Abstracts
Melasma is a common dermatosis that involves changes in normal skin pigmentation, resulting from the hyperactivity of epidermal melanocytes. The consequent hyperpigmentation is mostly induced by ultraviolet radiation. Clinically, melasma is characterized by light to dark brown macules that usually occur on the face, although they can also affect the cervical and anterior thoracic regions and upper members.Fertile age women and those with intermediate skin phototypes are most likely to develop melasma. Most of its physiopathogenics is not yet fully understood, but there is a relation with genetic and hormonal factors, drugs and cosmetics use, endocrinopathies and sun exposure. The authors discuss the main aspects associated with skin pigmentation and the development of melasma.
Melanosis; Pigmentation disorders; Skin pigmentation; Ultraviolet rays
Melasma é uma dermatose comum que cursa com alteração da cor da pele normal, resultante da hiperatividade melanocítica focal epidérmica de clones de melanócitos hiperfuncionantes, com consequente hiperpigmentação melânica induzida, principalmente, pela radiação ultravioleta. Clinicamente, caracteriza-se por manchas acastanhadas, localizadas preferencialmente na face, embora possa acometer também região cervical, torácica anterior e membros superiores.Mulheres em período fértil e de fototipos intermediários representam as populações mais acometidas. Grande parte de sua fisiopatogenia permanece desconhecida, havendo relação com fatores genéticos, hormonais, uso de medicamentos, cosméticos, endocrinopatias e fotoexposição. Os autores discutem os principais elementos relacionados à pigmentação da pele e ao desenvolvimento do melasma.
Melanose; Pigmentação da pele; Raios Ultravioleta; Transtornos da pigmentação
REVIEW
Physiopathology of melasma
Luciane Donida Bartoli MiotI; Hélio Amante MiotII; Márcia Guimarães da SilvaIII; Mariângela Esther Alencar MarquesIV
IIIPhD Assistant Professor of the Department of Dermatology and Radiation Therapy of Botucatu School of Medicine (Unesp) - Botucatu (SP), Brazil
IIIPhD Assistant Professor of the Department of Pathology of Botucatu School of Medicine (Unesp) - Botucatu (SP), Brazil
IVAdjunct Professor of the Department of Pathology of Botucatu School of Medicine (Unesp) - Botucatu (SP), Brazil
Mailing Address
ABSTRACT
Melasma is a common dermatosis that involves changes in normal skin pigmentation, resulting from the hyperactivity of epidermal melanocytes. The consequent hyperpigmentation is mostly induced by ultraviolet radiation. Clinically, melasma is characterized by light to dark brown macules that usually occur on the face, although they can also affect the cervical and anterior thoracic regions and upper members.Fertile age women and those with intermediate skin phototypes are most likely to develop melasma. Most of its physiopathogenics is not yet fully understood, but there is a relation with genetic and hormonal factors, drugs and cosmetics use, endocrinopathies and sun exposure. The authors discuss the main aspects associated with skin pigmentation and the development of melasma.
Keywords: melanosis; skin pigmentation; ultraviolet rays; pigmentation disorders
INTRODUCTION
Normal skin color
The skin is the most visible aspect of the human phenotype and its color is one of its most variable features. Little is known about the genetic, evolutional and cultural aspects related to the definition of human skin color patterns.1,2
We believe that variations in skin color are evolutional and are related to the regulation of the penetration of ultraviolet radiation (UVR). 3,4
The synthesis of vitamin D on the skin, degradation of folic acid by UVR, resistance to direct sun exposure and cultural elements are arguments that try to explain the phenotypical distribution of skin color in different latitudes of the planet.5,6
Normal human skin color is mainly influenced by the production of melanin, a dense high-molecular-weight brown pigment, that looks darker the more concentrated it is.7-9
However, exogenous yellow pigments, the carotenoids, also contribute to skin color, as well as endogenous red, oxygenated hemoglobin in capillary vessels in the dermis, and endogenous blue, reduced hemoglobin in venulas. 7,9
In humans, skin and hair pigmentation depends on the melanogenic activity inside melanocytes, melanin synthesis rate, as well as on the size, number, composition and distribution of cytoplasm particles of the melanocytes called melanosomes, in addition to the chemical nature of the melanin they have.8-11
Melanocytes and melanosomes have a relatively constant number in different races, as discussed ahead.9
Melanocytes
Melanocytes are phenotypically important cells, responsible for skin and hair pigmentation, contributing skin tone and providing direct protection against the damages caused by UVR.9
They are dendritic cells, embryologically derived from melanoblasts, which originate from the neural crest, migrating to the skin shortly after the closing of the neural tube. This migration may occur to many destinations, and the signalizer that direct this process still need to be better described.8,12
When they become fully developed cells, they spread through different sites: the eyes (retina pigment epithelium, iris and choroid), ears (vascular strias), central nervous system (leptomeninges), hair matrix, mucosas, and skin.8,12
On the skin, they are located on the basal layer of the epidermis and, occasionally, on the dermis. They project their dendrites through the Malpighii stratum where they transfer their melanosomes to keratinocytes (Figure 1). This melanocyte-keratinocyte association is called epidermal melanin unit and is formed, in humans, by one melanocyte and about thirty-six keratinocytes.8,13,14
Epidermal basal cells are united to adjacent cells by specific structures called desmosomes and to the basement membrane by hemidesmosomes. Melanocytes are not fixed on the epidermis; they can only be identified by a small unevenness in their position in relation to the alignment of the basal layer, projecting slightly towards the dermis (Figure 2).11
The density of melanocytes varies in different parts of the body. There are about 2,000 or more epidermal melanocytes per square millimeter of skin in the head and forearm, and about 1,000 in the rest of the body, in all races. This exact regulation of the number of melanocytes on the epidermis seems to be mediated by keratinocytes and by specific mediators like the fibroblast growth factor (FGF2).11
The number of melanocytes goes down with age, in areas not exposed to light, at the proportion of 6% to 8% per decade, and racial differences in pigmentation are not due to a significant variation in the number of melanocytes, but rather on their level of activity (melanosomes and melanin synthesis), in the proportion of melanin subtypes (pheomelanin and eumelanin), their distribution, and environmental factors like sun exposure, which directly stimulates the synthesis of melanin.11,15
The melanin produced in melanocytes is stored in specific intracytoplasmatic structures called melanosomes.
Melanosomes
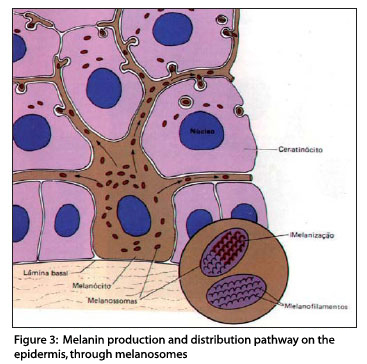
Melanosomes are highly specialized elliptical organelles, where there is synthesis and deposition of melanin (Figure 3), storage of the tyrosinase synthesized by ribosomes, and they are the site of the biochemical phenomena that originate melanin.7
Melanin synthesis takes place exclusively in melanosomes and dependents on many genes.
Melanosomes develop in a series of morphologically defined stages (Figure 4), from unpigmented structures (stage I) to striped organelles filled with melanin (stage IV).11,16
The fundamental phenotypical difference between the more pigmented and less pigmented races does not reside on the production of melanin, neither on the number of melanocytes, but mainly in the quality of the melanosomes (Table 1)16.
Melanosomes are larger and more mature in black people than in white people and are stored more as units than in clusters. The degradation of larger melanosomes in keratinocytes is delayed, which also contributes to the higher levels of skin pigmentation in these cases.8 The processes that lead to this behavior difference need to be further clarified.
In normal skin melanosomes, melanin is extremely dense. It is an insoluble high-molecular-weight nitrogenated polymer that forms a pigment, which, in addition to providing color to the skin, plays a protection role, filtering and absorbing UVR. It plays, therefore, an important photoprotective role against UVR damages, as shown by the inverse correlation between the content of melanin on human skin and the incidence of skin carcinomas and melanomas.11,17
Melanin
Melanin is the main biological pigment involved in skin pigmentation, and is determinant for the differences in skin color.11
The initial element of melanin biosynthesis is tyrosine, an essential amino acid. Tyrosine suffers the chemical action of tyrosinase, a copper protein enzyme complex synthesized in the ribosomes and transferred through the endoplasmic reticulum to the Golgi apparatus, and it is clustered in units surrounded by a membrane, i.e. the melanosomes.11
The three members of the tyrosinase-related family (tyrosinase, Tyrp 1 or tyrosinase-related protein 1, and Dct or dopachrome tautomerase) are involved in the melanogenesis process leading to the production of either eumelanin (brown-black) or pheomelanin (yellow-red).18
In the presence of molecular oxygen, tyrosinase oxidizes tyrosine into dopa (dihydroxyphenylalanine) and this into dopaquinone. From then on, the presence or absence of cysteine determines the course of the reaction for the synthesis of eumelanin or pheomelanin.19
In the absence of cysteine (glutatione), dopaquinone is converted into cyclodopa (leukodopachrom) and this into dopachrome. There are two degradation pathways for dopachrome, one that forms DHI (dopa,5,6 dihydroxyindole) at a larger proportion and another that forms a smaller quantity of DHICA (5,6 dihydroxyindole-2-carboxylic acid). This process is catalyzed by dopachrome tautomerase (Tyrp 2-Dct). Finally, these dihydroxyindoles are oxidized into melanin.19
Tyrosinase related protein 1 (Tyrp 1) seems to be involved in the catalysis of the oxidation of DHICA into eumelanin. On the other hand, in the presence of cysteine, dopaquinone rapidly reacts with this substance to generate 5-S-cysteinyldopa and a smaller proportion of 2-S-cysteinyldopa. Cysteinyldopas are then oxidized into benzothiazine intermediates and finally produce pheomelanin (Figure 5).19
Eumelanin is an insoluble brown alkaline polymer, and pheomelanin is a soluble yellowish alkaline pigment. Pigments similar to pheomelanin, however, can be structurally derived from eumelanin, as it can be oxidized in the presence of metallic ions, resulting in a lighter soluble pigment. Another sulfur-containing pigment, derived from pheomelanin, which can be found in small quantities in red human hair, and is called trichrome.13
In this manner, melanogenesis has three different and important steps. The first step is the production of cysteinyldopa, which continues as intense as the quantity of cysteine present. The second step is the oxidation of cysteinyldopa to form pheomelanin, a process that depends on the quantity of cysteinyldopa present. And the third and last step is the production of eumelanin, which only starts after the depletion of most of the cysteinyldopa. However, eumelanin seems to deposit on pre-formed pheomelanin and the ratio between pheomelanin and eumelanin is determined by the activity of tyrosinase and availability of cysteine.19
Eumelanin absorbs and disperses ultraviolet light, attenuating its penetration on the skin and reducing the harmful effects of the sun. In other words, people with more pigmentation tend to get less sun burnt and to tan more than those who are lighter skinned.15,20,21
Pheomelanin, on the other hand, has a great potential to generate free radicals in response to UVR, which are capable of causing damage to DNA, and, in this manner, may contribute to the phototoxic effects of UVR. This explains why people with light skin, who have relatively high quantities of pheomelanin, are at greater risk of ultraviolet-induced epidermal damage, including neoplasms. 20
Individual melanocytes typically synthesize eumelanins and pheomelanins, and their rate is determined by a balance of variables, including the expression of pigment enzymes and the availability of tyrosinase and specific reducing agents in the cell.9
Melanocortin 1 receptor (MC1-R) controls the amount of eumelanin and pheomelanin inside melanosomes. This proportion is an important determinant of sun sensitivity in humans. However, probably the total quantity of melanin produced is even more important than the ratio of the types of melanin.5 It is known that melanocytes derived from intensely pigmented skin present greater quantity of total melanin and also a greater rate of eumelanin than the melanocytes derived from light skins.10
Total skin melanin is the result of a combination of monomers of pheomelanin and eumelanin and the proportion between the two determines the final phenotypical expression of skin and hair color.
A reduction in eumelanin and the predominant presence of pheomelanin, as in red-haired people, are regaled chiefly by MC1-R.11
Two types of melanin pigmentation are the basis for normal skin color. Constitutive skin color is the genetically determined color of healthy skin not exposed to UVR and it plays a key role in photoprotection against the UVR that reaches the Earth, thus minimizing the damages to DNA that may lead to the emergence of skin cancer.7,17
Facultative skin color is the more intense skin color resulting from sun exposure or pigmentary diseases and reflects the genetically determined tanning capacity in response to UVR. In this manner, the "tanning" level is genetically determined and is the basis for the division of normal skin in adaptive response patterns called phototypes.7
After the complete synthesis of melanin, melanosomes filled with this pigment are injected in the interior of keratinocytes in the corresponding epidermal melanin unit through the dendritic extensions of melanocytes (cytocin activity). Once inside keratinocytes, melanosomes tend to spread through the cytoplasm, over the upper part of the nucleus, so as to protect it from ultraviolet radiations. It has been suggested that the pigment inside these cells also works as a scanner of photoproduced free radicals, always striving to protect cell DNA.12,22,23
The photoprotection properties of the melanin of human skin have been well documented and take place through the absorption and dispersion both of ultraviolet light and visible light. This absorption increases linearly at the range of 720-620 nm and then exponentially through shorter waves (300-600 nm). 17
Melanin has great affinity for DNA, and is capable of producing reactive oxygen species in response to ultraviolet radiation A. In people with light skin, the greater incidence of melanomas seems to result not only from poor natural protection, but also from increased mutations promoting the formation of pheomelanin and/or melanin intermediates.8,11,24
Ultra-structural studies revealed that eumelanosomes, which are usually produced by dark skins, remain intact on the epidermis after exposure to UVR, whereas in light skin, no intact melanosome can be detected after this radiation. 17
The main factors that regulate the quantity and quality of the melanin produced by melanocytes include UVR, -MSH ( -type melanocyte stimulating hormone or melanocortin), ASP (AGOUTI signaling protein), and MC1-R.8,25
α-MSH and MC1-R
The melanin pigmentation of human skin suffers intense hormonal control. In 1967, Snell summarized the prevailing consensus about hormonal action on the melanocytes of mammals, particularly humans.26,27
Injections of α-MSH and β-MSH in human individuals produced a darkening of the skin resulting from the high melanogenesis inside epidermal melanocytes and increased transportation of melanosomes derived from melanocytes, to keratinocytes, without the need of exposure to UVR. Hyperpigmentation of the skin is also seen when human individuals were injected with high doses of adrenocorticotropic hormone (ACTH).11,26,28,29
α-MSH is a tridecapeptide with a sequence identical to the first 13 amino acids of ACTH. The proteolytic cleavage of proopiomelanocortin (POMC), on the pituitary gland, is responsible for the origin of many byproducts, including α-MSH. It is also known that POMC is expressed and suffers cleavage in other places, including the brain and skin. α-MSH was the first of the peptides derived from POMC to be identified on the skin.20
Human keratinocytes are capable of synthesizing α-MSH and β-MSH at physiological quantities. α-MSH is also produced in melanocytes and Langerhans cells.9,11,17,25,30-34 Evidences indicate that these hormones play a paracrine role in the regulation of the functions of melanocytes. More than 120 genes have been identified and seem to regulate pigmentation; however, the effects of -MSH are mediated by MC1-R, which is expressed on the surface of melanocytes, and is considered the key point for pigmentation. It is also present in other cells like monocytes, neutrophiles, glioma cells, astrocytes, macrophages, fibroblasts, endothelial cells, and keratinocytes. As MC1-R has a wide tissue distribution, it probably is associated to a large number of biological functions.9,11,17,25,30,32-35
In 1992, Mountjoy et al.36 from the Oregon University of Health Sciences, in Portland, reported they had cloned an MC1-R hormone receptor, in humans and rats. They also demonstrated that mutations in the gene of this receptor caused hair color changes in rats. In 1995, Valverde et al.37 reported a similar association between abnormal forms of the receptor and variations in the skin and hair color of people.
In spite of the identification of more than 100 loci involved in the pigmentation of vertebrates, MC1-R is the chief determinant of the pigmentation phenotype. The extension of its locus was first identified in rats based on hair color changes. Recessive mutants had yellowish or pheomelanotic hair, while wild-type rats had dark/brown or eumelanotic hair.9
In the early 90's, molecular structure of the MSH receptor was described; it was then called MC1-R and its antagonist, AGOUTI signaling protein (ASP). For many years, two loci were known to be involved in the qualitative regulation (eumelanin and pheomelanin) and quantitative regulation of the pigmentation of mammals, and ASP was produced in the follicles and acting in follicular melanocytes through the inhibition of the synthesis of eumelanin.17,38,39 Before cloning, two melanocortin receptors, MSH and ACTH receptors, were discovered in classical pharmacological and physiological studies.
The melanocortin system consists of peptides with many forms of MSH (alpha, beta and gamma) and ACTH; a family with five melanocortin receptors, linked to protein G was described, with seven transmembrane domains (seven passages through the membrane) and ASP.17,38,39
MC1-R was the first ?-MSH receptor that was cloned and was isolated from a melanoma cell strain.34 Human MC1-R gene is located in chromosome 16q24.3 and has a reading frame of 951 base pairs that codify a protein with 317 amino acids. The human protein sequence shows all the characteristics of the receptors coupled to protein G, including the presence of seven transmembrane fragments and two sites of potential N-glicosilation. The occurrence of specific high-affinity binding sites in most of the human melanocytes was already known, even before the MC1-R gene was cloned.25,40,41 However, the number of binding sites is variable, and it can be as low as a few hundreds per cell, as detected by Scatchard's analysis using radiomarked probes. Not just α-MSH, but also ACTH, β and γ MSH34 bind to MC1-R.
It is then a highly polymorphic white gene population and these gene variations are associated with light skin and reddish hair and act in the reduction of the ability of the epidermis to respond to UVR.
Today, the MC1-R gene is considered one of the main markers of susceptibility to malignant skin neoplasms, as gene variants are associated to an increased risk of melanoma and non-melanoma skin cancers.17,30,34,42-44
Other studies demonstrated that solar ephelis and lentigines are different types of pigmented lesions that present significant differences in their etiologies, but gene variants of MC1-R are a necessary factor for the development of ephelis, while they play a less important role in the case of lentigines.3,30,45,46
Variations related to gene MC1-R are exceptionally high among Caucasians and have a significant impact on the pigment phenotype of this ethnic group. Red hair has been related to some alleles, but recent studies indicate that the same genotypes may express different hair colors depending on the population. 3,44
MC1-R is abundantly expressed in human and rat melanoma cells, and at significantly lower levels in rat melanocytes. More recently, it has been demonstrated in normal glands of human skin and hair follicles, as well as in skin malformations and neoplasms.34
In most individuals with light skin, who do not tan, there is a variation in the gene sequence of classical MC1-R, which normally determines dark hair and ease to tan.11 In individuals with reddish hair and light skin that have a predominance of pheomelanin in the hair and skin and/or reduced ability to synthesize eumelanin, a functional reduction of MC1-R with a resulting decrease in the melanotropin-induced tyrosinase activity, associated with eumelanogenesis, can be the key in the promotion of pheomelanin synthesis inside human melanocytes.
In this manner, the MC-1R of melanocytes is undoubtedly an important element in the regulation of pigmentation in mammals, but also one of the most polymorphic.11,20,30,32-34,47 Variant gene sequences are found in more than 80% of people with red hair and light skin, in less than 20% of individuals with brown or black hair and in less than 4% of those who respond easily to tanning. 34
α-MSH signals through MC1-R, activating adenyl cyclase (AC) and increasing intracellular cyclic adenosin monophosphate (AMPc), resulting in the production of dark eumelanin pigment (Figure 6). Whether MC1-R is involved in other signaling pathways, still remains unknown, but the activation of MC1-R influences the relative quantities of pheomelanin and eumelanin produced, and its activity loss is associated to red or yellow hair.20,34,38,39,48-52
Variants of MC1-R have been associated with red hair inheritance, in which more yellow-reddish pheomelanin pigment is produced and they present very small tanning capacity. MC1-R variants R160W, R151C, D294H, R142H, 86insA, and 537insC are the main determinants of the red hair and light skin phenotype. It is a phenotype typical of phototypes I and II, with greater chance of sun burn and development of skin neoplasms.10,20,34
The recently cloned murine gene AGOUTI is located in chromosome 2 and codes protein ASP formed by 131 amino acids and acts as a competitive antagonist of MC1-R, blocking its activation by α-MSH. However, the shift between eumelanogenesis and pheomelanogenesis involves the opposition to ASP and α-MSH effects as binders for MC1-R. Pheomelanogenesis can be stimulated by in vitro treatment with purified recombinant ASP. After treatment with ASP, the expression of genes that codify tyrosinase and other melanogenetic proteins is suppressed in melanocytes, which requires other physiological factors typical of in vivo pheomelanogenesis. In normal human melanocytes, where the number of expressed MC1-R is relatively low, ASP completely annuls α-MSH stimulating effects in melanocytic and melanogenesis proliferation.17,25,53
The incapacity to tan of individuals with variation in MC1-R is consistent with the critical role MSH/AMPc play in this response, but some studies indicate that damages to melanocyte DNA may mediate pigmentation induced by UVR.54
UVR and Pigmentation
The solar radiation spectrum is broad, ranging from cosmic rays (ultra X rays) to infrared radiation. Shorter wavelength radiations, up to 200 nm, do not reach the Earth, because they are absorbed by atmospheric oxygen and ozone.17,55 UVR and visible light are between 200 nm and 760 nm and form the photobiological spectrum, with ultraviolet between 200 and 400 nm and visible light between 400 and 760 nm. Beyond this limit, up to 17000 nm is infrared, which is a heat inducer.17,55
The acute effects of exposure to UVR are basically two: skin burn and/or tanning. The individual response to exposure to UVR, that is, how tanned one may get, is one of the best examples of human adaptation to the environment. 9
After one single exposure to UVR, an increase in the size of melanocytes can be observed, followed by an increase in tyrosinase activity. Repeated exposures to UVR lead to an increase in the number of stage IV melanosomes transferred to keratinocytes, as well as an increase in the number of active melanocytes. Moreover, the density of melanocytes, in comparative studies, is greater in photoexposed areas.16
Therefore, UVR is an efficient stimulant of skin pigmentation in humans and is responsible for the initiation of the tanning response. Many mechanisms may be involved and the response is believed to be the result of a combination of different signals acting both directly and indirectly in melanocytes. Indirect UVR action involves the release of keratinocyte mediators in the skin.33,40
Ultraviolet-B radiation (UVB) on human skin induces the production of α-MSH and ACTH in melanocytes and keratinocytes. α-MSH stimulates the activity of tyrosinase and in vivo melanin synthesis, and the synthesis of melanocytes through MC1-R. Other reports indicate that the irradiation of melanocytes with UVR increases MC1-R RNAm levels. Moreover, the synthesis of many epidermal factors, including α-MSH, ACTH and endothelin-1, is increased by the exposure to UVR, suggesting an important influence of these mediators on the response of melanocytes to sun light.32,56-58
Ultraviolet C (UVC) (200-290 nm) is basically germicide; UVB (290-320 nm) causes erythema, pigmentation and especially alterations that induce skin cancer; and ultraviolet A (UVA) (320-400 nm) penetrates deeper in the skin, causes pigmentation and cancerigenous alterations, and is the main inducer de photosensitivity. 55
UVB is the chief cause of sun burn, causing erythema after a latency period of 2 to 7 hours. UVA causes erythema that appears later and may gradually become more intense.55
The interaction of hormones and UVR can be illustrated in melasma. UVR stimulates the production of melanocortin inside melanocytes and keratinocytes, which explains the involvement of this hormone in the pathogenesis of melasma that is basically characterized by increased epidermal melanization in melanocytic proliferation.11
Melasma
Melasma is a common, acquired and symmetrical hypermelanosis characterized by more or less dark brownish maculae, with irregular contour, but clear limits, on photoexposed areas, especially the face, forehead, temples, and more rarely on the nose, eyelids, chin, and upper limbs (Figure 7).59-62
It is a dermatological disease easily diagnosed by clinical examination, typically chronic, with frequent recurrences, great refractoriness to existing treatments, and with many unknown physiopathological aspects.57
The word melasma comes from the Greek melas that means black. Chloasma is a term used with the same meaning, also derived from the Greek cloazein, meaning greenish. The word melasma is, therefore, the most appropriate designation for the condition.59
Although it may affect both sexes and all races, it is more often in intermediate phototypes and people of Asian or Hispanic origin that live in tropical areas. It is more common in adult women in childbearing age, but its onset can also be after menopause. The age of onset is usually between 30-55 years and men account for only 10% of cases.7,57,61,63,64
Even though melasma is more frequent among Hispanics, its exact prevalence is unknown. Approximately 66% of Mexican women develop melasma during pregnancy and one third of these women keep the pigmentation for the rest of their lives.65-67
To provide an idea of this disease, according to a 2000 census in the United States, Hispanics accounted for 12.6% of the population and this number is estimated to grow annually to 15.5% in 2010 and 24.4% in 2050. 65,67
There is no consensus as to the clinical classification of melasma. Two patterns of facial melasma are recognized: central-facial, which affects the central region of the forehead, mouth, lips, supra labial area, and chin; and malar, which affects the zygomatic region. Some authors also add a third and less frequent pattern, called mandibular. Ponzio & Cruz observed in a study, that 78.7% of the melasmas were central and 21.3% were peripheral.59,63,68
There are countless factors involved in the etiology of the disease, but none of them can be mentioned as the only factor leading to its development. They include: genetic influences, exposure to UVR, pregnancy, hormone therapy, cosmetics, phototoxic drugs, endocrinopathies, emotional factors, anti-convulsive drugs, and others with historic value. However, it seems that genetic predisposition and exposure to sun radiation play an important role, considering that melasma lesions are more evident during or shortly after periods of exposure to the sun.7,60,62,63,68-70
Sacre et al., investigating idiopathic melasma, concluded that thyrotropin, prolactin, and gonadotrophin reserves are normal and that, as ovary and thyroid functions were also normal, it was not possible to establish a correlation between hormone levels and this form of melasma.71
In contrast with what happens in pregnancy, melasma induced by anovulatory drugs does not regress with the suspension of the drug and, among the patients that had melasma because of birth control pills, 87% had also had it in previous pregnancies.63
Genetic predisposition has been suggested by reports of family occurrence. A racial factor has been reported due to the frequent occurrence of melasma Hispanic patients. Sanchez et al. Identified family history in more than 20% of the cases studied, and all patients reported exacerbation due to sun light and the use of cosmetics.59,72
It is worth highlighting that melasma is one of the unaesthetic dermatoses that lead to great demand for specialized dermatological care, even though they are just a common and benign pigmentation abnormality. This might be explained by its cosmetically compromising nature and the associated emotional and psychological effects in individuals affected by this problem, who often, because of dissatisfaction with their appearance, eventually reduce their social lives, even with cases of suicide reported.65-67,69
Although this condition often has only aesthetical implications, such concern can be very important and impacting on the social, family and professional lives those affected, causing psychological effects that cannot be neglected.65,73
In 2003, Melasqol, a new tool for assessing health-related quality of life in women with melasma, was published by Balkrishnan et al.. This instrument was validated and demonstrated utility to monitor the impact caused by melasma on the quality of life of patients. The main quality of life domains that showed to be affected by melasma were social life, recreation, leisure, and emotional well being. In 2006, this tool was translated into Portuguese and culturally adapted, according to the rules defined by the World Health Organization.65-67,74
The treatment of melasma is usually dissatisfactory due to the great recurrence of lesions and due to the absence of a definitive whitening alternative. Controlled clinical trials indicate photoprotection and the use of whiteners as the first line elements of its treatment.75-77 However, the discussion about the many treatment modalities, in spite of the great clinical and academic interest, is outside the scope of this paper.
This being the case, contributing to the understanding of the mechanism through which melanocytes promote localized phenotypical coloration or how skin color is genetically pre-determined and changes color induced by factors like sun light, hormones, inflammations, and others, is a task of major importance, and such clarifications may provide relevant support to treatment innovations and, consequently, improve the quality of life of patients.
Jointly, comparative studies on skin affected by melasma and normal adjacent skin found that this condition is characterized by epidermal hyperpigmentation without increase in the number of melanocytes, increase in the quantity of melanin in all layers of the epidermis, increase in the number of melanosomes, and augmented dermal elastosis.57,78 Dermal pigmentation does not differ on the epidermis with melasma and on healthy adjacent skin, this finding goes against the classification of melasmas in epidermal, dermal and combined, as proposed by Wood.2,57,59,63
Recent studies indicate that countless peptides play an autocrine or parocrine regulation in human skin melanocytes and in many pigmentary diseases. They are represented mainly by: endothelin 1 (ET-1), granulocyte macrophage colony-stimulating factor, and membrane-bound stem cell factor (SCF). Growth related oncogene- is also known to regulate the interactions between melanocytes and keratinocytes, hepatocyte growth factor, and soluble stem cell factor for interactions between fibroblasts and melanocytes.1,79,80
This inter-relation also involves some specific receptors expressed in melanocytes, like endothelin-B receptor, stem cell factor receptor, and c-KIT. Up or downregulation of this interconnected network is intrinsically involved in the stimulation melanocytic functions in many epidermal disorders that evolve with pigmentation alterations.79,80
Immunohistochemical findings suggest that a strong immunoreactivity to α-MSH on skin with melasma is one of the leading factors in the genesis of this disease. The relationship between the photoexposed area and enhanced immunoreactivity to α-MSH on affected skin has not yet been clarified. However, the existence of a yet unknown signaling pathway, with increased expression of MC1-R, which may play a significant role in this greater immunoreactivity to α-MSH should be investigated. There are evidences that a strong expression of α-MSH antigen in the keratinocytes of the skin affected with melasma, suggesting that α-MSH plays a key role in the hyperpigmentation of skin with melasma.57,81
In this manner, the evaluation of the expression of α-MSH and MC1-R in the epidermis with melasma lesions, compared to healthy perilesional skin, would permit estimating the role of MC1-R pathway in the physiopathogenesis of the disease.
Moreover, β-estradiol increases the expression of α-MSH and MC1-R in melanocytes.51 In addition to that, a recent study demonstrated an increased expression of estrogen receptors on skin with melasma, as compared to normal skin, but only two patients were qualitatively evaluated, which does yet not permit determining the real function of this receptor and of estrogen in the physiopathogenesis of melasma.82
FINAL CONSIDERATIONS
Melasma is a frequent disease in the general population that causes great impact in the quality of life of patients and drives great efforts to clinical and pharmaceutical research for the development of treatments. However, the knowledge of its physiopathogenesis is still very limited.65,83
The investigation of estrogen receptors on the epidermis and in melanocytes of healthy and affected skins would enlighten the role sexual steroid hormones play in the process of localized hyperpigmentation of this disease. 84-88
Research on allele variants of MC1-R that express differently in healthy and affected skin could explain the more effective pigmentation in certain photoexposed areas than in others.30,46,89
The culture of keratinocytes and melanocytes from healthy and melasma skin and of populations not affected by the disease, under different exposures, would permit a comparative study of the expression of many genes to demonstrate the bases of the different phenotypical behavior of these groups of adjacent cells on the same tissue.90,91
Clinical experimentation with AGOUTI proteins in melasma lesions that compete with α-MSH in MC1-R receptors, could provide physiopathological grounds for the understanding of the role of -MSH/MC1-R system in the physiopathogenesis of the disease.79,92
Profiles of melanogenetic cytokines expressed in healthy and affected skins, as well as their cells of origin, local consequences, and triggering stimulus would provide the understanding of the elements involved in the genesis of melasma.79,80,93,94
Finally, population-based epidemiological studies or with subgroups of patients with melasma, like pregnant women, post-menopausal women or men would also contribute to the design of new hypotheses for the natural history and physiopathogenesis of melasma.
REFERENCES
- 1. Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. Faseb J. 2007;2:976-94.
- 2. Miot LDB, Miot HA, Silva MG, Marques MEA. Estudo comparativo morfofuncional de melanócitos em lesőes de melasma. An Bras Dermatol. 2007;82:529-64.
- 3. Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443-52.
- 4. Ortonne JP [homepage on the Internet]. Skin color variations in humankind: an explanation? Nice: Pigmentary Disorders Academy; 2005 [cited 2009 Jun 23]. Available from: http://www.pigmentarydisordersacademy.org/guest_editorials_ortonne_skincolorjsp.
- 5. Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000;39:57-106.
- 6. Relethford JH. Hemispheric difference in human skin color. Am J Phys Anthropol.1997;104:449-57.
- 7. Mosher DB, Fitzpatrick TB, Ortonne JP, Hori Y. Normal skin color and General Considerations of Pigmentary Disorders. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF. Dermatology in General Medicine. v. 1. New York: Mcgraw-Hill; 1999. p. 936-44.
- 8. Sulaimon SS, Kitchell BE. The biology of melanocytes. Vet Dermatol. 2003;14: 57-65.
- 9. Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007; 445:843-50.
- 10. Abdel-Malek ZA, Scott MC, Furumura M, et al. The melanocortin 1 receptor is the principal mediator of the effects of agouti signaling protein on mammalian melanocytes. J Cell Sci. 2001;114:1019-24.
- 11. Jimbow K, Quevedo Jr WC, Fitzpatrick TB et al. Biology of Melanocytes. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF. Dermatology in General Medicine. v. 1. New York: Mcgraw-Hill; 1999. p.192-220.
- 12. Boissy RE. The melanocyte. Its structure, function, and subpopulations in skin, eyes, and hair. Dermatol Clin. 1988;6:161-73.
- 13. Bleehen SS, Ebling FJG, Champion RH. Disorders of Skin Color. In: Champion RH, Burton JL, Ebling FJG. Eds Rook / Wilkinson / Ebling textbook of Dermatology. v. 2. Oxford: Blackwell Scientific publications; 1992. p.1561-2.
- 14. Storm CA, Elder DE. Pele. In: Rubin E, Gorstein F, Rubin R, Scwarting R, Strayer D. Patologia: Bases clínico-patológicas da medicina. vl. 1. Rio de Janeiro: Guanabara-Koogan; 2006. p.1224-91.
- 15. Jones K, Hughes J, Hong M, Jia Q, Orndorff S. Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15:335-40.
- 16. Bolognia JL, Orlow SJ. Melanocyte biology. In: Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. v. 1. New York: Mosby; 2003.
- 17. Rouzaud F, Kadekaro AL, Abdel-Malek ZA, Hearing VJ. MC1R and the response of melanocytes to ultraviolet radiation. Mutat Res. 2005;57:133-52.
- 18. Murisier F, Beermann F. Genetics of pigment cells: lessons from the tyrosinase gene family. Histol Histopathol. 2006;2:567-78.
- 19. Ito S. A chemist's view of melanogenesis. Pigment Cell Res. 2003;16:230-6.
- 20. Thody AJ, Graham A. Does alpha-MSH have a role in regulating skin pigmentation in humans? Pigment Cell Res. 1998;1:265-74.
- 21. Wagner JK, Parra EJ, LNorton H, Jovel C, Shriver MD. Skin responses to ultraviolet radiation: effects of constitutive pigmentation, sex, and ancestry. Pigment Cell Re. 2002;15:385-90.
- 22. Hearing VJ. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J Dermatol Sci. 2005;37:3-14.
- 23. Boissy RE. Melanosome transfer to and translocation in the keratinocyte. Exp Dermato. 2003;12 Suppl 2:5-12.
- 24. Szabo G, Hirobe T, Flynn EA, Garcia RI. The biology of the melanocyte. Prog Clin Biol Res.1988;256:463-74.
- 25. Rouzaud F, Hearing VJ. Regulatory elements of the melanocortin 1 receptor. Peptides. 2005;26:1858-70.
- 26. Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155-228.
- 27. Klaus SN, Snell RS. The response of mammalian epidermal melanocytes in culture to hormones. J Invest Dermatol. 1967;48:352-8.
- 28. Barsh G, Attardi LD. A healthy tan? N Engl J Med. 2007;356:2208-10.
- 29. Wickelgren I. Skin biology. A healthy tan? Science. 2007; 315:1214-6.
- 30. Bastiaens M, ter Huurne J, Gruis N, Bergman W, Westendorp R, Vermeer BJ, et al. The melanocortin-1-receptor gene is the major freckle gene. Hum Mol Genet. 2001;10:1701-8.
- 31. Prusis P, Schioth HB, Muceniece R, Herzyk P, Afshar M, Hubbard RE, et al. Modeling of the three-dimensional structure of the human melanocortin 1 receptor, using an automated method and docking of a rigid cyclic melanocyte-stimulating hormone core peptide. J Mol Graph Model.1997;15:307-17,334.
- 32. Abdel-Malek Z, Scott MC, Suzuki I, Tada A, Im S, Lamoreux L, et al. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Res. 2000;13 Suppl 8:156-62.
- 33. Thody AJ. alpha-MSH and the regulation of melanocyte function. Ann N Y Acad Sci. 1999;885:217-29.
- 34. Voisey J, Carroll L, van Daal A. Melanocortins and their receptors and antagonists. Curr Drug Targets. 2003;4:586-97.
- 35. Prusis P, Frandberg PA, Muceniece R et al. A three dimensional model for the interaction of MSH with the melanocortin-1 receptor. Biochem Biophys Res Commun. 1995;210:205-10.
- 36. Mountjoy KG, Robbins LS, Mortrud MT, Kalvinsh I, Wikberg JE. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248-51.
- 37. Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328-30.
- 38. Rees JL. The melanocortin 1 receptor (MC1R): more than just red hair. Pigment Cell Res. 2000;13:135-40.
- 39. Tan CP, McKee KK, Weinberg DH, MacNeil T, Palyha OC, Feighner SD, et al. Molecular analysis of a new splice variant of the human melanocortin-1 receptor. FEBS Lett.1999;451:137-41.
- 40. Rouzaud F, Costin GE, Yamaguchi Y, Valencia JC, Berens WF, Chen KG, et al. Regulation of constitutive and UVRinduced skin pigmentation by melanocortin 1 receptor isoforms. Faseb J. 2006; 20:1927-9.
- 41. Schaffer JV, Bolognia JL. The melanocortin-1 receptor: red hair and beyond. Arch Dermatol. 2001;137:1477-85.
- 42. Loir B, Perez Sanchez C, Ghanem G, Lozano JA, García-Borrón JC, Jiménez-Cervantes C. Expression of the MC1 receptor gene in normal and malignant human melanocytes. A semiquantitative RT-PCR study. Cell Mol Biol (Noisy-le-grand).1999;45:083-92.
- 43. Leonard JH, Marks LH, Chen W, Cook AL, Boyle GM, Smit DJ, et al. Screening of human primary melanocytes of defined melanocortin-1 receptor genotype: pigmentation marker, ultrastructural and UVsurvival studies. Pigment Cell Res. 2003;16:198-207.
- 44. Branicki W, Brudnik U, Kupiec T, Wolańska-Nowak P, Wojas-Pelc A. Determination of phenotype associated SNPs in the MC1R gene. J Forensic Sci. 2007;52:349-54.
- 45. Branicki W, Brudnik U, Kupiec T. J Invest Dermatol. 2004;123:414.
- 46 Motokawa T, Kato T, Hashimoto Y, Katagiri T. Effect of Val92Met and Arg163Gln variants of the MC1R gene on freckles and solar lentigines in Japanese. Pigment Cell Res. 2007;20:140-3.
- 47. Abdel-Malek Z, Suzuki I, Tada A, Im S, Akcali C. The melanocortin-1 receptor and human pigmentation. Ann N Y Acad Sci. 1999;885:117-33.
- 48. Ha T, Rees JL. Melanocortin 1 receptor: what's red got to do with it? J Am Acad Dermatol. 2001;45:961-4.
- 49. Ha T, Naysmith L, Waterston K, Oh C, Weller R, Rees JL. Defining the quantitative contribution of the melanocortin 1 receptor (MC1R) to variation in pigmentary phenotype. Ann N Y Acad Sci. 2003;994:339-47.
- 50. Scott MC, Wakamatsu K, Ito S, Kadekaro AL, Kobayashi N, Groden J, et al. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J Cell Sci. 2002;115:2349-55.
- 51. Scott MC, Suzuki I, Abdel-Malek ZA. Regulation of the human melanocortin 1 receptor expression in epidermal melanocytes by paracrine and endocrine factors and by ultraviolet radiation. Pigment Cell Res. 2002;15:433-9.
- 52. Gantz I, Yamada T, Tashiro T, Konda Y, Shimoto Y, Miwa H, et al. Mapping of the gene encoding the melanocortin-1 (alpha-melanocyte stimulating hormone) receptor (MC1R) to human chromosome 16q24.3 by Fluorescence in situ hybridization. Genomics. 1994;19:394-5.
- 53. Hunt G, Thody AJ. Agouti protein can act independently of melanocyte-stimulating hormone to inhibit melano genesis. J Endocrinol. 1995;147:R1-4.
- 54. D'Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340-4.
- 55. Sampaio SAP, Rivitti EA. Fotodermatoses. In: Sampaio SAP, Rivitti EA. Dermatologia. v. 1. Săo Paulo: Artes médicas; 1998. p. 629-42.
- 56. Funasaka Y, Chakraborty AK, Hayashi Y, Komoto M, Ohashi A, Nagahama M, et al. Modulation of melanocyte-stimulating hormone receptor expression on normal human melanocytes: evidence for a regulatory role of ultraviolet B, interleukin-1alpha, interleukin-1beta, endothelin-1 and tumour necrosis factor-alpha. Br JDermatol. 1998;139:216-24.
- 57. Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, Yim H, et al. Melasma: histopathological characteristics in 56 Korean patients. Br J Dermatol. 2002;146:228-37.
- 58. Bohm M, Metze D, Schulte U, Becher E, Luger TA, Brzoska T. Detection of melanocortin-1 receptor antigenicity on human skin cells in culture and in situ. Exp Dermatol. 1999;8:453-61.
- 59. Sanchez NP, Pathak MA, Sato S, Fitzpatrick TB, Sanchez JL, Mihm MC Jr. Melasma: a clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol. 1981;4:698-710.
- 60. Johnston GA, Sviland L, McLelland J. Melasma of the arms associated with hormone replacement therapy. Br J Dermatol. 1998;139:932.
- 61. Piamphongsant T. Treatment of melasma: a review with personal experience. Int J Dermatol. 1998;3:897-903.
- 62. Grimes PE. Melasma. Etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-7.
- 63. Ponzio HAS. Contribuiçăo ŕ classificaçăo clínica e histopatológica dos melasmas [dissertaçăo]. Porto Alegre: UFRGS; 1995. p. 157.
- 64. Guevara IL, Pandya AG. Melasma treated with hydroquinone, tretinoin, and a fluorinated steroid. Int J Dermatol. 2001;40:212-5.
- 65. Balkrishnan R, McMichael AJ, Camacho FT, Saltzberg F, Housman TS, Grummer S, et al. Development and validation of a health-related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-7.
- 66. Cestari TF, Hexsel D, Viegas ML, Azulay L, Hassun K, Almeida AR, et al. Validation of a melasma quality of life questionnaire for Brazilian Portuguese language: the MelasQoL-BP study and improvement of QoL of melasma patients after triple combination therapy. Br J Dermatol. 2006;156 Suppl 1:13-20.
- 67. Dominguez AR, Balkrishnan R, Ellzey AR, Pandya AG. Melasma in Latina patients: cross-cultural adaptation and validation of a quality-of-life questionnaire in Spanish language. J Am Acad Dermatol. 2006;55:59-66.
- 68. Ponzio HAS, Cruz MF. Acurácia do exame sob a lâmpada de Wood na classificaçăo dos cloasmas. An Brás Dermatol. 1993;68:325-8.
- 69. Wolf R, Wolf D, Tamir A, Politi Y. Melasma: a mask of stress. Br J Dermatol. 1991; 125: 192-3.
- 70. Robins AH. Melanosis after prolonged chlorpromazine therapy. S Afr Med J. 1975;49:1521-4.
- 71. Sacre RC, Fernandes NC, Vaisman M, Tendrich M. Melasma idiopático: avaliaçăo das funçőes tireoidiana, prolactínica e gonadal feminina. An Bras Dermatol. 1996;71:195-8.
- 72. Scheinfeld NS. Melasma. Skinmed. 2007;6:35-7.
- 73. Rigopoulos D, Gregoriou S, Katsambas A. Hyperpigmentation and melasma. J Cosmet Dermatol. 2007;6:195-202.
- 74. Grimes P, Nordlund JJ, Pandya AG, Taylor S, Rendon M, Ortonne JP. Increasing our understanding of pigmentary disorders. J Am Acad Dermatol. 2006;54:S255-61.
- 75. Pandya A, Berneburg M, Ortonne JP, Picardo M. Guidelines for clinical trials in melasma. Pigmentation Disorders Academy. Br J Dermatol. 2006;156 Suppl 1: 21-8.
- 76. Hexsel D, Arellano I, Rendon M. Ethnic considerations in the treatment of Hispanic and Latin-American patients with hyperpigmentation. Br J Dermatol. 2006; 156 Suppl:7-12.
- 77. Rendon M, Berneburg M, Arellano I, Picardo M. Treatment of melasma. J Am Acad Dermatol. 2006;54:S272-81.
- 78. Toyoda M, Morohashi M. Morphological alterations of epidermal melanocytes in photoageing: an ultrastructural and cytomorphometric study. Br J Dermatol. 1998;139:444-52.
- 79. Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17:96-110.
- 80. Kang HY, Hwang JS, Lee JY, Ahn JH, Kim JY, Lee ES, et al. The dermal stem cell factor and c-kit are overexpressed in melasma. Br J Dermatol. 2006;154:1094-9.
- 81. Im S, Kim J, On WY, Kang WH. Increased expression of alpha-melanocyte-stimulating hormone in the lesional skin of melasma. Br J Dermatol. 2002;146: 165-7.
- 82. Lieberman R, Moy L. Estrogen receptor expression in melasma: results from facial skin of affected patients. J Drugs Dermatol. 2008;7:463-5.
- 83. Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- 84. Pache M, Glatz-Krieger K, Sauter G, Meyer P. Expression of sex hormone receptors and cell cycle proteins in melanocytic lesions of the ocular conjunctiva. Graefes Arch Clin Exp Ophthalmol. 2006;244:113-7.
- 85. Matsumura R, Takeuchi S, Takahashi S. Effect of estrogen on melanocortin-3 receptor mRNA expression in mouse pituitary glands in vivo and in vitro. Neuroendocrinology. 2004;80:143-51.
- 86. Chowers I, Livni N, Frucht-Pery J, Pe'er J. Immunostaining of the estrogen receptor in conjunctival primary acquired melanosis. Ophthalmic Res. 1999;31:210-2.
- 87. Jee SH, Lee SY, Chiu HC, Chang CC, Chen TJ. Effects of estrogen and estrogen receptor in normal human melanocytes. Biochem Biophys Res Commun. 1994;199:1407-12.
- 88. Sawaya ME, Garland LD, Rothe MJ, Honig LS, Hsia SL. Oestrogen and progesterone receptors in lentigo maligna. Br J Dermatol. 1988;118:69-71.
- 89. Naysmith L, Waterston K, Ha T, Flanagan N, Bisset Y, Ray A, et al. Quantitative measures of the effect of the melanocortin 1 receptor on human pigmentary status. J Invest Dermatol.2004;122:423-8.
- 90. Carroll L, Voisey J, van Daal A. Gene polymorphisms and their effects in the melanocortin system. Peptides. 2005;26:1871-85.
- 91. Rees JL. Genetics of hair and skin color. Annu Rev Genet. 2003;37:67-90.
- 92. Carlson JA, Linette GP, Aplin A, Ng B, Slominski A. Melanocyte receptors: clinical implications and therapeutic relevance. Dermatol Clin. 2007;25:541-57, viii-ix.
- 93. Sriwiriyanont P, Ohuchi A, Hachiya A, Visscher MO, Boissy RE. Interaction between stem cell factor and endothelin-1: effects on melanogenesis in human skin xenografts. Lab Invest. 2006;86:1115-25.
- 94. Tada A, Suzuki I, Im S et al. Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth Differ. 1998;9:575-84.
Publication Dates
-
Publication in this collection
25 Feb 2010 -
Date of issue
Dec 2009
History
-
Accepted
30 June 2009 -
Received
30 June 2009