren KM. Failure to demonstrate the true resection margins of excised skin tumors: a case for routine marking. Br J Dermatol. 1992;127:19-21.
- 11. Hruza GJ. Mohs micrographic surgery local recurrences. J Dermatol Surg Oncol. 1994;20:573-7.
- 12. Tritsch H. Das Wachstumsverhalten der Basaliome. In: Eichmann F, Schnyder UW. editors. Das Basaliom Derhäufigste Tumor der Haut. Berlin Heidelberg New York: Springer-Verlag; 1981. p.55-62.
Publication Dates
-
Publication in this collection
03 Nov 2005 -
Date of issue
June 2005
History
-
Received
14 Dec 2004 -
Accepted
17 May 2005