Abstracts
BACKGROUND: Leprosy remains a public health problem. Episodes of erythema nodosum leprosum (ENL) are acute events that occur before, during and after polychemotherapy. In the last decade, the use of thalidomide as an immunomodulating agent was expanded to other diseases. OBJECTIVES: To perform a systematic review of published clinical trials on efficacy and side effects of thalidomide in ENL. To describe the methodology and screening results of recruiting for a clinical trial performed in Brazil, which aimed to assess the dose-response of thalidomide followed by tapering regimen in severe and moderate cases of ENL. METHODS: Published clinical trials on the use of thalidomide in ENL were analyzed. A randomized, double-blind clinical trial was designed to evaluate the doses of 100mg versus 300mg/day thalidomide during the acute stage of ENL, followed by thalidomide tapering regimen versus placebo. For this clinical trial, the methodology and data for enrollment of patients were described, with an emphasis on severity of ENL episodes. RESULTS: Six clinical trials published in the 1960's and 1970's indicated the benefits of thalidomide in ENL, although methodological differences made comparison difficult. In the enrollment stage of the Brazilian trial, 65% of patients were potentially eligible out of 143 ENL patients screened. The association with neuritis in 56.4% of moderate and severe cases of ENL required the co-intervention with steroids. CONCLUSION: The patients' enrollment pattern demonstrated high frequency of neuritis in ENL episodes. The treatment regimen with thalidomide in monotherapy for ENL was considered infrequent in the clinical practice in Brazil. The current challenge is to accumulate evidence about efficacy and side effects of thalidomide in combination with steroids.
Erythema nodosum; Leprosy; Neuritis; Thalidomide; Clinical Trial
FUNDAMENTOS: A hanseníase persiste como problema de saúde pública, e episódios de ENH são eventos agudos que ocorrem antes, durante e após PQT. Na última década, o uso da talidomida como agente imunomodulador foi expandido a outras doenças. OBJETIVOS: realizar revisão sistemática dos ensaios clínicos publicados sobre a eficácia e efeitos colaterais da talidomida no ENH. Descrever metodologia e resultados da triagem para recrutamento de ensaio clínico visando avaliar dose-resposta da talidomida seguida de desmame no ENH moderado e grave, realizado no Brasil. MÉTODOS: Analisaram-se ensaios publicados sobre talidomida no ENH. Foi delineado um ensaio clínico duplo-cego randomizado para avaliar dose de 100 thalid 300mg/dia de talidomida durante fase aguda de ENH, seguida de desmame da talidomida, thalid placebo. Para este ensaio clínico descreve-se metodologia e dados de recrutamento de pacientes, com ênfase na gravidade dos episódios de ENH. RESULTADOS: Os seis ensaios clínicos publicados nas décadas de 1960 e 1970 apontam para o benefício da talidomida no ENH, embora diferenças metodológicas dificultem a comparação. Na fase de recrutamento do ensaio brasileiro, dos 143 pacientes de ENH triados, 65% eram potencialmente elegíveis. A associação com neurite em 56,4% dos ENH moderados e graves exigiu co-intervenção com corticosteróide. CONCLUSÃO: O padrão de recrutamento dos pacientes evidenciou alta freqüência de neurite nos episódios de ENH. O esquema de talidomida isolada no ENH foi avaliado como infreqüente na prática clínica brasileira. O desafio atual é acumular evidências sobre a eficácia e efeitos colaterais da talidomida em associação com corticosteróides.
Eritema nodoso; Hanseníase; Neurite; Talidomida; Ensaio clínico
REVIEW ARTICLE
Thalidomide in the treatment of erythema nodosum leprosum (ENL): systematic review of clinical trials and prospects of new investigations* * This study was performed at the Núcleo de Medicina Tropical da Universidade de Brasília, the Instituto de Patologia Tropical e Saúde Púbica da Universidade Federal de Goiás (UFG) - Goiânia (GO), at the Fundação Alfredo da Matta - Manaus (AM) and at the Hospital de Doenças Tropicais de Goiânia (GO), Brazil.
Gerson Oliveira PennaI; Celina M. T. MartelliII; Mariane M. A. StefaniII; Vanize O. MacedoIII; Maria de Fátima MarojaIV; Aiçar ChaulV
IPhysician, Ph.D. Associate Researcher, Núcleo de Medicina Tropical - Universidade de Brasília (UnB) - Brasília (DF), Brazil
IIPh.D., Professor at the Instituto de Patologia Tropical e Saúde Pública - Universidade Federal de Goiás (UFG) - Goiânia (GO), Brazil
IIIPh.D., Professor at the Núcleo de Medicina Tropical - Universidade de Brasília (UnB) - Brasília (DF), Brazil
IVPhysician - Fundação Alfredo da Matta - Amazonas (AM), Brazil
VPhysician - Professor do Instituto de Patologia Tropical e Saúde Púbica - Universidade Federal de Goiás (UFG) - Goiânia (GO), Brazil
Correspondence Correspondence Gerson Oliveira Penna SQN 112 Bloco J aptº 307 70762-100 - Brasília - DF Tel: (61) 274-4941 / Fax: (61) 340-1670 E-mail: gpenna@unb.br or gpenna@terra.com.br
ABSTRACT
BACKGROUND: Leprosy remains a public health problem. Episodes of erythema nodosum leprosum (ENL) are acute events that occur before, during and after polychemotherapy. In the last decade, the use of thalidomide as an immunomodulating agent was expanded to other diseases.
OBJECTIVES: To perform a systematic review of published clinical trials on efficacy and side effects of thalidomide in ENL. To describe the methodology and screening results of recruiting for a clinical trial performed in Brazil, which aimed to assess the dose-response of thalidomide followed by tapering regimen in severe and moderate cases of ENL.
METHODS: ublished clinical trials on the use of thalidomide in ENL were analyzed. A randomized, double-blind clinical trial was designed to evaluate the doses of 100mg versus 300mg/day thalidomide during the acute stage of ENL, followed by thalidomide tapering regimen versus placebo. For this clinical trial, the methodology and data for enrollment of patients were described, with an emphasis on severity of ENL episodes.
RESULTS: Six clinical trials published in the 1960's and 1970's indicated the benefits of thalidomide in ENL, although methodological differences made comparison difficult. In the enrollment stage of the Brazilian trial, 65% of patients were potentially eligible out of 143 ENL patients screened. The association with neuritis in 56.4% of moderate and severe cases of ENL required the co-intervention with steroids.
CONCLUSION: The patients' enrollment pattern demonstrated high frequency of neuritis in ENL episodes. The treatment regimen with thalidomide in monotherapy for ENL was considered infrequent in the clinical practice in Brazil. The current challenge is to accumulate evidence about efficacy and side effects of thalidomide in combination with steroids.
Keywords: Erythema nodosum; Leprosy; Neuritis; Thalidomide; Clinical Trial [Publication Type].
INTRODUCTION
In Brazil, leprosy remains a public health problem, with increasing detection coefficients in the last decade and a prevalence of 4.4/10000 inhabitants, in 2003; therefore, this prevalence is higher than one case/10000 inhabitants, which is the goal in public health.1-7 There is a spectrum of clinical forms, including polar, tuberculoid and lepromatous, as well as intermediary forms, which are called borderline and determined by the patient immune response. Type 1 and 2 reactions are acute inflammatory events that occur during the chronic course of the disease,8,9 and may affect the skin and nerves due to tropism of Mycobacterium leprae for Schwann cells and macrophages.10,11
The most frequently reported type 2 reaction clinical manifestation is erythema nodosum leprosum (ENL), which may occur before, during or after chemiotherapeutic treatment, mainly in lepromatous patients.12 ENL is commonly associated with systemic symptoms and it constitutes a medical emergency with immediate need for anti-inflammatory and immunomodulating drug due to its potential to cause disabilities. The disabilities are a consequence of neural damage with decreased peripheral nerve function because of motor and sensory loss.13-15 In a previous publication, the epidemiological, clinical and immunopathological aspects of ENL were addressed, and the Brazilian recommendation for the use of thalidomide (a-N-pthali-midoglutarimide) as the drug of choice to treat ENL was discussed. The use of steroids is mandatory when there is associated neural involvement, reactional lesions on hands and feet, neuritis, iritis, iridocyclitis, orchitis, nephritis and necrotizing ENL.16,17 Nowadays, the use of thalidomide in women at child-bearing age is regulated by Brazilian Law No. 10,651, of April 16, 2003.
Thalidomide was synthesized in Germany, in 1954, from the glutamic acid, to be used as a sedative and hypnotic antiemetic drug, indicated to treat morning sickness in the first trimester of gestation. It was marketed in 46 countries, except in North America. The first cases of congenital malformations associated with the use of thalidomide during pregnancy were described in Germany, United Kingdom and Australia in the beginning of the 1960's. The drug was withdrawn from the market in 1962, when over 10000 cases of congenital defects associated with its use had already occurred all over the world.18,19 Almost 40 years after its prohibition in the international market, thalidomide reemerges as a powerful agent for various clinical autoimmune and inflammatory situations.20 It is now used in AIDS, graft versus host disease (GVHD), rheumatoid arthritis, lupus and multiple myeloma, among other conditions.16,21,22 The antiangiogenic potential of thalidomide was described in the 1990's, opening he possibility to potentially use it in the treatment of several types of cancer. The drug theratogenic mechanism is partially known and it apparently involves the inhibition of angiogenesis and/or generation of free radicals that would lead to oxidative damage in DNA.20,23,24
The mechanism of anti-inflammatory action of thalidomide involves the inhibition of selective gene expression of TNF-a and, consequently, of its functions.24-28 TNF-a is a potent proinflammatory cytokine capable of immunostimulating pleiotropic effects, thus producing beneficial or deleterious results depending on the amount and time of its production. The inhibitory effect seems to be related to higher messenger RNA(mRNA) degradation rate into TNF-a.29-31 TNF-a has been involved in pathogenesis of neural damage in leprosy.25
The therapeutic potential of thalidomide in Dermatology was shown in 1963, based on a case report of Hyde's nodular prurigo, published only in 1973.32 After that, Jacob Sheskin, an Israeli dermatologist, showed the efficacy of this drug in treating ENL cases.33 Later, the clinical benefit in ENL was demonstrated in Brazil.34 A clinical trial published in 1971 and sponsored by the WHO evaluated the efficacy of thalidomide versus acetylsalicylic acid in the treatment of ENL, with study sites in India, Spain, Mali and Somalia.35 There are six different trials as a group and their results point out the beneficial use of thalidomide in ENL, although the scientific evidences are difficult to be evaluated due to the diverse methodologies employed, the criteria to define cases, the measurements of effects and co-interventions.
In the 1960's, the use of thalidomide was regulated by the Brazilian Ministry of Health (MoH) and, more recently, by the National Agency for Health Surveillance (ANVISA).36 Until the end of last decade, Brazil was the only manufacturer of thalidomide in a commercial scale in order to meet the demand for the National Leprosy Control Program (LCP). It is estimated that between 1965 and 2001 about 91000 patients with ENL may have received thalidomide supplied by the LCP (personal communication, 2002). In the last five years, the Ministry of Health bought approximately four million thalidomide capsules per year, manufactured by a national public laboratory, for the treatment of ENL. The official recommendation of thalidomide for treating ENL varies from 100 to 400 mg/day; the dose is adjusted according to severity of the clinical picture and there is no indication for tapering in the official regulations. This is the largest use of thalidomide in a public health service in the world, considering that Brazil is the only endemic country for leprosy that has this drug available. Despite its extensive use, there is no official record about severity characteristics of ENL or the combination of thalidomide and steroid. The World Health Organization does not include thalidomide among the drugs recommended to treat ENL.
Under pressure due to illegal trade of thalidomide in the USA for use in AIDS patients, the U. S. Food and Drug Administration (FDA) registered the thalidomide manufactured in the USA (Thalomid®), in 1998, for use in ENL.20,37 Before this registration for commercialization, a randomized clinical trial, sponsored by the American pharmaceutical industry Celgene, was initiated in the Philippines, in 1996, to evaluate the use of two treatment regimens, 100mg versus 300mg/kg/day for 7 days to treat ENL,38 In 2000, the authors were contacted by the industry to evaluate the possibility of discussing a protocol in Brazil, in order to compare the safety and efficacy of two drug regimens with thalidomide (Thalomid®) in the treatment of ENL. The present article contains a systematic review of clinical trials on thalidomide in the treatment of ENL, with a summary of the evidences published. It emphasizes design, methodological questions, eligibility criteria, screening data for inclusion, as well as aspects in the preparation and conduction of field39 regarding the clinical trial of 100 and 300mg/day doses of thalidomide to treat moderate and severe ENL.
CLINICAL TRIALS WITH THALIDOMIDE: A SYSTEMATIC REVIEW OF THE LITERATURE
A systematic review of the literature was carried out based on data from Medline, Cochrane and Lilacs, from January 1966 to October 2004, using the following keywords: Trial, thalidomide, Erythema Nodosum Leprosum, ENL, Leprosy, Neuropathy, Nerve function Impairment e NFI.
A total of 82 articles were identified comprising all publications from Medline containing the combination of the keywords trial and thalidomide in different diseases, such as cancer, AIDS, GVHD, rheumatoid arthritis, Behcet's syndrome, multiple myeloma, and others. Among the 82 clinical trials, 63 (77%) were published in the last 8 years, showing increased investments in research in order to expand the therapeutic use of thalidomide to other specialties in addition to Dermatology. In the Cochrane database, only two reviews were retrieved between 1999 and 2002, emphasizing the use of thalidomide in Behcet's syndrome40 and toxic epidermal necrosis.41 In the Lilacs database, no publication was retrieved by the selected keywords.
Regarding the use of thalidomide in ENL, six clinical trials were published in the last four decades with sample size ranging from 10 to 92 patients, and a total of 262 cases of ENL (Chart 1). A short-term, double-blind clinical trial, coordinated and sponsored by the World Health Organization (WHO), compared thalidomide with acetylsalicylic acid in the treatment of ENL.35 In three clinical trials with thalidomide, the control group received placebo.42-45 In one trial, clofazimine was administered to the control group.45 In an open, uncontrolled trial, all patients received steroid, and an internal comparison was made between the groups with and without thalidomide.46
In these trials, the dosage ranged from 300 to 400 mg/day; only one study46 met the requirement of supervised intake of the medication for efficacy purposes. In three trials the sample size was larger than 50 patients and males were the vast majority of those enrolled. The eligibility criteria were subjective and the selected patients presented different clinical pictures, including "moderately severe" ENL with or without neuritis, erythema multiforme, orchitis, iritis, iridocyclitis and skin ulcers. This heterogeneity in the definition of eligible case among the studies hinders comparison of results. The concomitant use of steroids, paracetamol, anti-histamines and antimonial drugs clearly represents co-intervention in the trials. These other drugs, known for having immunomodulating and/or inflammatory effects, lead to improved clinical manifestations of ENL; therefore, it is impossible to distinguish if therapeutical efficacy observed could be solely attributed to thalidomide. Treatment with thalidomide was considered well tolerated, with no significant side effects and no report of peripheral neuropathy caused by the drug. Additionally, the methodological differences of the trials, especially regarding eligibility criteria for patients, make it impossible to perform a meta-analysis. It is important to mention that all published clinical trials were conducted before introduction of multidrogatherpy (MDT).
In the era of evidence-based medicine, randomized and controlled clinical trials are the design of choice to establish the efficacy and safety of interventions for use of drugs in human experiments.47-52 As to the ethical aspects in these experiments, investigations in general, and particularly the experimental designs involving humans, are subject to national and international laws about research ethics.53-55 According to current recommendations,56,57 the published clinical trials about thalidomide in the treatment of ENL do not meet the quality criteria established for reporting this type of experimental design.57 Thus, the six trials analyzed could be classified as level B of scientific evidence, i.e., experimental studies of lesser?? consistency. However, these trials conducted in the 1960's and 1970's were fundamental in the process of reutilization of thalidomide as a beneficial drug, and in fact preceding the description of its immunomodulating action, in the 1990's.25
Several reviews about thalidomide have been published in various fields, including (a) immunology, biology and molecular genetics,31,58-66 (b) pharmacology,67-69 ((c) clinical aspects, therapy,70-74 (d) different diseases,75-92 (e) teratogenicity93,94,74 and (f) analogous drugs.24 The discussion of these articles is not in the scope of this study. Despite the extensive literature available, the present study is the first systematic review of clinical trials with thalidomide in the treatment of ENL, in conformity with the current methodological recommendations.95
An assessment of the publications about clinical trials with thalidomide for the treatment of ENL shows many questions that remain as gaps in knowledge about the drug, which justifies the need to conduct new randomized and double-blind clinical trials. Some of these gaps are management of patients in the acute stage of ENL, therapeutic dose and recommended length of treatment to achieve clinical efficacy. In addition to these gaps, there is the need or not to perform tapering regimen similarly to what is done with steroids. It is still necessary to evaluate the effect of the concomitant use of thalidomide and steroids with potential synergism of action. Another relevant issue is an objective evaluation of the main side effects caused by thalidomide, such as peripheral neuropathy, particularly in the treatment of ENL, and somnolence, as well as the influence of the dose and duration of treatment in occurrence of adverse effects.
Under these assumptions, the clinical trial named "Controlled study about safety, efficacy and comparison of thalidomide doses administered for 2 weeks in the treatment of erythema nodosum leprosus (ENL)" was designed, aiming to fill some of the existing gaps about the use of thalidomide in ENL, as discussed above.
PATIENTS AND METHODS
Methodological issues of the clinical trial with two doses of thalidomide in the treatment of ENL
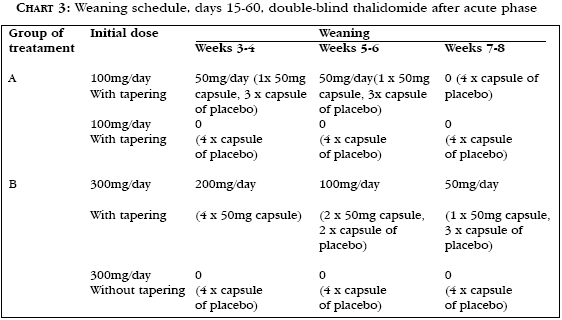
The main purpose of the protocol was to evaluate the dose-response of thalidomide used in the acute treatment of moderate and severe skin manifestations of ENL (Charts 2 and 3). Safety and efficacy are assessed in doses of 100mg/day and 300mg/day, daily administered for 14 days (Chart 2). The secondary objectives were to compare drug withdrawal with and without tapering regimen, and the endpoint was the time to relapse of ENL. The trial also included a meticulous and standardized evaluation of peripheral neuropathy in the pre-, during and post-intervention stages. This evaluation, in addition to clinical examinations, included a complementary test that is objectively carried out with the use of an American instrument called Vibraton II, which quantifies the perception of the patient's uptake of electrical impulses in the halluces (right and left) and in the forefingers (right and left). The endpoint is defined as objective improvement or worsening of the condition. Complete or partial clinical resolution or treatment failure is categorized by the number of skin lesions during acute treatment, and the complete response to treatment is defined as absence of acute erythematous and/or painful inflammatory lesions; the partial response is classified as a reduction by 50 to 90% in number of lesions; and treatment failure corresponds to reduction by less than 50% or no change in number of acute lesions.
As to tapering regimen of thalidomide as of the 14th day, the patients who presented complete response to treatment (Complete Response) should be submitted to a double-blind study with tapering regimen according to the description in chart 3. During this stage, patients would receive four capsules per day. Patients who required continued treatment, i.e., those who presented partial response or treatment failure, would participate in the extension protocol with known treatment. There is scarce literature about the need of thalidomide tapering regimen. One of the scientifically applicable hypothesis is that genetically prone patients, such as those presenting reactional lesions, would require a "maintenance dose" to maintain TNF-a levels very low, so that they are unable to cause any clinical repercussion.28,31,64
Study design
It is a double-blind, randomized study comparing groups with fixed doses of the drug. Patients with moderate and severe erythema nodosum leprosum (ENL) were randomized to one of the treatment groups. Randomization was performed in such a way that patients received 100 mg/day thalidomide for 14 days in the acute stage of treatment, followed without a tapering regimen period (placebo) or with a tapering regimen period (thalidomide) for 4 weeks, or 300mg/day for 14 days followed without a tapering regimen period (placebo) or with a tapering regimen period (thalidomide) for 6 weeks (Figure 1). The treatment regimens proposed in the acute stage of treatment (days 1-14) and in the tapering regimen (days 15-60), for maintaining the double-blind status, are described in chart 3 and table 1. Clinical monitoring of all participants was programmed for 12 months in order to assess relapses of ENL.
Ethical considerations
The project was adjusted to the national scientific and ethical requirements, with a research agreement signed by Celgene and Núcleo de Medicina Tropical da Universidade de Brasília, assuring independence in the performance and presentation of the research results. The clinical trial was approved by the Research Ethics Committee (RHC) at each enrollment site (Fundação Alfredo da Matta - AM and Faculdade de Medicina da Universidade Federal de Goiás - GO), by Conep, US FDA and by Anvisa for import of the drug.
As requested by the US FDA, the enrolled patients received condoms free of charge and they were instructed to wear them while taking the medication and up to four weeks after discontinuation of the drug.74 The presence of thalidomide in the semen of few patients being treated for HIV, as demonstrated in recent studies, was the basis for this recommendation,74 although this advice is not part of the Brazilian routine regarding the use of thalidomide.
Eligibility and enrollment criteria
Male patients aged 18-65 years and suffering from borderline leprosy (BL), lepromatous leprosy (LL) and skin lesions typical of ENL were enrolled. The patients were assigned according to clinical criterion with histological confirmation. Severity was objectively classified by means of number of skin lesions and evaluation of the systemic involvement as follows: moderate (10 to 20 ENL nodules per body segment) or severe (more than 20 ENL nodules per body segment involved), as proposed by Penna et al., in Guerra et al., in 2002.17
The exclusion criteria were: females; mild skin lesions (fewer than 10 ENL nodules per body segment involved) which were treatable with aspirin or non-steroidal anti-inflammatory drugs (NSAID); severe lesions causing death risk; neuritis requiring steroid treatment; use of thalidomide in the last 30 days or previous intolerance to thalidomide; use of clofazimine in a dose higher than 50mg/day in the last month; use of steroids in the last two weeks; concurrent use of medicine that may potentialize somnolence; other associated diseases (HIV+). Additionally, since the study's primary endpoint was to evaluate the efficacy of thalidomide dose administered in the acute stage of ENL, a minimum washout period was established for the steroid, since it could interfere in the analysis of efficacy of thalidomide, resulting in co-intervention. For clofazimine, the standardized dosage schedule of the MDT standard regimen was considered acceptable.
The enrollment target was 180 patients (90 patients in each group) according to the eligibility parameters described above, for the period of one year. This sample size was considered enough to detect a difference of 20% in cure between the group treated with 300mg thalidomide/day (80%) and the group treated with 100mg thalidomide/day (60%), with a statistical power of 80% and alpha error of 0.05.
The recruitment sites were located in Goiânia and Manaus, both cities highly endemic for leprosy. The first recruitment site was Goiânia, at the Hospital de Doenças Tropicais e Centro de Referência em Diagnóstico e Terapêutica.
RESULTS
From June to October 2001, seven patients met the eligibility criteria and were included in the study in Goiânia. Although the initial forecast for recruitment was at least five patients per week, in a demand study based on the number of ENL episodes in previous years in those units, the inclusion of patients presented a much slower pace than it was initially expected. The greatest hindrances to enrollment were the use of steroids concomitantly with thalidomide, in patients with moderate to severe ENL and the washout period for steroid and thalidomide required by the protocol. In a recent series of ENL cases, it was observed that the indication of steroid associated with thalidomide was frequent,96 with scarce data about the treatment routine in Brazil for comparison.
Taking into account that the enrollment pattern at the beginning of the study was considered an important indicator/predictor for meeting the inclusion criteria of patients in the trials,97 it was decided to open a second enrollment site at Fundação Alfredo da Matta (Fuam), in Manaus, which was already included in the research protocol. A standardized form was created with the purpose of characterizing the patients with ENL receiving medical care. This assessment enabled analyzing the eligibility potential for enrollment among the patients screened at the outpatient's clinic. The standardized card was filled out with data relative to inclusion and exclusion criteria, and place of residence. The patients were also submitted to clinical examination by the main investigator in collaboration with the local staff that had been previously trained. Data about the start date of MDT and the number of previous episodes of reactional ENL were collected retrospectively through review of medical records by the same staff.
A total of 253 diagnoses of ENL were made at the Dermatology Outpatient Clinic at Fuam, from May to October 2002. There were 253 episodes of ENL in 143 patients, corresponding to approximately two appointments per subject. There was a predominance of male adult patients with ENL (83.2%), with a mean age of 32.5 years (SD = 13.8). More than 80% of patients lived in the capital city and 77% of patients had follow-up visits during this period (Table 2). Table 2 shows the clinical characteristics of ENL patients seen at the outpatient clinic. The diagnosis of ENL was detected predominantly during the use of MDT, in 21.4% of cases after MDT and in 9.8% at the moment of diagnosis of leprosy. About 98% of patients presented recurrent episodes of ENL, and in only 3 patients medical care was delivered due to the first reactional episode, demonstrating the relapsing aspect of this event. Approximately 65% of patients presented moderate and severe ENL. Roughly half of those ENL patients had been prescribed thalidomide and steroids, indicating the high frequency of combination therapy in this outpatient's clinic. Thalidomide as monotherapy accounted for 32.0% of all prescriptions, and steroids as monotherapy, for only 7%. As to the washout period, only 22.4% and 17.5% of patients had been off anti-inflammatory medication, either steroids or thalidomide, for the last 30 days.
Figure 2 shows the flowchart for screening ENL patients at the Fuam outpatient's clinic between May and October 2002. The criteria established in the protocol regarding ENL severity, male sex and age range 18-65 years could be met. Living in the capital city and agreeing to participate in the study were considered essential conditions to include patients in the clinical trial, were also analyzed as appropriate for the enrollment of patients. However, the data obtained at clinical screening and characterization of ENL patients from the enrollment sites showed that moderate and severe cases of ENL are frequently associated with neuritis and clinical events demanding use of steroids, which made co-intervention mandatory. Among the exclusion criteria of patients specified in the protocol that may render the enrollment unfeasible, we could mention neuritis and events associated with moderate and severe cases of ENL, which require corticotherapy as well as the washout period for anti-inflammatory drugs.
DISCUSSION
The primary objective of this study was to evaluate the efficacy of two different doses of thalidomide with no co-intervention of steroids. Most ENL patients enrolled presented signs/symptoms of peripheral neuritis confirmed by specialists, and were prescribed systemic corticotherapy. According to Brazilian regulations, every patient with peripheral neuropathy must, compulsorily, be submitted to systemic corticotherapy, in addition to receiving thalidomide.4 For this reason, the eligibility criterion could not be observed, even in two enrollment sites located in two highly endemic leprosy areas, where patients presenting with ENL episodes in outpatient's clinics are frequent. Although thalidomide is the first line drug to treat ENL and is available public healthcare units in Brazil, its use associated with corticotherapy is practically universal at these enrollment sites.
Patients recruited for this study showed demographic characteristics similar to those from outpatient clinics of other Brazilian healthcare services.16 There was a predominance of male patients, adults, and recurrent episodes of ENL.16 Houve predominância de homens, adultos, e episódios de ENH recorrentes.6,9,15 Additionally, multibacillary patients present a high risk of developing neuritis and potential disabilities, as demonstrated in large cohort studies.14
Routine treatment with thalidomide in the management of ENL is well known among physicians in the Brazilian Control Program. However, the concurrent neuritis in ENL patients with are not frequently described, recorded or reported by Brazilian general practitioners. The authors observed high frequency of neuritis among patients with moderate or severe ENL and the washout period needed, which were considered the main limitations in recruitment of patients.
Classically, neuritis with or without reaction signs must be treated with steroid agents. This is also the official recommendation of the WHO and Ministry of Health. These results bring important reflexions for the clinical practice and for the Leprosy Control Program: (a) the urgent need to evaluate the frequency and severity of neuritis among ENL patients by using standardized and objective criteria; (b) the pertinence of use of thalidomide as monotherapy in patients with ENL. Hence, the data obtained allow us to raise the question: could steroids prevent the neuropathy caused by thalidomide? The measurement of electrophysiological signs of neuropathy is the most reliable measurement of incidence of this side effect originated from the treatment with thalidomide.91 In spite of that, neuropathy caused by thalidomide in leprosy patients has not been described because the usual clinical measures during the follow-up of patients with ENL are not able to show the differences between neuropathy caused by thalidomide or by ENL. Reports of isolated cases described sensory loss due to thalidomide treatment (personal communication), although there is still a distinction gap between neuropathy of ENL and that considered as an adverse effect of thalidomide.
Another important consideration is the fact that the eligibility criterion adopted in this protocol is scientifically strict as to patient enrollment, even in highly endemic areas. Furthermore, if patients could be enrolled, they would not be representative of the majority of moderate and severe cases of ENL. These data suggest that, in clinical practice, few ENL patients are eligible to receive anti-reactional treatment with thalidomide as monotherapy, not associated with any steroids.
The six clinical trials with thalidomide published in the 1960's and 1970's suggested some beneficial outcome with the drug to treat moderate and severe ENL.35,41-45 However, these studies are difficult to be interpreted taking into account the current guidelines for clinical trials.56 The main limitations are the great variety of manifestations of the disease in the affected patients and the absence of standardized criteria to define severity and the clinical response to the drug, in addition to different doses of medications and unsupervised medicine intake.
In the majority of these studies there was concomitant use of steroids and other anti-inflammatory drugs that could interfere with the clinical response, potentially altering the cure rates, in addition to the small sample sizes, which are common in these published controlled trials. The exception is a controlled, double-blind clinical trial (n=92), sponsored by the WHO, comparing thalidomide (300mg) and acetylsalicylic acid (ASA), which was used to replace placebo with antipyretic and analgesic effects.45 Nowadays, ASA is recommended only for patients with mild ENL. Heterogeneous enrolled patients, absence of standardized criteria for cure and small sample sizes make it difficult to compare efficacy and side effects in these previous studies, which, it is worth mentioning, were conducted before the implementation of multiple drug therapy. Moreover, the recurrent nature of ENL episodes constitutes another hindrance to evaluate outcomes of clinical trials.
In Brazil, weaning has been performed in clinical practice and its rationale has been justified by recent laboratory findings.28,31,63
It remains a challenge to gather enough scientific evidence in terms of efficacy of the optimal dose of thalidomide, need of weaning and frequency of neuropathy as a side effect among ENL patients.
Note: In 2005, after this article was submitted and accepted, a randomized double-blind trial was published comparing two fixed doses of thalidomide followed by weaning in the treatment of moderate ENL. It was performed in Cebu, in the Philippines (1996-1998). The protocol, a requirement from the FDA for application of the thalidomide in the USA, was financed by Celgene and it is similar to the Brazilian study. It included 22 patients, and it was not possible to evaluate the efficacy due to the small sample size and lack of statistical power of this study, as explained by its authors.98
FINAL REMARKS
These clinical screening results presented show that moderate and severe cases of ENL are usually associated with neuritis and other events that require the use of steroids. This explains why it is impossible to meet the enrollment goals established in the research protocol. In face of these evidences, some scientific and ethical questions deserve further discussion. Would the patients enrolled based on eligibility criteria adopted by this current protocol be representative of the demand of moderate and severe ENL to Brazilian, or other countries, healthcare services? These results show that the number of patients with moderate or severe ENL to whom thalidomide as monotherapy could be recommended is very low. Therefore, the eligibility criterion adopted includes a restricted subgroup of patients. Even if the enrollment period to include the necessary number of eligible cases were extended for years, the evidences obtained could not be generalized to the majority of patients affected by moderate and severe ENL. The presence of neuritis in an exacerbated inflammatory picture, such as those described in most cases of ENL, is compatible with the immunopathogenesis of the disease, since the neural damage seems to be an early event in leprosy patients.11
In this context, a different question must be asked. Which possible combination therapies with thalidomide and steroids and other anti-inflammatory agents available for the treatment of ENL would be more effective, in the shortest period of time and causing minimum side effects? Therefore, changing the eligibility criteria of patients to be included in this clinical trial could meet the representativeness of those patients, gathering evidence about dose-response and co-interventions. q
ACKNOWLEDGEMENTS
To Dr. Steve Thomas, from Celgene Corporation, sponsor of the Brazilian Clinical Trial; to Dr. Karin Kook; to the dermatologists Dr. Jackeline Guerra and Dr. Marilene Silvestre, from the Hospital de Doenças Tropicais, in Goiânia; to the graduate student Gisner Pereira, from the Instituto de Patologia Tropical de Saúde Pública; to Dr. Ilma Mondnez, from CRDT-SES-GO; to Emília Pereira and Valderiza Pedroza, from Fundação Alfredo da Mata - AM, for the technical and operational support.
REFERENCES
Received on February 22, 2005.
Approved by the Consultive Council and accepted for publication on September 08, 2005.
- 1. Noordeen SK. Elimination of leprosy as a public health problem: progress and prospects. Bull World Health Organ. 1995:73:1-6.
- 2. Jacobson RR, Krahenbuhl JL. Leprosy. Lancet. 1999:353:655-60.
- 3. Martelli CMT, Stefani MMA, Penna GO, Andrade ALSS. Endemias e Epidemias Brasileiras, Desafios e Perspectivas de Investigação Científica: Hanseníase. Rev Bras Epidemiol. 2002:5:273-85.
- 4. Penna GO, Pereira G, Moreira M. A guide to leprosy control. Brasília (DF): Ministério da Saúde; 2002.
- 5. Ministério da Saúde. Fundação Nacional de Saúde. Guia de Vigilância Epidemiológica. Brasília, 2002. vol. 1 e 2.
- 6. Brasil. Ministério da Saúde. Guia para o controle da hanseníase. Brasília (DF), 2002 (Cadernos da Atenção Básica, 10).
-
7Report of the International Leprosy Association Technical Forum. Paris, France, 22-28 February 2002. Int J Lepr Other Mycobact Dis. 2002:70:S1-62.
- 8. Modlin RL, Mehra V, Jordan R, Bloom BR, Rea TH. In situ and in vitro characterization of the cellular immune response in erythema nodosum leprosum. J Immunol. 1986:136:883-6.
- 9. Croft RP, Richardus JH, Nicholls PG, Smith WC. Nerve function impairment in leprosy: design, methodology, and intake status of a prospective cohort study of 2664 new leprosy cases in Bangladesh (The Bangladesh Acute Nerve Damage Study). Lepr Rev. 1999:70:140-59.
- 10. Ottenhoff TH. Immunology of leprosy. New developments. Trop Geogr Med. 1994:46:72-80.
- 11. Sarno EN, Pessolani MC. Leprosy. Oldest and most feared disease. Lancet. 2001;358:S39.
- 12. Goulart IM, Penna GO, Cunha G. Immunopathology of leprosy: the complexity of the mechanisms of host immune response to Mycobacterium leprae Rev Soc Bras Med Trop. 2002:35:365-75.
- 13. Talhari S, Neves RG. Hanseníase. 3. Manaus: Instituto Superior de Estudos da Amazônia; 1997.
- 14. Croft RP, Nicholls PG, Steyerberg EW, Richardus JH, Cairns W, Smith S. A clinical prediction rule for nerve-function impairment in leprosy patients. Lancet. 2000:355:1603-6.
- 15. Lockwood D. Leprosy. Clin Evid. 2002:709-20.
- 16. Penna GO, Pinheiro AMC, Hajjar AL. Talidomida: mecanismo de ação, efeitos colaterais e uso terapêutico. An Bras Dermatol. 1998:73:501-17.
- 17. Guerra JG, Penna GO, Castro LCM, Martelli CMT, Stefani MMA. Eritema nodoso hansênico: atualização clínica e terapêutica. An Bras Dermatol. 2002:77:389-407.
- 18. Mellin GW, Katzenstein M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N Engl J Med. 1962:267:1184-92.
- 19. Lary JM, Daniel KL, Erickson JD, Roberts HE, Moore CA. The return of thalidomide: can birth defects be prevented? Drug Saf. 1999:21:161-9.
- 20. Marriott JB, Muller G, Dalgleish AG. thalidomide as an emerging immunotherapeutic agent. Immunol Today. 1999:20:538-40.
- 21. Tseng S, Pak G, Washenik K, Pomeranz MK, Shupack JL. Rediscovering thalidomide: a review of its mechanism of action, side effects, and potential uses. J Am Acad Dermatol. 1996:35:969-79.
- 22. Penna GO, Ramos AMC, Proença N. Contribuição da SBD a revisão da Portaria 354 - Agosto 1997. An Bras Dermatol. 2001:76:632-33.
- 23. Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med. 1999:5:582-5.
- 24. Sampaio EP, Hernandez MO, Carvalho DS, Sarno EN. Management of erythema nodosum leprosum by thalidomide: thalidomide analogues inhibit M. leprae- induced TNFalpha production in vitro. Biomed Pharmacother. 2002:56:13-9.
- 25. Sampaio EP, Sarno EN, Galilly R, Cohn ZA, Kaplan G. thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991:173:699-703.
- 26. Neubert R, Nogueira AC, Neubert D. thalidomide and the immune system. 2. Changes in receptors on blood cells of a healthy volunteer. Life Sci. 1992:51:2107-16.
- 27. Neubert R, Nogueira AC, Neubert D. thalidomide derivatives and the immune system. I. Changes in the pattern of integrin receptors and other surface markers on T lymphocyte subpopulations of marmoset blood. Arch Toxicol. 1993:67:1-17.
- 28. Sarno EN, Santos AR, Jardim MR, Suffys PN, Almeida AS, Nery JA, Vieira LM, Sampaio EP. Pathogenesis of nerve damage in leprosy: genetic polymorphism regulates the production of TNF alpha. Lepr Rev. 2000:71:S154-S58.
- 29. Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993:177:1675-80.
- 30. Moraes MO, Sarno EN, Almeida AS, Saraiva BC, Nery JA, Martins RC, Sampaio EP. Cytokine mRNA expression in leprosy: a possible role for interferon-gamma and inter leukin-12 in reactions (RR and ENL). Scand J Immunol. 1999:50:541-9.
- 31. Moraes MO, Duppre NC, Suffys PN, Santos AR, Almeida AS, Nery JA, Sampaio EP, Sarno EN. Tumor necrosis factor-alpha promoter polimorphism TNF2 is associated with a stornger delayed-type hypersensivity reaction in the skin of borderline tuberculoid leprosy patients. Immunogenetics. 2001:53:45-7.
- 32. Mattos O. Prurigo Nodular de Hyde tratado com Talidomida. Bol Div Nac Lepra. 1973:32:71-75.
- 33. Sheskin J. thalidomide in the treatment of lepra reaction. Clin Pharmacol Ther. 1965:6:303-06.
- 34. Sampaio SAP, Proença NG. Tratamento da reação leprótica pela talidomida. Revista Paulista de Medicina. 1966:68:301.
- 35. Iyer CG, Languillon J, Ramanujam K, Tarabini-Castellani G, De las Aguas JT, Bechelli LM, Uemura K, Martinez Dominguez V, Sundaresan T. WHO co-ordinated shortterm double-blind trial with thalidomide in the treatment of acute lepra reactions in male lepromatous patients. Bull World Health Organ. 1971:45:719-32.
- 36. Oliveira MA, Bermudez JA, Souza AC. thalidomide in Brazil: monitoring with shared responsibility? Cad Saude Publica. 1999:15:99-112.
- 37. Zeldis JB, Williams BA, Thomas SD, Elsayed ME. S.T.E.P.S.: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21:319-30.
- 38. Kook K, Villaermosa L, Downs M, Fajardo Jr. T, Walsh GST. A double blind, controlled, dose comparison study of thalidomide in the acute treatment of erythema nodosum leprosum ENL. 15th International Leprosy Congress. Abstracts of Congress Papers, Beijing; 1998.
- 39. Powell RJ, Gardner-Medwin JM. Guideline for the clinical use and dispensing of thalidomide. Postgrad Med J. 1994:70:901-4.
- 40. Saenz A, Ausejo M, Shea B, Wells G, Welch V, Tugwell P. Pharmacotherapy for Behcet's syndrome. Cochrane Database Syst Rev. 2000:CD001084.
- 41. Majumdar S, Mockenhaupt M, Roujeau J, Townshend A. Interventions for toxic epidermal necrolysis (Cochrane Review). Cochrane Database Syst Rev. 2002:CD001435.
- 42. Pearson JM, Vedagiri M. Treatment of moderately severe erythema nodosum leprosum with thalidomide-a double-blind controlled trial. Lepr Rev. 1969:40:111-6.
- 43. Sheskin J, Convit J. Results of a double blind study of the influence of thalidomide on the lepra reaction. Int J Lepr Other Mycobact Dis. 1969:37:135-46.
- 44. Waters MF. An internally-controlled double blind trial of thalidomide in severe erythema nodosum leprosum. Lepr Rev. 1971:42:26-42.
- 45. Iyer CG, Ramu G. An open trial with clofazimine in the management of recurrent lepra reaction using thalidomide as a control drug. Lepr India. 1976:48:690-4.
- 46. Cazort RJ, Ye Kun S. A trial of thalidomide in progressive lepra reactions. Curr Ther Res Clin Exp. 1966:8:299-311.
- 47. Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. Br Med J. 1996:312:71-2.
- 48. Knatterud GL, Rockhold FW, George SL, Barton FB, Davis CE, Fairweather WR, Honohan T, Mowery R, O'Neill R. Guidelines for quality assurance in multicenter trials: a position paper. Control Clin Trials. 1998:19:477-93.
- 49. Palmer CR, Rosenberger WF. Ethics and practice: alternative designs for phase III randomized clinical trials. Control Clin Trials 1999:20:172-86.
- 50. MacMahon S, Collins R. Reliable assessment of the effects of treatment on mortality and major morbidity, II: observational studies. Lancet. 2001:357:455-62.
- 51. Gibson JR, van Schie PM. Clinical Trial Measuring Systems in Dermatology. In: Bashir MC, Kibbon BC. Evidence Based Dermatology. Maibach: London; 2002.
- 52. Grimes DA, Schulz KF. Cohort studies: marching towards outcomes. Lancet. 2002:359:341-5.
-
53Brasil. Ministério da Saúde. Resolução do Conselho Nacional de Saúde Nº 196. 1996.
- 54. Judge MR, Kobza-Black A, Hawk JL. Guidelines for the clinical use and dispensing of thalidomide. Postgrad Med J. 1995:71:123.
- 55. Vieira CL. Brazil. Tough placebo rules leave scientists out in the cold. Science. 2002:295:264.
- 56. Meinert CL. Clinical trials and treatment effects monitoring. Control Clin Trials. 1998:19:515-22; discussion 23-43.
- 57. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001:357:1191-4.
- 58. Sampaio EP, Moreira AL, Sarno EN, Malta AM, Kaplan G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in lepromatous leprosy patients. J Exp Med. 1992:175:1729-37.
- 59. Sampaio EP, Kaplan G, Miranda A, Nery JA, Miguel CP, Viana SM, Sarno EN. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J Infect Dis. 1993:168:408-14.
- 60. Gardner-Medwin JM, Powell RJ. thalidomide - the way forward. Postgrad Med J. 1994:70:860-2.
- 61. Zwingenberger K, Wnendt S. Immunomodulation by thalidomide: systematic review of the literature and of unpublished observations. J Inflamm.1996:46:177-211.
- 62. Sampaio EP, Sarno EN. Expression and cytokine secretion in the states of immune reactivation in leprosy. Braz J Med Biol Res. 1998:31:69-76.
- 63. Argiles JM, Carbo N, Lopez-Soriano FJ. Was tumour necrosis factor-alpha responsible for the fetal malformations associated with thalidomide in the early 1960s? Med Hypotheses. 1998:50:313-8.
- 64. Santos AR, Almeida AS, Suffys PN, Moraes MO, Mattos HJ, Nery JAC, et al. Tumor Necrosis Factor Promoter Polymorphism (TNF2) Seems to protect Against Development of Severe Forms of Leprosy in a Pilot Study in Brazilian Patients. Int J Lepr. 2000:68:325-27.
- 65. Shaw MA, Donaldson IJ, Collins A, Peacock CS, Lins-Lainson Z, Shaw JJ, et al. Association and linkage of leprosy phenotypes with HLA class II and tumour necrosis factor genes. Genes Immun. 2001:2:196-204.
- 66. Shannon EJ, Sandoval FG. thalidomide can costimulate or suppress CD4+ cells' ability to incorporate [H3]-thymidine--dependence on the primary stimulant. Int Immunopharmacol. 2002:2:1143-53.
- 67. Hastings RC, Trautman JR, Enna CD, Jacobson RR. thalidomide in the treatment of erythema nodosum leprosum. With a note on selected laboratory abnormalities in erythema nodosum leprosum. Clin Pharmacol Ther. 1970:11:481-7.
- 68. Chen TL, Vogelsang GB, Petty BG, Brundrett RB, Noe DA, Santos GW, et al. Plasma pharmacokinetics and urinary excretion of thalidomide after oral dosing in healthy male volunteers. Drug Metab Dispôs. 1989:17:402-5.
- 69. Teo SK, Colburn WA, Thomas SD. Single-dose oral pharmacokinetics of three formulations of thalidomide in healthy male volunteers. J Clin Pharmacol. 1999:39:1162-8.
- 70. Levy L, Fasal P, Levan NE, Freedman RI. Treatment of erythema nodosum leprosum with thalidomide. Lancet. 1973:2:324-5.
- 71. Ochonisky S, Verroust J, Bastuji-Garin S, Gherardi R, Revuz J. thalidomide neuropathy incidence and clinico-electrophysiologic findings in 42 patients. Arch Dermatol.1994:130:66-9.
- 72. Jacobson JM, Spritzler J, Fox L, Fahey JL, Jackson JB, Chernoff M, et al. thalidomide for the treatment of esophageal aphthous ulcers in patients with human immunodeficiency virus infection. National Institute of Allergy and Infectious Disease AIDS Clinical Trials Group. J Infect Dis. 1999:180:61-7.
- 73. Calabrese L, Fleischer AB. thalidomide: current and potential clinical applications. Am J Med. 2000:108:487-95.
- 74. Neiger BL. The re-emergence of thalidomide: results of a scientific conference. Teratology. 2000:62:432-5.
- 75. Barnhill RL, McDougall AC. thalidomide: use and possible mode of action in reactional lepromatous leprosy and in various other conditions. J Am Acad Dermatol. 1982:7:317-23.
- 76. Naafs B, Faber WR. thalidomide therapy. An open trial. Int J Dermatol. 1985:24:131-4.
- 77. Proença NG. Talidomida. Uma medicação eclética em dermatologia. An Bras Dermatol. 1990:65:11-14.
- 78. Williams I, Weller IV, Malni A, Anderson J, Waters MF. thalidomide hypersensitivity in AIDS. Lancet. 1991:337:436-7.
- 79. Calderon P, Anzilotti M, Phelps R. thalidomide in dermatology. New indications for an old drug. Int J Dermatol. 1997:36:881-7.
- 80. Wolkenstein P, Latarjet J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, et al. Randomised comparison of thalidomide thalid placebo in toxic epidermal necrolysis. Lancet 1998:352:1586-9.
- 81. Stirling DI. thalidomide and its impact in dermatology. Semin Cutan Med Surg. 1998:17:231-42.
- 82. Quilitz R. thalidomide in oncology: The Peril and the Promise. Cancer Control. 1999:6:483-95.
- 83. Kaplan G, Thomas S, Fierer DS, Mulligan K, Haslett PA, Fessel WJ, et al. thalidomide for the treatment of AIDS-associated wasting. AIDS Res Hum Retroviruses. 2000:16:1345-55.
- 84. Molloy FM, Floeter MK, Syed NA, Sandbrink F, Culcea E, Steinberg SM, et al. thalidomide neuropathy in patients treated for metastatic prostate cancer. Muscle Nerve. 2001:24:1050-7.
- 85. Rajkumar SV. Current status of thalidomide in the treatment of cancer. Oncology (Huntingt). 2001:15:867-74; discussion 77-9.
- 86. Yong-Gee SA, Muir JB. Long-term thalidomide for actinic prurigo. Australas J Dermatol. 2001:42:281-3.
- 87. Steins MB, Padro T, Bieker R, Ruiz S, Kropff M, Kienast J, et al. Efficacy and safety of thalidomide in patients with acute myeloid leukemia. Blood. 2002:99:834-9.
- 88. Hwu WJ, Krown SE, Panageas KS, Menell JH, Chapman PB, Livingston PO, et al. Temozolomide plus thalidomide in patients with advanced melanoma: results of a dose-finding trial. J Clin Oncol. 2002:20:2610-5.
- 89. Singhal S, Mehta J. thalidomide in cancer: potential uses and limitations. BioDrugs. 2001:15:163-72.
- 90. Singhal S, Mehta J. thalidomide in cancer. Biomed Pharmacother. 2002:56:4-12.
- 91. Proença NG. Emprego de talidomida em dermatologia. An Bras Dermatol. 1995:70:61-67.
- 92. Silva SR, Viana PC, Lugon NV, Hoette M, Ruzany F, Lugon JR. thalidomide for the treatment of uremic pruritus: a crossover randomized double-blind trial. Nephron. 1994:67:270-3.
- 93. Crawford CL. Use of thalidomide in leprosy. Adverse Drug React Toxicol Rev. 1994:13:177-92.
- 94. D'Arcy PF, Griffin JP. thalidomide revisited. Adverse Drug React Toxicol Rev. 1994:13:65-76.
- 95. Clark OA, Castro AA. Searching the Literatura Latino Americana e do Caribe em Ciencias da Saude (LILACS) database improves systematic reviews. Int J Epidemiol. 2002:31:112-4.
- 96. Guerra JG, Penna GO, Castro LCM, Martelli CMT, Stefani MMA, Costa MB. Avaliação da série de casos de eritema nodoso hansênico: perfil clínico, base imunológica e tratamento instituído nos serviços de saúde. Rev Soc Bras Med Trop. 2004:37:384-90.
- 97. Haidich AB, Ioannidis JP. Patterns of patient enrollment in randomized controlled trials. J Clin Epidemiol. 2001:54:877-83.
- 98. Villahermosa LG, Fajardo Jr. TT, Abalos RM, Balagon MV, Tan EV, Cellona RV, et al. A randomized, double-blind, double-dummy, controlled dose comparison of thalidomide for treatment of erythema nodosum leprosum. Am J Trop Med Hyg. 2005; 72: 518 -26.
Publication Dates
-
Publication in this collection
13 Dec 2005 -
Date of issue
Oct 2005
History
-
Received
22 Feb 2005 -
Accepted
08 Sept 2005