Abstracts
Urticaria has diverse clinical presentations and causes. It is one of the most frequent dermatological conditions: 15% to 20% of population has at least one acute eruption during their lifetime, resulting in 1% to 2% of dermatological and allergological visits. Urticaria is classified based on its temporal evolution as acute (less than 6 weeks) or chronic (more than 6 weeks). Management strategies may involve non-pharmacological measures and drug interventions, which are grouped into first- (antihistamines), second- (corticosteroids and anti-leukotrienes) and third-line therapies (immunomodulators). Stronger, but potentially riskier, second- and third-line management may be justified for patients who do not respond to first-line therapy, or whenever a specific etiology cannot be determined, such as in autoimmune urticaria.
Adrenal Cortex Hormones; Cyclosporine; Histamine; Histamine Antagonists; Histamine H1 Antagonists; Histamine H2 Antagonists; Mast cells; Prostaglandins; Urticaria
A urticária apresenta-se com diversas formas clínicas e causas distintas. Constitui uma das dermatoses mais freqüentes: 15% a 20% da população têm pelo menos um episódio agudo da doença em sua vida, resultando em percentual que varia de um a 2% dos atendimentos nas especialidades de Dermatologia e Alergologia. A urticária é classificada do ponto de vista de duração da evolução temporal em aguda (inferior a seis semanas) ou crônica (superior a seis semanas). O tratamento da urticária pode compreender medidas não farmacológicas e intervenções medicamentosas, as quais são agrupadas em tratamentos de primeira (anti-histamínicos), segunda (corticosteróides e antileucotrienos) e terceira linha (medicamentos imunomoduladores). As medidas terapêuticas de segunda e terceira linha apresentam maiores efeitos adversos, devendo ser reservadas aos doentes que não apresentaram controle da doença com os de primeira linha, ou àqueles a respeito dos quais não é possível estabelecer uma etiologia, tal como nas urticárias auto-imunes.
Antagonistas de Histamina; Antagonistas dos receptores H1 de histamina; Antagonistas dos receptores H2 de histamina; Corticosteróides; Ciclosporina; Histamina; Mastócitos; Prostaglandinas; Urticária
REVIEW ARTICLE
Urticaria* * Work done at Division of Dermatology of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), Skin Allergy Outpatient´s Clinic, Sao Paulo, Brazil.
Paulo Ricardo CriadoI; Roberta Fachini Jardim CriadoII; Celina W. MarutaIII; Jose Eduardo Costa MartinsIV; Evandro A. RivittiV
IMasters degree in Medicine, Division of Dermatology of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (FMUSP) and Hospital do Servidor Público Estadual de São Paulo - São Paulo (SP), Brazil
IIAllergologist, Masters degree in Medicine, Volunteer physician of the Discipline of Dermatology of the Faculdade de Medicina do ABC - Santo André (SP), Brazil
IIIDermatologist, Ph.D. in Medicine, Lecturer of the Department of Dermatology of the Faculdade de Medicina da Universidade de São Paulo (FMUSP) - São Paulo (SP), Brazil
IVAssociate Professor of the Department of Dermatology, Faculdade de Medicina, Universidade de Sao Paulo - USP - Sao Paulo (SP), Brazil
VFull professor of the Department of Dermatology of the Faculdade de Medicina da Universidade de São Paulo (FMUSP) - São Paulo (SP), Brazil
Mailing address Mailing address: Paulo Ricardo Criado Rua Xingu, 245/182 - Bairro Valparaíso 09060-050 Santo André SP Tel/Fax: (11) 4426-8803 E-mail: prcriado@uol.com.br
ABSTRACT
Urticaria has diverse clinical presentations and causes. It is one of the most frequent dermatological conditions: 15% to 20% of population has at least one acute eruption during their lifetime, resulting in 1% to 2% of dermatological and allergological visits. Urticaria is classified based on its temporal evolution as acute (less than 6 weeks) or chronic (more than 6 weeks). Management strategies may involve non-pharmacological measures and drug interventions, which are grouped into first- (antihistamines), second- (corticosteroids and anti-leukotrienes) and third-line therapies (immunomodulators). Stronger, but potentially riskier, second- and third-line management may be justified for patients who do not respond to first-line therapy, or whenever a specific etiology cannot be determined, such as in autoimmune urticaria.
Keywords: Adrenal Cortex Hormones; Cyclosporine; Histamine; Histamine Antagonists; Histamine H1 Antagonists; Histamine H2 Antagonists; Mast cells; Prostaglandins; Urticaria
INTRODUCTION
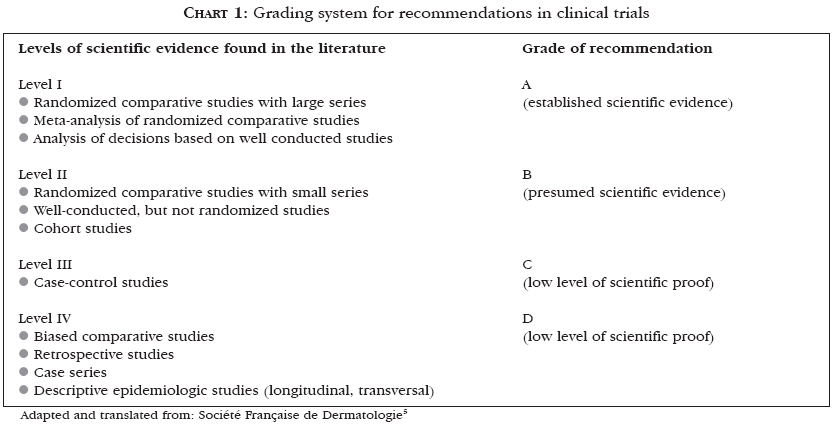
Urticaria was described by Hippocrates as a distinct entity. As the understanding of molecular mechanisms involved in the pathogenesis of this affection improves, there is increasing evidence of its heterogeneity.1 Some studies demonstrated that about 0.1% of the population has urticaria, and that the cumulative prevalence rates vary between 15% and 20%.2,3 Among those affected by urticaria, 50% will still be presenting the condition one year after the first visit to the doctor, and 20% will go on experiencing episodes of the disease for over 20 years.2-4 In 2003, the Consensus of the French Society of Dermatology for the Management of Chronic Urticaria evaluated the literature concerning the matter using a recommendation grading system5 (Chart 1), adapted here for this review.
DEFINITIONS
Urticaria is characterized by the fast arising of wheals, which may be accompanied by angioedema.1,2 Edema of the superficial dermis is named urticaria, whereas edema of the profound dermis, the subcutaneous layer and the gastrointestinal tract, is named angioedema.1,2 A wheal is an elementary dermatological lesion comprising three typical features: (i) central edema of varying size, surrounded by a reflex erythema; (ii) associated pruritus; (iii) ephemeral nature, with the skin usually returning to its normal aspect within one to 24 hours.1,2 Angioedema is defined by: (i) sudden and pronounced edema of the profound dermis and the subcutaneous layer; (ii) pain as a more frequent symptom than pruritus; (iii) frequent involvement of the mucous membranes; and (iv) resolution of the condition within approximately 72 hours, which is slower than in wheals.1
CLASSIFICATION OF URTICARIA AND CLINICAL PICTURE
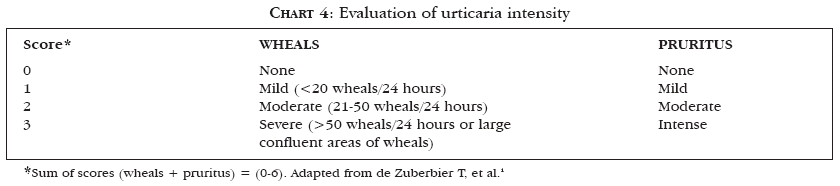
Urticaria and angioedema can be classified from the point of view of their etiopathogenic mechanisms, as shown in chart 2, proposed by Grattan et al.6 Another way of classifying urticaria and angioedema is based on the spectrum of clinical manifestations of the different subtypes of urticaria and is represented in chart 3.1 Another important factor in urticaria is its classification according to the intensity of the disease. Zuberbier et al. proposed a simple scoring system (Chart 4).1
Sequential clinical evaluations have to be made at pre-established times of the day to assure accuracy of the score obtained. Large wheals generally indicate a disease that is more severe and more resistant to treatment.1
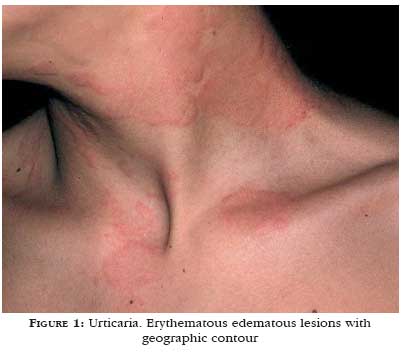
Another relevant aspect is the color of the wheal. Wheals induced by histamine are of light color, surrounded by a pink erythema that is secondary to skin vessel dilation (Figure 1). Wheals with a pronounced red, purple or violet erythema, like in urticarial vasculitis, indicate intense vascular damage and plasma leakage forming the lesion.1 Angioedema produces skin paleness and an increase in volume (Figure 2).
ETIOPATHOGENIC MECHANISMS
In a summarized manner, chart 5 describes the possible mechanisms involved in the etiopathogenesis of urticarias.1
A. The role of mast cells and other cell elements
The basic mechanism of wheal formation resides in Lewiss triple reaction: initial erythema by capillary dilation; secondary response produced by an arteriolar dilation mediated by axonal nervous reflexes; and formation of the wheal, caused by leakage of fluid from the intravascular to the extravascular space, secondary to increased vascular patency.7 These reactions can be reproduced by intradermal injection of several vasoactive mediators which are common to mast cells and are considered as the leading actors in most urticaria and angioedema cases.7
Mast cell stimulation in human skin can occur: (i) by antigenic stimulation of the IgE bound to the high-affinity IgE receptor (FceRIa); (ii) by activation of the complement (fractions C3a and C5a); (iii) by direct stimuli such as acetylcholine; (iv) by direct histamine releasers (chemicals named secretagogues) which cause calcium mobilization, such as codeine, morphine, meperidine, succinylcholine, d-tubocurarine, polymyxin B, acetylsalycilic acid, quinine, thiamine, dextran, iodine contrast media, compound 48/80, calcium ionophores; (v) by other compounds such as crustaceans, strawberries and dyes; (vi) by physical stimuli (heat, cold, vibration, light, pressure, water); (vii) by neuropeptides (substance P); (viii) by the eosinophil main basic protein (MBP); and (ix) by cytokines (IL-1, IL-3, IL-8, GM-CSF, platelet Factor 4) (Figure 3)8
The immune stimulus best characterized to mast cells is the binding of specific divalent antigens to their FceRI receptors.8 All tissue mast cells express FceRI on their surface, which bind to the Fc portion of the IgE antibodies.9,10 After the immune stimulus, the mast cells undergo a refractory period, thus enabling regeneration of the mediators associated to their granules.8
The degranulation process can be inhibited by adrenalin, theophylline and dibutyl cyclic AMP (AMPc), which act on the cyclic nucleotides, and by cytochalasin B and colchicine, which, in turn, interfere with the functioning of the microtubules and formation of the protein tubuline.7 The released histamine can inhibit a future degranulation by a negative feedback mechanism, involving the H2 receptors and increasing the AMPc levels.7 Acetylcholine, in turn, increases the release of histamine, due to the fact that it raises the levels of cyclic guanosine monophosphate (GMPc).7
Mast cell degranulation produces the release of preformed mediators and the generation of new lipid-derived metabolites.8 The preformed mediators are composed of histamine (producing pruritus, vasodilation, vascular permeability, contraction of the smooth musculature, mucous secretion, leukocyte chemokinesis, prostaglandine production, gastric acid secretion and immunoregulation); natural proteases (tryptase, which may cause C3 cleavage and fibrinolysis; chymase and carboxypeptidase A); heparin (local anticoagulation, inhibition of complement activation and neutralization of the main basic protein), eosinophil chemotactic factor, neutrophil chemotactic factor and acid hydrolases (arylsulfatase, beta-glucuronidase and beta-hexosaminidase, which are responsible for enzymatic degradation).8
The metabolites derived from lipids generated by mast cell degranulation comprise the following substances: prostaglandin D2 (PGD2) (vasodilation, inhibition of platelet aggregation, vasopermeability, smooth muscle contraction properties), leukotrienes LTC4, LTD4, LTE4 (vasopermeability, smooth muscle contraction, and mucous secretion) and LTB4 (vasopermeability, chemotaxis, adherence, neutrophil activation, and degranulation), platelet activating factor (vasopermeability, smooth muscle contraction, mucous secretion, platelet aggregation, chemotaxis, and neutrophil and eosinophil activation), thromboxane A2 (smooth muscle contraction, platelet aggregation), oxygen (cell cytotoxicity) and adenosine metabolites (vasopermeability, smooth muscle contraction).8 Histamine is probably the main mediator, since it presents higher tissue concentrations after degranulation.8
The succession of mediators secreted by the activated mast cells starts an immediate response in urticaria and may be able to trigger a more persistent state of inflammation, named according to Schwartz (1991) urticaria late-phase response (Figure 4).9
The eosinophils are cells that are associated to the mast cell-dependent allergic diseases, although in routine histopathology examinations they are found in few subtypes of urticariform lesions.10
B. Autoimmunity in urticaria
Chronic idiopathic urticaria accounts for approximately 70% of all chronic urticarias; in that, 25% to 50% of them displays histamine-releasing auto-antibodies directed against the FceRIa receptors or, less frequently, against IgE, or against both (Figure 5).11-14 The presence of these autoantibodies characterizes the so-called chronic autoimmune urticaria.11-14 The isotypes of these autoantibodies are IgG1 and IgG3.11-14 The clinical diagnosis of autoimmune urticaria (anti-FceRIa or anti-IgE antibodies) is infered by the autologous serum skin test.11-14 This test is done by drawing blood from the patient during an urticaria eruption, and then separating the serum by centrifugation.11-14 Afterwards, 0.05ml of the serum are injected intradermally into skin clinically not affected by urticaria.11-14 The reaction shown by the inoculated skin is submitted to reading after 30 minutes.11-14 Formation of a wheal with a diameter at least 1.5mm larger than the edema caused by the injection of sterile saline solution, used as control, is considered a positive test (grade of recommendation B).11-14
C. Participation of Helicobacter pylori and other bacterial infections
Hizal et al.15 demonstrated positivity in the autologous serum skin test and high anti-Helicobacter pylori IgG antibody levels in patients with chronic urticaria and concluded that the relationship between autoimmunity and Helicobacter pylori infection deserves larger studies.15,16 The French Consensus5 on Urticaria, held in 2003, suggested there is no relationship between Helicobacter pylori infection and the course of chronic urticaria, recommending investigation of the bacterium only in the presence of digestive symptoms (grade of recommendation B). In the same Consensus5 no evidence was found of an association between occult bacterial infections (dental or sinusal infections, for example) and chronic urticaria.
D. Autoimmunity to thyroid
In up to 20% of the patients with chronic urticaria that is refractory to treatment, high titers of antithyroid antibodies (antiperoxidase and antithyroglobulin) can be found, while in the general population they are expected to be found in no more than about 3% to 4% of normal individuals.17 The fact that the simultaneous presence of antithyroid antibodies and anti-FceRIa antibodies was detected in some patients with chronic urticaria seems to indicate the existence of a state of disease that is secondary to an autoimmune process and/or a rupture in immune regulation.17 This idea is emphasized by Rottem, who did not correlate the direct participation of the antithyroid autoantibodies with the etiopathogenesis of chronic urticaria, postulating that they are probably correlated in a parallel manner, as autoimmune events18 (grade of recommendation C).
E. Latex contact urticaria
Contact urticaria by natural rubber latex affects persons who are routinely exposed to products made of this material.19 The highest-risk groups comprise healthcare professionals, workers in the rubber industry and people submitted to multiple surgical procedures, such as patients with spina bifida.19 Rubber latex contact urticaria consists of a type I Gel and Coombs reaction, mediated by IgE, which can lead to anaphylaxis and death. The reactions to rubber latex occur within one hour from exposure to latex.19
The clinical manifestations of latex allergy depend on exposure: through the skin (urticaria, dermatitis and pruritus), air-borne (rhinitis, conjunctivitis, asthma), and through mucosa (anaphylaxis, tachycardia, angioedema, nausea, vomiting, abdominal pain, low blood pressure).19 Latex causes at least 10% of all intraoperative anaphylactic reactions. Anaphylaxis was reported after contact with nursing bottle nipples, pacifiers, vaginal vibrators, Foley catheters, latex condoms, inflatable balloons, dental elastics, endotracheal tubes, electrocardiogram stickers, and food prepared with rubber gloves.19
F. Other possible etiopathogenic mechanisms
Tharp et al.20 suggested that gastrin, a 17-aminoacid peptide residue, released by the G cells of the gastric anthrum and proximal duodenum immediately after eating, might be involved in the anaphylactic reactions and in urticarias reported after ingestion of certain foods. To reinforce this theory, they observed that a direct correlation between clinical symptoms and detection of a specific antigen IgE is not always possible in cases suspected of food allergy.20 These authors demonstrated that an intradermal injection of gastrin or pentagastrin caused the release of skin mast cell mediators. It is known that the ingestion of proteins produces a significantly higher secretion of gastrin than carbohydrates and lipids.20 Moreover, they speculated that postprandial immediate hypersensitivity-like syndromes, such as exercise-induced anaphylaxis only after meals, postprandial hypotension in the elderly, and unexplained urticaria after eating could have gastrin as an underlying factor, influencing the clinical expression of mast cell mediator release in these patients20 (grade of recommendation C).
In recurrent and chronic urticarias it is postulated that a histamine intolerance might be involved, determined by an overload of histamine contained in diet and/or due to an abnormal histamine metabolism (diamine oxidase deficiency).21 Diamine oxidase is the main enzyme involved in histamine degradation, with predominant activity in the intestinal mucosa.21 Alcohol and some drugs [imipenem, dobutamine, pancuronium, pentamidine, verapamil, isoniazid, clavulanic acid, dihydralazine, chloroquine, acetylcysteine, metoclopramide and cefuroxime ] can reduce the activity of this enzyme and determine greater sensitivity to histamine-rich or histamine-producing foods [fish (tuna, sardine, anchovy), cheese (parmesan, Emmenthal, Gouda), salami, sausage, certain vegetables (tomato), wine and beers ].21 Several experiments have demonstrated a diamine oxidase deficiency in the enterocytes of patients with chronic or recurrent urticaria21 (grade of recommendation C).
The mechanism associating dental infections and chronic urticaria remains undefined.22 There are reports of transient urticaria flares occurring along with high fever, after dental treatment, suggesting that bacteremia and/or toxemia resulting from dental treatment might induce urticaria, both by the immune path and non-immune mechanisms.22 The release of histamine by mast cells through lipopolyssaccharides of gram-negative bacteria of the oral flora, such as Veilonella sp, could be relevant as a pathogenic factor in urticaria of patients with odontogenic infection; in addition, these anaphylotoxins may have a direct acute vasodilating effect determining flares of urticaria22 (grade of recommendation C).
In 2001, Kozel et al.23 evaluated 220 adult patients with urticaria. Of these, 72 (33.2%) presented physical urticaria, 24 (10.9%) an association of physical and chronic idiopathic urticaria, 78 (36%) chronic idiopathic urticaria, 20 (9%) drug-related urticaria, 15 (6.8%) food-related, 4 (1.8%) due to infections, 3 (1.4%) due to internal diseases, and 2 (0.9%) had contact urticaria. A cause could be identified in 53.1% of patients. Thirty-five percent of the patients were cured after a year, and within this period the symptoms decreased in 28.9% of them.23 Spontaneous remission occurred in 47.4% of patients in which no cause for the disease was identified, and in only 16.4% of those with physical urticaria.23 In this study, the patients with physical urticaria presented the poorest prognosis regarding duration of the disease: 84% of them still had symptoms after one year.23
HISTOPATHOLOGICAL ASPECTS
The histopathological examination of the classical wheal shows edema of the epidermis and of the superficial and intermediate dermis, with dilated postcapillary venules and lymph vessels of the superficial dermis.7 In angioedema, similar alterations occur in the profound dermis and in the subcutaneous layer.1 Depending on the duration of the wheal, there is a mixed perivascular inflammatory infiltrate of variable intensity, composed of neutrophils and/or eosinophils, macrophages and helper T-lymphocytes.1 In delayed pressure urticaria, the infiltrate is preferentially located in the intermediate and profound dermis.1
CLINICAL PICTURE
Acute urticaria
The lesions are large, pruriginous, erythematous and edematous plaques, of sudden onset and short duration, frequently accompanied by general phenomena. An acute urticaria episode can persist for hours and even days.24 The triggering factor is not difficult to find, as it is usually related to the causes listed in chart 6.24 In general, it does not require investigation, except for that suggested by the history taken.24 When IgE-mediated reactions to environmental allergens (such as latex, nuts or fish) are the cause of acute or contact urticaria, they can be investigated by the skin prick test or by Rast (radioallergosorbent test) in blood.24 It should be pointed out that, in order to be duly valued, the result of both these tests must be correlated to the clinical context.
Chronic common urticaria
Urticaria is considered chronic when it persists for more than six weeks.25 Approximately 30% of patients with urticaria present chronic urticaria.25 Of recurrent nature, it can even last for years; there is a tendency to spontaneous cure.26 REven with proper investigation, its etiology is rarely found.26 By and large, it affects adult females and its treatment is difficult and only symptomatic. According to the 2001 Guidelines of the British Association of Dermatologists, investigation is not recommended in most patients with mild chronic urticaria who respond to the use of antihistamines.26 For those with a more severe disease that does not improve with conventional treatment, a useful guide for investigation consists of requesting a complete blood count (which helps detecting hematological neoplasm or eosinophilia indicating intestinal worm infections) and erythrocyte sedimentation rate (normal in idiopathic chronic urticaria and usually elevated in urticarial vasculitis and Schnitzler syndrome).26 Thyroid autoantibodies and thyroid function tests can be indicated if a disease of this gland is suspected26 (Chart 7). Currently there is no standardized laboratory test to assess the presence of histamine-releasing autoantibodies, but in experienced health units the intradermal test with autologous serum provides reasonable sensitivity and specificity.27
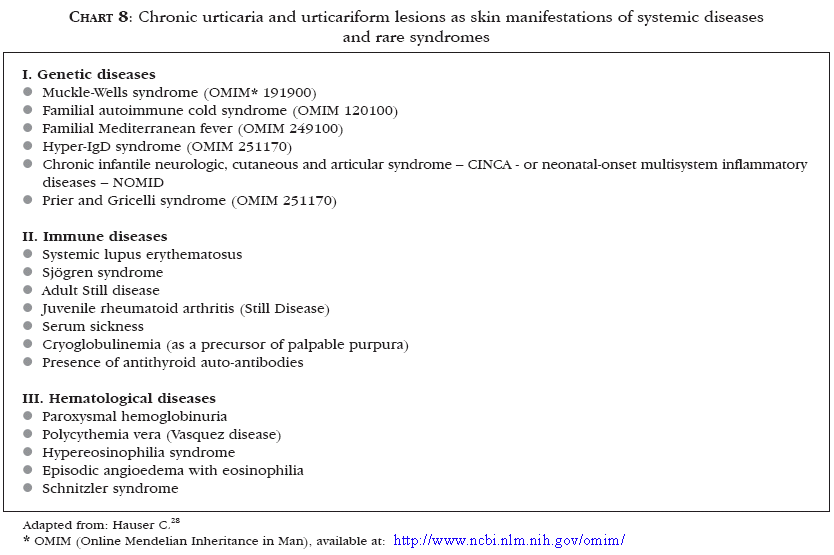
Chronic urticaria or urticarial vasculitis may be associated to a great number of systemic immune diseases or rare syndromes, besides autoimmune connective tissue diseases (Chart 8).28
Physical urticarias
Physical urticarias comprise a heterogeneous group of diseases due to broad variability of triggering stimuli and clinical forms, as well as to their association with other types of urticaria.29 This suggests that non-specific mechanisms take part in the traditional forms, such as lower threshold of mast cell activation or higher reactivity of the target cells.29 This has been observed in patients with dermographism, who may also suffer from bronchial hyperreactivity to histamine or metacholine.29 On the other hand, more specific mechanisms can be relevant in solar and heat-related urticaria. However, many questions regarding the pathogenic mechanisms of physical urticarias remain unclear.29
The international standardization for the investigation of physical urticarias is summarized below.30
Immediate symptomatic dermographism (Urticaria facticia): application of pressure of less than 36g/mm2.The test is performed on the dorsum, with an instrument named dermographometer or with a blunt-tip object.
Acquired cold contact urticaria: application of an ice cube wrapped in a plastic bag to the skin for a period of five minutes. The response occurs after 10 minutes. If the ice cube test is negative, an arm may be immersed in cold water (5 to 10ºC) for 10 minutes.
Reflex cold urticaria: only exposure of the body to cold induces the wheals, and the ice cube test is negative. The wheals can be produced by cooling of the body in a room at 4ºC, for 30 minutes.
Cold urticaria (CU) is a distinct clinical entity characterized by erythema, pruritus and wheals on the skin exposed to cold. The lesions may be limited exclusively to the area of contact with the cold or be generalized, with a higher risk of presenting systemic symptoms such as headache, chills, tachycardia and diarrhea. The oral mucosa may be involved. Different clinical manifestations of CU have been described, including cold-induced dermographism, localized CU, perifolicular CU, cholinergic CU, and delayed familiar autosomal dominant CU. Delayed CU has a latency from 3 to 24 hours after cold exposure and lasts approximately 24 hours.
In systemic familiar CU, there is a maculopapular eruption, different from classical urticaria, that can be triggered by cold windss.31 Diagnosis could be made by ice cube test or cold water (15 minutes, 8ºC) test.31 CU in turn may precede the presence of cryoglobulins or multiple myeloma by several years, and disappear with the reduction of cryoglobulins. CU has been described in association with infectious mononucleosis, hepatitis, measles, HIV, borreliosis, syphilis and bacterial infections.31 Other tests include a blood count, serological reactions for syphilis, hepatitis, HIV, Epstein-Baar virus, cryoglobulintest, cold agglutinins and cryofibrinogen.31
Delayed pressure urticaria: a wheel of 1.5cm-diameter weighing 2.5 to 4.5kg is applied perpendicularly to the patients dorsum or thigh for 20 or 15 minutes, respectively. The arising of wheals in the test area within a six-hour period indicates a positive result.
Solar urticaria: it can be tested for by challenge test with natural sun light, monochromatic light or an artificial sun simulator, for 10 minutes, when the arising of wheals is expected.
Aquagenic urticaria: gauze soaked in water at 37ºC is applied for 20 minutes, or the patient is bathed in water at body temperature.
Acquired heat contact urticaria: a flask containing heated water at 38ºC-50ºC is applied for one to 5 minutes. The wheals arise within a few minutes at the application site.
Vibratory angioedema: application of a vibratory stimulus (preferably a laboratory vortex vibrator) on the patient forearm for 15 minutes. Wheals arise at the application site within 10 minutes after the test.
Cholinergic urticaria: the patient is submitted to physical exercise (for example, running) up to the point of sweating, or to partial immersion of the body in heated water at 42ºC for 10 minutes. The test causes quick arising of wheals. If the test is negative, it should be repeated on another day, for confirmation.
Angioedema
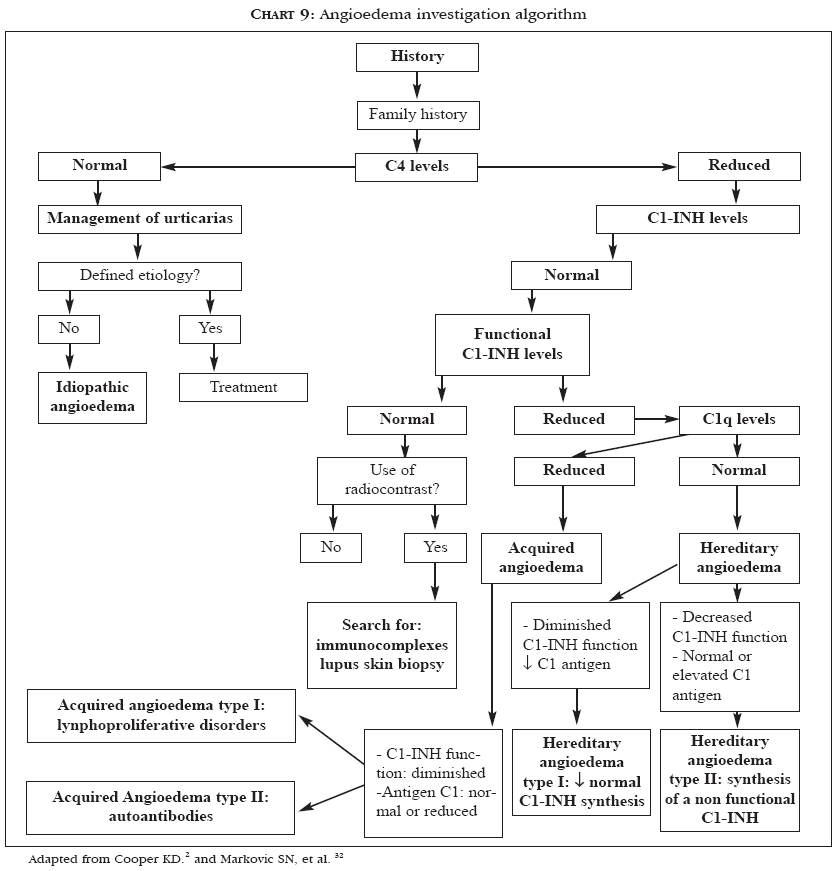
Urticaria often occurs with angioedema.2 QWhen this is observed, the prognosis is poorer, with 75% of patients presenting recurrent episodes for more than five years.2,4 The approach for patients with angioedema can be similar to that for patients with urticaria.2,4 However, various diagnostic possibilities may be involved and should be kept in mind: for example, in hereditary angioedema, which is caused by a deficiency in the C1 esterase inhibitor, the use of anabolic steroids is effective.2,32Chart 9 presents a procedure algorithm for investigating angioedema.2,32
The first step consists of a careful history taking on existence of family history of the affection and a detailed physical examination.2 If the levels of the fourth complement component (C4) are normal, the management is the same used for urticaria.2 If C4 levels are diminished, dosage of C1 esterase inhibitor protein (C1-INH) has to be performed. If the amount of protein is normal and C4 levels are still low, a functional test of C1 esterase inhibitor has to be done, since there is a subgroup of patients with hereditary angioedema producing normal amounts of C1-INH, but with abnormal function. If the result is normal, it can be concluded that the patient does not have hereditary angioedema.2
Diminished C2 or C4 levels along with normal C1-INH levels may result from exposure to radiological contrasts, as well as from syndromes involving formation of immunocomplexes.2 If the results of the functional tests or of the C1-INH dosage show reduced values, serum C1q levels must be measured, in order to distinguish hereditary angioedema, which is easier to treat, from the rare association of acquired angioedema with neoplasm, such as B cell lymphomas.2
A reduced C1q level can result from a paraneoplastic syndrome that consumes C1q, thus secondarily depleting C1-INH.32 Therefore, low C1q levels should lead to a search for internal neoplasms. In addition to this condition, in 1973, McDuffie described the low-complement urticarial vasculitis syndrome, in which antibodies (preceptines) directed against C1q are present, with or without decreased levels of the first complement component.33 These antibodies are directed against the collagen-like portion of C1q and are found in 100% of patients with this syndrome.33 The C3 and C4 levels can vary from undetectable to normal.33
A diagnosis of hereditary angioedema can be made if the C1q level is normal and the C2 and/or C4 and C1-INH levels are diminished.2,32 Except for the age of onset and the family history, acquired and hereditary angioedema are two clinically undistinguishable syndromes.32 This diagnosis is crucial, since it is a potentially fatal disease and responds well to treatment with anabolic steroids, such as danazol and stanozolol.2,32 Acquired angioedema occurs in 0.1% to 0.5% of patients in use of angiotensin-converting enzyme inhibitors (ACE inhibitors).34 The greater occurrence of angioedema among Afro-Americans seems to be due to lower levels of endogenous bradikinine of these patients and their consequently higher sensitivity to bradikinine elevations induced by ACE inhibitors.34 In patients whose angioedema is due to an ACE inhibitor, the drug has to be withdrawn and replaced by another therapeutic alternative.34
Angioedema can represent one of the clinical manifestations of anaphylaxis that can be graded according to the parameters displayed in chart 10.35
Etiologic diagnosis of urticarias
A detailed patient history and a complete physical examination are fundamental for the etiologic diagnosis of urticarias.1 Laboratory and challenge tests based on history and physical examination complement the investigation.1
The data to be obtained from patients must include the following items:1
1. Onset of the disease
2. Frequency and duration of the lesions
3. Variation during day/night
4. Shape, size and distribution of the lesions
5. Associated angioedema
6. Symptoms associated with lesions (pruritus, burning, pain)
7. Family and personal history of urticaria and atopy
8. Previous or concomitant history of allergy, infections, internal diseases or other possible causes
9. Induction by physical agents or exercises
10. Urticaria-related foods and dietary habits
11. Exposure to inhaled drugs
12. Use of drugs (non-steroidal anti-inflammatory drugs, betablockers, angiotensin-converting enzyme inhibitors, immunizations, hormones, laxatives, suppositories, eye, nasal and ear drops and alternative medicine medications)
13. Smoking habits
14. Occupation
15. Leisure activities
16. Occurrence with regard to weekends, vacations or travels abroad
17. Surgical implants
18. Reactions to insect stings
19. Relation with menstrual cycle
20. Treatment response
21. Stress
22. Quality of life with regard to urticariaurticária
The next steps depend on the nature of urticaria subtype and are summarized in chart 11. Several authors insist upon the recommendation against the use of long and expensive general protocols in the etiologic investigation of urticaria.1 The most recommended complementary tests for the several urticaria subtypes are summarized in chart 12.1 Additional tests should be reserved for selected patients. Type I Gel & Coombs allergy is a rare cause of chronic continuous urticaria, but it has to be investigated in intermittent chronic urticaria, while pseudoallergic reactions to foods and food additives can be relevant in chronic continuous urticaria1 (grade of recommendation B). The frequency of infectious diseases varies among the different groups of patients and in different regions of the planet. For instance, hepatitis B and C virus infections are frequent causes of chronic urticaria in Ssoutheastern Europe and uncommon in the Northeastern region of the same continent1 (grade of recommendation B). The need to investigate intestinal infestations seems to vary among the different groups of patients according to endemicity of these diseases.1 Currently, the only test available to infer presence of auto-antibodies against IgE receptor is the autologous serum skin test.1
Differential diagnosis of urticarias
Even though identifying a skin eruption such as urticaria is not difficult, some conditions may be morphologically similar to urticaria, thus leading to confusion (Chart 13)2
Differential diagnosis of angioedema
Typical cases of angioedema are easily diagnosed. Acute edema of deep tissues has to be distinguished from anaphylaxis, and the possibility of airway obstruction must be considered.2 While angioedema lesions typically last 48 to 96 hours, the diseases included in differential diagnoses (Chart 14) last longer.2
TREATMENT OF URTICARIA
The standard therapeutic approach for acute urticarias is based on the use of second-generation (non-sedating) antihistamine agents.36,37 The use of second-generation anti-H1 in acute urticaria is the only therapeutic intervention presenting grade of recommendation B, due to the existence of randomized controlled studies.36 An alternative treatment for patients who do not respond to the use of anti-H1 or in whom the presentation of the acute disease is severe with associated angioedema is the use of an oral steroid (prednisolone), 50mg/day PO for adults and 1mg/kg/day for children, for three days.36,38 Poon & Reid39 reviewed the literature searching for the best scientific evidence on the use of corticosteroids in acute urticaria and concluded that the addition of prednisolone to the anti-H1 treatment in urticaria produces quicker control of the symptoms and faster resolution of the disease (grade of recommendation B).
If there is angioedema with signs of progress toward anaphylaxis (edema of the larynx, edema of the glottis, bronchospasm, nausea, vomiting, blood hypotension):40 epinephrine (first drug intervention to be performed) in a 1:1000 (1mg/ml) solution by subcutaneous or preferably intramuscular administration in the anterolateral thigh (faster absorption and better plasma levels than by subcutaneous or intramuscular injection in the arm), 0.2 to 0.5ml for adults every five minutes; 0.01mg/kg (maximum of 0.3mg total dose) for children. Anaphylaxis can be graded according to chart 10.41 Depending on the response to epinephrine, the following measures may be necessary:40
-
Raising lower limbs, which prevents orthostatic hypotension and helps deviating the blood circulation from the periphery towards the head, heart and kidneys.
-
Maintaining the airways patent - unidirectional face mask with oxygen entrance. Endotracheal intubation or cricoidectomy be considered by duly trained physicians.
-
Administrating oxygen at 6 to 8 liter/minute.
-
Performing venous access.
Using intravenous saline solution for fluid replacement.40 Great volumes of crystalloids may be necessary (1 to 2 liters of saline solution for adults, which can be administered at a volume of 5 to 10ml/kg within the first 5 minutes;40 children can receive approximately 30ml/kg within the first hour.40 If hypotension persists, the use of expanders (colloidal solutions) may be necessary.40
Other measures to be considered:40
-
epinephrine infusion prepared by adding 1mg (1ml) of epinephrine diluted at 1:1000 to 250ml of 5% glucose solution, determining a concentration of 4mg/ml. This solution is intravenously infused at a rate of 1 to 4mg/minute (15 to 60 drops per minute with a microdrop device [60 microdrops per minute = 1ml = 60 ml/hour ]), increasing up to the maximum of 10mg/min min for adults and adolescents. Due to the risk of potentially lethal arrhythmia, epinephrine administration should only be intravenous during cardiovascular collapse or in profoundly hypotensive patients, who did not respond to volume infusion and to several doses of epinephrine injected IM, and always under cardiac monitoring;
-
consider ranitidine, 1mg/kg, that can be diluted in 5% dextrose in a total volume of 20ml and injected intravenously within approximately 5 minutes. Cimetidine (4mg/kg) can be used intravenously in adults;
-
epinephrine-resistant bronchospasm: albuterol 2.5 to 5mg in 3ml saline solution and repeat whenever necessary;
-
hypotension that is refractory to volume infusion and epinephrine injections, use dopamine 400mg in 500ml of 5% glucose serum, which can be administered intravenously at 2 to 20mg/kg/minute, with strict hemodynamic control;
-
in patients using betablockers, which complicates treatment: glucagon 1 to 5mg (20-30mg/kg [maximum of 1mg ]) intravenously for about 5 minutes, followed by infusion (5-15mg/minute);
-
consider the use of glucocorticoids for patients with a history of idiopathic anaphylaxis and asthma and in patients who experience severe and prolonged anaphylaxis. Glucocorticoids do not act acutely, but they can prevent recurrent or protracted anaphylaxis. They should be given every 6 hours at a dose equivalent to methylprednisolone (1 to 2mg/kg/day). Oral use of prednisone 0.5mg/kg can be sufficient for less critical situations;
-
consider removal to an intensive care unit.
In chronic urticarias10,42 management should include general information given to patients (directions) and drug therapy. Various drug and non-pharmacological interventions are possible; however, none of them is consistently successful. Treatment must be customized to patient characteristics.
I) General directions for the patient:
-
remove the identified cause;
-
explain about the disease;
-
reduce emotional stress, body overheating and alcohol ingestion;
-
avoid the use of acetylsalicylic acid, non-steroidal anti-inflammatory drugs, codeine and morphine. Analgesic drugs aggravate chronic urticaria in 30% of patients. Those on aspirin at low doses for antithrombotic purposes can usually proceed with their regular treatment, although alternatives to aspirin, such as clopidogrel, are available;
-
patients with angioedema should avoid the use of angiotensine-converting enzyme inhibitors (ACE inhibitors). Angioedema can occur several months after the beginning of treatment;
-
exclusion diets (only if the history suggests causal nexus) and exclude, whenever possible, food additives such as preservatives, natural salicylates and dyes including: sodium metabisulfite, sodium benzoate, monosodium glutamate, sodium nitrate, tartrazine, erythrosine, sorbic acid and butylhydroxyanisol.
43 With regard to monosodium glutamate a causal relation was not proven in a controlled study of patients with chronic urticaria
44 (grade of recommendation B). True food allergy is an exception in chronic urticaria, in contrast to the acute form, and there are no specific complementary tests (grade of recommendation B).
II) Drug treatment (Chart 15):
-
First-line drug treatment
Oral antihistamines are the essential drugs in treatment of chronic urticaria, and good or reasonable response is obtained in 44%-91% of patients, when all types of urticaria are evaluated.45 Second-generation antihistamines which are non-sedating or little sedating can be used, such as cetirizine, fexofenadine, loratadine, mizolastine and, more recently, levocetirizine, desloratadine and ebastine, all by oral route (grade of recommendation A). Treatment can be started with one of the following drugs: cetirizine 10mg/day, fexofenadine 180mg/day, desloratadine 5mg/day, loratadine 10mg/day or epinastine 20mg/day. They present similar effectiveness. However, because of the absence of hepatic metabolism, fexofenadine and desloratadine are indicated in patients with liver disease.
If the response to non-sedating anti-H1 is not satisfactory, a classical anti-H1 can be introduced at night, in view of its more sedating properties, of which the authors prefer hydroxyzine 25mg before going to bed; when there is associated angioedema, the drug of choice is also a classical anti-H1, particularly hydroxyzine, 25mg to 100mg/day orally, in 25mg fractions every 8 or 6 hours. Other options are clemastine, dexchlorpheniramine and cyproheptadine. Doxepine, a tricyclic antidepressant with a strong antihistamine effect, can also be used (but must not be combined with cimetidine).
The use of first-generation antihistamines during pregnancy should be limited. Chlorpheniramine and diphenidramine are considered category B drugs by the FDA (drugs for which animal studies did not show adverse effects, but with no data available in humans).46,47 As to the second-generation antihistamines, cetirizine and loratadine are also considered category B drugs by the FDA.46
Fexofenadine and loratadine (and consequently desloratadine) are considered drugs compatible with breastfeeding.46
The association of H1 and H2 receptor antagonists (cimetidine, ranitidine) has a theoretical rationale, but its effectiveness is discussed in the literature (grade of recommendation C). The H2 receptors influence skin vasodilation and vasopermeability, but they do not produce pruritus or erythema.
-
Second-line drug treatment
The use of oral corticosteroids can be necessary for short periods (7 to 14 days) in cases of major exacerbation of chronic urticaria that does not respond completely to antihistamines. Long-term use should be avoided.
Tedeschi et al.42 found good response to antileukotrienes (montelukast) in about 20% to 50% of patients who did not respond to treatment with antihistamines only (grade of recommendation B).
-
Third-line drug treatment (immunosuppressors/immunomodulators)
In patients with severe disease and persistent course, with therapeutic failure of the previous measures, or in cases where the investigation demonstrated that the urticaria had an autoimmune basis, immunosuppressive therapy has become an option, especially in the context of studies carried out at universities. Some studies aimed to reduce the use of systemic corticosteroids and assessed cyclosporine, plasmapheresis and intravenous immunoglobulin (grade of recommendation C). Cyclosporin can be used at an initial dose of 4mg/kg/day during four weeks, then reducing to 3mg/kg/day for six weeks and finally to 2mg/kg/day for another six weeks.37 Intravenous immunoglobulin is administered at 0.4g/kg/day for five days, in slow infusion.48
Other drugs, such as sulfasalazine, hydroxychloroquine, methotrexate, warfarin, colchicine and sulfone, which have an immunomodulating effect, but with no controlled studies with small samples and completely proven efficacy, are employed as an alternative to failures of the conventional therapy (grade of recommendation C).36,42 
REFERENCES
Received on October 24, 2005.
Approved by the Editorial Council and accepted for publication on October 31, 2005.
- 1. Zuberbier T, Greaves MW, Juhlin L, Kobza-Black A, Maurer D, Stingl G, et al. Definition, classification, and routine diagnosis of urticaria: a consensus report. J Investig Dermatol Symp Proc. 2001; 6:123-7.
- 2. Cooper KD. Urticaria and angioedema: diagnosis and evaluation. J Am Acad Dermatol. 1991;25:166-76.
- 3. Criado RFJ, Criado PR, Sittart JAS, Pires MC, Mello JF, Aun WT. Urticária e doenças sistêmicas. Rev Assoc Med Bras. 1999;45:349-56.
- 4. Champion RH, Roberts SO, Carpenter RC. Urticaria and angioedema: a review of 554 patients. Br J Dermatol. 1969;81:588-97.
- 5. Société Française de Dermatologie. Consensus Conference Management of chronic urticaria. Eur J Dermatol.2003;13:385-92.
- 6. Grattan C, Powell S, Humphreys F. Management and diagnostic guidelines for urticaria and angio-edema. Br J Dermatol. 2001;144:708-14.
- 7. Criado PR. Resposta inflamatória na urticária aguda desencadeada por exposição a medicamentos: estudo ultra-estrutural [tese ]. São Paulo: Hospital do Servidor Público Estadual de São Paulo; 2002.
- 8. Huston DP, Bressler RB. Urticaria and angioedema. Med Clin North Am. 1992;76:805-40.
- 9. Schwartz LB. Mast cells and their role in urticaria. J Am Acad Dermatol. 1991;25:190-203.
- 10. Haas N, Motel K, Czarnetzki BM. Comparative immunoreactivity of the eosinophil constituents MBP and ECP in different types of urticaria. Arch Dermatol Res. 1995;287:180-5.
- 11. Grattan CEH, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol. 2002;46:645-57.
- 12. Greaves MW. Chronic urticaria in childhood. Allergy. 2000;55:309-20.
- 13. Greaves MW. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664-72.
- 14. Greaves MW. Immunology and inflammation: type I allergy and intolerance. J Dermatol Treat. 2000;11:S27-S30.
- 15. Hizal M, Tuzun B, Wolf R, Tuzun Y. The relationship between Helicobacter pylori IgG antibody and autologous serum test in chronic urticaria. Int J Dermatol. 2000;39:443-5.
- 16. Federman DJ, Kisner RS, Moriarty JP, Concato J. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. J Am Acad Dermatol 2003;49:861-4.
- 17. Turktas I, Gockora N, Dermisoy S, Cakir N, Onal E. The association of chronic urticaria and angioedema with autoimmune thyroiditis. Int J Dermatol. 1997;36:187-90.
- 18. Rottem M. Chronic urticaria and autoimmune thyroid disease: is there a link? Autoimmun Rev. 2003;2:69-72.
- 19. Warshaw EM. Latex allergy. J Am Acad Dermatol. 1998;39:1-24.
- 20. Tharp MD, Thirlby R, Sullivan TJ. Gastrin induces histamine release from human cutaneous mast cells. J Allergy Clin Immunol. 1984;74:159-65.
- 21. Lessof MH, Gant V, Hinuma K, Murphy GM, Dowling RH. Recurrent urticaria and reduced diamine oxidase activity. Clin Exp Allergy. 1990;20:373-6.
- 22. Goga D, Vaillant L, Mateu J, Ballon G, Beutter P. The elimination of dental and sinusal infectious foci in dermatologic pathology. A double-blind study in 27 cases confined to chronic urticaria. Rev Stomatol Chir Maxillofac. 1988;89:273-5.
- 23. Kozel MMA, Mekkes JR, Bossuyt PMM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45;387-91.
- 24. Zuberbier T, Iffländer J, Semmler C, Henz BM. Acute urticaria: clinical aspects and therapeutic responsiveness. Acta Dermatol Venereol (Stockh).1996;76:295-7.
- 25. Greaves MW, Sabroe RA. Allergy and the skin. I - Urticaria. BMJ. 1998;316:1147-50.
- 26. Grattan C, Powell S, Humphreys F. British Association of Dermatologists. Management and diagnostic guidelines for urticaria and angio-oedema. Br J Dermatol. 2001;144:708-14.
- 27. Bindslev-Jensen C, Finzi A, Greaves M, Camarasa J, Ortonne J-P, Schöpf E, et al. Chronic urticaria: diagnostic recommendations. J Eur Acad Dermatol Venereol. 2000;14:175-80.
- 28. Hauser C. Chronic Urticaria. In: Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B, Williams H, editors. Evidence based Dermatology [Monograph on the Internet ]. England: BMJ Publishing; 2003. [cited 2005 Oct 12 ]. Available from: http//:www.bmjpb.com/chapters/0727914421_sample.pdf
- 29. Grabbe J. Pathomecanisms in Physical Urticarias. J Invest Dermatol Symp Proc. 2001;6:135-6.
- 30. Kobza Black A, Lawlor F, Greaves MW. Consensus meeting on the definition of physical urticarias and urticarial vasculitis. Clin Exp Dermatol. 1996;21:424-6.
- 31. Claudy A. Cold urticaria. J Invest Dermatol Symp Proc. 2001;6:141-2.
- 32. Markovic SN, Inwards DJ, Frigas EA, Phyliky RP. Acquired C1 esterase inhibitor deficiency. Ann Intern Med. 2000;132:144-50.
- 33. Fiorentino DF. Cutaneous vasculitis. J Am Acad Dermatol. 2003;48:311-40.
- 34. Karim MY, Masood A. ACE-inhibitors and angioedema. J Allergy Clin Immunol. 2002; 110:539.
- 35. Brown SGA. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371-6.
- 36. Zuberbier T. Urticaria. Allergy. 2003;58:1224-34.
- 37. Simons FER. Prevention of acute urticaria in young children with atopic dermatitis. J Allergy Clin Immunol. 2001;107:703-6.
- 38. Zuberbier T, Ifflander J, Semmler C, Czarnetzki BM. Acute urticaria clinical aspects and therapeutical resposnsiveness. Acta Dermatol Venereol (Stockh). 1996;76:296-7.
- 39. Poon M, Reid C. Do steroids help children with acute urticaria? Arch Dis Child. 2004; 89:85-6.
- 40. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol. 2005;115:S483-S523.
- 41. Brown SGA. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371-6.
- 42. Tedeschi A, Airaghi L, Lorini M, Asero R. Chronic urticaria: a role for newer immunomodulatory drugs? Am J Clin Dermatol. 2003;4:297-305.
- 43. Asero R. Multiple intolerance to food additives. J Allergy Clin Immunol. 2002;110:531-2.
- 44. Simon RA. Additive-induced urticaria: experience with monosodium glutamate (MSG). J Nutr. 2000;130:1063S-6S.
- 45. Kozel MAM, Sabroe RA. Chronic Urticaria. Aetiology, management and current and future treatment options. Drugs. 2004;64:2516-36.
- 46. Golightly LK, Greos LS. Second-generation antihistamines. Actions and efficacy in the management of allergic disorders. Drugs. 2005;65:341-84.
- 47. Simons FER. Advances in H1-Antihistamines. N Engl J Med. 2004;351:2203-17.
- 48. Grattan C. Chronic urticaria: general principles and management. In: Greaves MW, Kaplan AP, editors. Urticaria and angioedema. Nova York: Marcel Dekker; 2004. p.343-68.
Publication Dates
-
Publication in this collection
16 Mar 2006 -
Date of issue
Dec 2005
History
-
Accepted
31 Oct 2005 -
Received
24 Oct 2005