Abstracts
BACKGROUND: Cetuximab and erlotinib, epidermal growth factor receptor inhibitors, often cause peculiar adverse cutaneous reactions. OBJECTIVES: Our aim was to evaluate adverse cutaneous reactions and their management in patients undergoing treatment with cetuximab and erlotinib. PATIENTS AND METHODS: Between March/2005 and September/2009, we observed 14 patients with a mean age of 59.6 years undergoing treatment with cetuximab (7) or erlotinib (7), due to lung(10) or colorectal cancer (4). We evaluated the interval between introduction of the drug and onset of symptoms, treatment response, and the clinical pattern of evolution of the cutaneous reaction retrospectively. RESULTS: Twelve patients presented papular-pustular eruption typically affecting the face, chest and back, which appeared in average 13.5 days after starting the drug treatment. The patients underwent oral treatment with minocycline or doxycycline and topical treatment with metronidazole, benzoyl peroxide and/or corticosteroids. All patients showed improvement of the lesions. Five patients presented periungual pyogenic granulomas, which were associated with paronychia in 4 cases, after an average of 8 weeks of treatment. There was improvement of the lesions with topical treatment (antibiotics, corticosteroids and antiseptics). Xerosis was observed in some patients. Other less frequent adverse side effects such as telangiectasia and angiomas, hair and eyelash alterations, and eruptive melanocytic nevi were also described. Treatment with epidermal growth factor receptor inhibitor was maintained in most patients. CONCLUSION: The increasing use of these targeted therapies requires knowledge of their adverse cutaneous side effects to ensure timely intervention in order to allow the continuation of the therapy
Drug eruptions; Drug toxicity; Epidermal growth factor; Receptor, epidermal growth factor
FUNDAMENTOS: O cetuximab e o erlotinib, inibidores do receptor do factor de crescimento epidérmico, provocam frequentemente reacções cutâneas adversas peculiares. OBJETIVOS: Caracterizar do ponto de vista clínico-evolutivo as reacções cutâneas adversas e avaliar a sua abordagem terapêutica. METODOLOGIA: Entre março/2005 e setembro/2009 foram seguidos 14 doentes com idade média de 59,6 anos, em tratamento com cetuximab (7) ou erlotinib (7), por neoplasia pulmonar (10) ou colorrectal (4). Retrospectivamente foi avaliado o padrão clínico evolutivo de reacção cutânea, o intervalo entre a introdução do fármaco e o início dos sintomas e a resposta ao tratamento. RESULTADOS: Doze doentes apresentaram erupção papulopustulosa predominantemente na face, decote e dorso, em média 13,5 dias após o início do fármaco. Efectuaram tratamento oral com minociclina ou doxiciclina e tópico com metronidazol, peróxido de benzoílo e/ou corticoide. Ocorreu melhoria das lesões em todos os doentes. Cinco doentes, em média oito semanas após o início da terapia, apresentaram granulomas piogénicos periungueais, em quatro casos associados a paroníquia, melhorados com tratamento tópico (antibióticos, corticoides e antissépticos). Observou-se xerose em alguns doentes e, de forma isolada, outros efeitos adversos, como telangiectasias e angiomas, alterações dos cabelos e cílios e nevos melanocíticos eruptivos. Na maioria dos doentes, a terapêutica com o inibidor do receptor do factor de crescimento epidérmico foi mantida. CONCLUSÃO: Com o crescente uso destas terapêuticas-alvo, torna-se obrigatório reconhecer e tratar os seus efeitos cutâneos adversos, assegurando uma intervenção atempada de forma a permitir a manutenção desta terapêutica
Erupção por droga; Fator de crescimento epidérmico; Toxicidade de drogas; Receptor do factor de crescimento epidérmico
INVESTIGATION
Adverse cutaneous reactions to epidermal growth factor receptor inhibitors - a study of 14 patients*
Felicidade SantiagoI; Margarida GonçaloII; José Pedro ReisIII; Américo FigueiredoIV
IInternal Complementary Dermatology - Dermatology Service of the University Hospitals of Coimbra - Coimbra, Portugal
IIMD - Head of the Dermatology Service, Hospitais da Universidade de Coimbra - Coimbra, Portugal
IIIMD - Consultant in Dermatology, Dermatology Service, Hospitais da Universidade de Coimbra - Coimbra, Portugal
IVM.D. Ph.D. - Director of the Dermatology Service, Hospitais da Universidade de Coimbra - Coimbra, Portugal
Mailing address
ABSTRACT
BACKGROUND: Cetuximab and erlotinib, epidermal growth factor receptor inhibitors, often cause peculiar adverse cutaneous reactions.
OBJECTIVES: Our aim was to evaluate adverse cutaneous reactions and their management in patients undergoing treatment with cetuximab and erlotinib.
PATIENTS AND METHODS: Between March/2005 and September/2009, we observed 14 patients with a mean age of 59.6 years undergoing treatment with cetuximab (7) or erlotinib (7), due to lung(10) or colorectal cancer (4). We evaluated the interval between introduction of the drug and onset of symptoms, treatment response, and the clinical pattern of evolution of the cutaneous reaction retrospectively.
RESULTS: Twelve patients presented papular-pustular eruption typically affecting the face, chest and back, which appeared in average 13.5 days after starting the drug treatment. The patients underwent oral treatment with minocycline or doxycycline and topical treatment with metronidazole, benzoyl peroxide and/or corticosteroids. All patients showed improvement of the lesions. Five patients presented periungual pyogenic granulomas, which were associated with paronychia in 4 cases, after an average of 8 weeks of treatment. There was improvement of the lesions with topical treatment (antibiotics, corticosteroids and antiseptics). Xerosis was observed in some patients. Other less frequent adverse side effects such as telangiectasia and angiomas, hair and eyelash alterations, and eruptive melanocytic nevi were also described. Treatment with epidermal growth factor receptor inhibitor was maintained in most patients.
CONCLUSION: The increasing use of these targeted therapies requires knowledge of their adverse cutaneous side effects to ensure timely intervention in order to allow the continuation of the therapy.
Keywords: Drug eruptions; Drug toxicity; Epidermal growth factor; Receptor, epidermal growth factor
INTRODUCTION
There has been an increasing use of targeted therapies in cancer treatment, which include epidermal growth factor receptor inhibitors (EGFR-I). These drugs are usually well tolerated and do not present the systemic toxicity of traditional antineoplastic agents.1-3 However, they are often associated with a peculiar spectrum of adverse cutaneous reactions (ACR) which should be recognized and evaluated so that therapy compliance is not threatened. 2.3 Knowledge of these recently described ACR is critical, especially when one considers that their presence reflects anti-tumor activity. 1
EPIDERMAL GROWTH FACTOR RECEPTOR INHI-BITORS
EGFR (or ErbB1), a transmembrane glycoprotein connected to an intracellular tyrosine kinase, plays a critical role in regulating cell growth and proliferation. 4-7 In some neoplasms, there is an overexpression and/or misregulation of the receptor, thus triggering a series of intracellular events, including cell proliferation, blockade of apoptosis, invasion and metastasis, and tumor-induced neovascularization the hallmarks of carcinogenesis. 1,4,8,9 Therefore, blockade of EGFR represents a new strategy in cancer treatment. 3 - 4
EGFR-I can be classified into two categories. Monoclonal antibodies recognize the extracellular portion of the receptor with subsequent inhibition of signaling pathways. These include cetuximab and panitumumab. Tyrosine kinase inhibitors, such as erlotinib, gefitinib and lapatinib, act through intracellular binding to this portion of the EGFR. 1,2,4,5,6,9,10
Cetuximab is indicated for metastatic or unresectable colorectal and squamous-cell tumors on the head and neck in combination with chemotherapy or radiotherapy. Erlotinib is used as monotherapy in pancreatic and lung tumors in palliative situations. 1,4,10,11
EGFR is largely expressed in the skin, especially in the corneocytes of the basal layer, sweat glands and hair follicles. Therefore, its inhibition can significantly interfere with normal functions of the skin, thus explaining the large number of ACR, as we intend to demonstrate in this study. 3.7
PATIENTS AND METHODS
Between March/2005 and September/2009, 14 patients (9 male and 5 female) with a mean age of 59.6 years (34-77) were evaluated at the Dermatology Service of the Hospitais da Universidade de Coimbra - HUC (Coimbra's University Hospitals). Patients were referred from the Oncology Unit of the HUC or the Centro Hospitalar de Coimbra (Hospital Center of Coimbra) due to intense ACR and/or ACR that interfered with quality of life. They were being treated with cetuximab (7 patients) and erlotinib (7 patients) for lung tumor (10 patients) or colorectal tumor (4 patients) in stage IV.
Retrospectively and using a photographic record, we studied the types of ACR, the interval between introduction of the EGFR-I and onset of symptoms, response to treatment and its evolution.
RESULTS
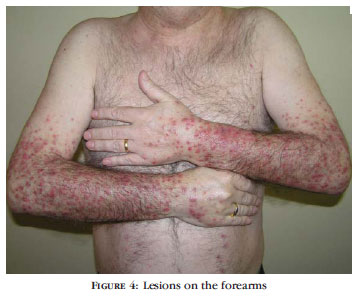
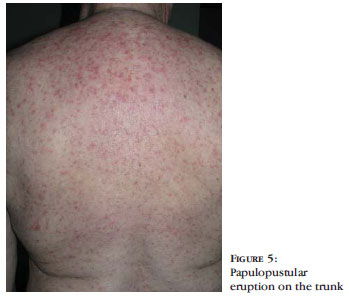
The most frequently observed ACR (12 patients) was papulopustular eruption (PPE), affecting the face (12 patients), presternal region and back (8 patients), scalp (4 patients), and also the abdomen, thighs and forearms (1 patient) (Figures 1 to 4 and Table 1). It came about 1 to 6 weeks (mean 13.5 days) after introducing EGFR-I in 6 patients treated with cetuximab and six other patients treated with erlotinib. The interval until the onset of the reaction and intensity were similar in both groups. In one patient it coexisted with itchy eczematous eruption of the face, with melicerous crusts, following the application of multiple inadequate topical drugs (promethazine, clotrimazole and povidone-iodine) for treatment of PPE (Figure 5).
None of these patients reported personal or family history of severe acne.
All patients with PPE were treated with oral antibiotics (minocycline or doxycycline - 50 to 100 mg/day) for a period of 4 to 8 weeks, combined with topical treatment (metronidazole, benzoyl peroxide and/or corticosteroids in the initial phase). There was a rapid improvement of skin lesions in all patients, and one patient presented with transient hyperpigmentation in some lesions.
The EGFR-I was discontinued in two patients due to progression of neoplastic disease and maintained in the others, although the dose was reduced in 2 and temporarily interrupted in 4 patients due to skin toxicity.
Periungual pyogenic granulomas (5 patients) with paronychia (4 patients) in fingernails (2), toenails (1) or both (2) were observed on average after 8 weeks of treatment with EGFR-I (Figure 6). A histopathological examination of one of these lesions revealed granulation tissue and inflammatory infiltrate with predominance of neutrophils. Treatment consisted of local disinfection and application of antibiotics and corticosteroids, leading to improvement in all patients. In patient No. 14, there was an initial improvement of the lesions with local care, but exuberant pyogenic granulomas with paronychia and purulent drainage with significant limitation of movement appeared later, which led to performing cryotherapy. So far, it has not been necessary to resort to nail surgery.
Some patients presented xerosis and it was necessary to reinforce the use of emollients.
Three of the 14 patients showed unusual ACR. After 6 months of therapy with EGFR-I, patient No. 1 started to present eruptive melanocytic nevi of dark brown color and dimensions between 2 and 4mm in diameter on the trunk. Patient No. 6 presented telangiectasia in the chest area and two angiomas, venous lakes of 3 and 5mm, on the helix. After 3 months of treatment, patient No. 14 complained of slow-growing hair (with less frequent need of haircuts) and changes in hair texture (coarse and curly hair) with frequent shedding while combing the hair. After 4 months, she had bilateral abnormalities of the cilia, with trichomegaly, uneven texture and trichiasis, with no conjunctival inflammation so far.
DISCUSSION
Despite the small number of patients in this study, the results obtained are similar to those described in the literature in terms of type and chronology of ACR and therapeutic approach.
As in this study, PPE (also called acneiform eruption or "rosacea-like") is the most frequent ACR associated with EGFR-I. It is estimated to occur in 60 to 80% of the patients, most with mild to moderate forms of the condition.4,6,12 It is characterized by monomorphic, papulo-erythematous, follicular lesions, which may develop into pustules, with associated itch. It usually appears 10 to 14 days after beginning treatment (on average 8 to 21 days). It is typically located in seborrheic areas (face, scalp, presternal region and upper back), although patient No. 1 presented lesions in less typical areas such as the forearms, thighs and abdomen. 2,4,13 There are no reports of healing with scar, although residual hyperpigmentation may occur in some patients, as it occurred to one of our patients.
PPE appears to be dose-dependent and is usually more frequent, severe and early in patients receiving monoclonal antibodies, especially cetuximab, although this has not been our experience. 1,3,4,6,12,14 The severity of the PPE is not associated with skin type or history of acne. 15 Some authors argue that the presence and intensity of a PPE have a positive correlation with the effectiveness of the therapy, suggesting a gradual increase in dose until a severe PPE is produced ("dose to rash).3,4,6, 11,14,16
From the histological viewpoint, PPE corresponds to a suppurative neutrophilic folliculitis and/or a perifollicular inflammatory infiltrate. 4,6,12,13
Another complication often associated with EGFR-I is paronychia, i.e. inflammation of the proximal and/or lateral nailfolds. It occurs later (20 days to 6 months) in less than 15% of the patients, causing pain, erythema and, in some cases, abscesses and friable neoformations clinically and histopathologically consistent with pyogenic granuloma, as in our study. 4,6,17,18 It usually involves multiple fingers and often the hallux, which may prevent the patient from moving, due to pressure of a shoe or the performance of tasks requiring fine motor manipulation. 1.6 In our study, this reaction was present in 5/12 patients, and it was the late-onset type of paronychia.
Xerosis, especially on the trunk and extremities, occurs in approximately 4 to 35% of the patients. 4.6 It may be severe and cause great discomfort, with painful fissures in the pulps of the fingers and "eczema craquelée". Older patients or patients with a history of atopic eczema and subjected to prior conventional chemotherapy are the most susceptible. 2,4,11 Because the majority of the patients in our study was informed of the importance of carrying out proper personal hygiene care on a daily basis and the need of applying prophylactic emollients, a large number of patients with xerosis or eczema was not observed.
Another late effect, which occurs in up to 20% of the patients, is change in hair texture and growth. The hair can become thin, brittle or curled; slow hair growth, frontal alopecia and hypertrichosis may also occur. However, patients can be reassured that resolution of these conditions occurs within 1 month after cessation of treatment. 2,4,16 In our study, this effect was reported by only one patient. However, this number would certainly increase with longer follow-up of these patients receiving EGFR-I, as it is known that the frequency of the various ACR to EGFR-I differs in the short and long term. The PPE tends to decrease over time, unlike xerosis and hair changes, which arise later. 19
Ocular toxicity may occur in about one third of the patients, manifesting as blepharitis, trichomegaly (the most documented ocular effect, though rare and late), trichiasis, dry eye and conjunctivitis, among others. 11
In the course of therapy with EGFR-I, 2 to 36% of the patients may still present with mild to moderate mucositis or stomatitis, which unlike PPE are not dose-dependent, and also telangiectasia associated with acneiform eruption, which tend to disappear with time and which may cause residual hyperpigmentation aggravated by sun exposure. 2, 4, 6
The late appearance of eruptive melanocytic nevi in one of our patients was only described in the literature with the use of sorafenib, a vascular endothelium growth factor receptor inhibitor. 1,20,21
The treatment of skin toxicity induced by EGFR-I is still no consensual. 3.22 Its approach is based on two options: preventive/proactive treatment and treatment of ACR according to their degree of severity. 16
Before beginning the treatment, it is necessary to inform patients about the potential skin toxicities associated with therapy with EGFR-I and establish preventive measures including hygiene with mild soap, quick showers with warm water and repeated and regular use of emollients, preferably all over the skin and with products containing no alcohol or perfume, in addition to photoprotection. Patients must also wear comfortable shoes to avoid friction and pressure on the nailfold, and avoid cutting the nails flush. 2,5,10-12
Treatment of mild or asymptomatic PPE is based on topical agents with anti-inflammatory properties that also reduce the risk of secondary infection (metronidazole, clindamycin and benzoyl peroxide). 6.11
Topical corticosteroids (hydrocortisone or methylprednisolone aceponate cream) can be very effective, but must not be used for more than 14 days because they may decrease skin thickness and increase the risk of secondary infection. 6.10 The use of topical retinoids (tretinoin , adapalene), although considered useful by some authors, has been questioned due to the possibility of irritation and aggravation of xerosis.2.3, 6,14,23 The use of topical immunomodulators (pimecrolimus, tacrolimus) is also controversial. 5,8,23
For more extensive and symptomatic PPE, topical treatment should be associated with an oral tetracycline (minocycline or doxycycline, 50 to 100 mg/day). The four-week treatment, to be prolonged for as long as the eruption is symptomatic, is not consensual. 2,5,10 Tetracycline is usually well tolerated and response to antibiotics is usually rapid, compared to what is observed in case of acne or rosacea. 13, 24 The doses of the antibiotic can be duplicated if the ACR worsens.
Oral isotretinoin in low doses can be considered for patients not responding to the previous measures, but its use is controversial. 24.25 It may exacerbate xerosis and paronychia, and it is unknown whether it interferes with the mechanism of action of EGFR-I.2,5,6,14,24 Oral corticosteroids may be beneficial in the treatment of severe PPE8, but its use is contested due to the adverse effects that may result. 2.23
Oral antihistamines may help fight itches. 2.4
The treatment chosen for the patients in our study proved to be well tolerated and effective, and all patients showed improvement of their lesions. We emphasize the use of corticosteroids in the initial phase for the rapid relief of PPE associated with metronidazole, which contributes to its anti-inflammatory effect. The choice of tetracycline was based on individual and institutional experience, and it was well tolerated by patients, who showed a rapid clinical response.
Pyogenic Granulomas/ paronychia may be the most difficult ACR to treat and tend to persist throughout the therapy with EGFR-I, but rarely condition its termination. Mild to moderate cases can be treated with antiseptics (potassium permanganate), topical antibiotics and topical corticosteroids that relieve pain and inflammation.3 Other procedures may include the application of silver nitrate, intra-lesional corticosteroids, cryotherapy or partial matricectomy.11,18,23 In our study, the patients were treated with combined topical medication that has proved useful in improving these lesions, and it was associated with cryotherapy in only one patient.
In the case of xerosis, associated with pulpitis or eczema, the indicated treatments are emollients and topical corticosteroids. It is worth emphasizing the importance of early prophylactic measures in this ACR.
With regard to therapy with EGFR-I, it is recommended to continue treatment without dose modification in cases of mild to moderate ACR.4 Dose modification must be considered when the eruption is resistant to treatment, symptomatic and severe, reaching a body surface area exceeding 50%4. In this case, treatment may be temporarily suspended for 7 to 10 days (until clinical resolution), and then reintroduced at lower doses. Other options may be continuation of the treatment with low doses or the adoption of an alternate scheme. 4.5 It should be stressed that given the fact that the PPE is a marker of effectiveness of therapy with EGFR-I, all efforts should be made to maintain the anti-neoplastic therapy at optimal doses.5
CONCLUSION
ACRs to EGFR-I form a single and specific group of adverse effects, with a well-established and predictable chronology, and as such the patient should be informed and prepared for their appearance. In most patients, ACRs are handled smoothly and does not imply the interruption of EGFR-I23.
The growing use of EGFR-I, some already approved by the FDA and others undergoing clinical trials, implies that oncologists and dermatologists should improve their knowledge about the secondary cutaneous side effects inherent to this therapy. Multidisciplinary and proactive management of ACRs to EGFR-I is essential to limit the incidence of severe symptoms, improve tolerance to the drug and thus therapeutic compliance, consequently prolonging the survival of patients with better quality of life. 10.16, 19
REFERENCES
- 1. Deslandres M, Sibaud V, Chevreau C, Delord JP. Cutaneous side effects associated with epidermal growth factor receptor and tyrosine kinase inhibitors. Ann Dermatol Venereol. 2008; Spec No 1:16-24.
- 2. Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16:1425-33.
- 3. Roé E, García Muret MP, Marcuello E, Capdevila J, Pallarés C, Alomar A. Description and management of cutaneous side effects during cetuximab or erlotinib treatments: a prospective study of 30 patients. J Am Acad Dermatol. 2006;55:429-37.
- 4. Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-70.
- 5. Lacouture ME, Melosky BL. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007;12:1-5.
- 6. Hu JC, Sadeghi P, Pinter-Brown LC, Yashar S, Chiu MW. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-26.
- 7. Nanney LB, Stoscheck CM, King JR, Underwood RA, Holbrook KA. Immunolocalization of epidermal growth factor receptors in normal developing human skin. J Invest Dermatol. 1990;94:742-8.
- 8. Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803-12.
- 9. Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11:689-708.
- 10. Melosky B, Burkes R, Rayson D, Alcindor T, Shear N, Lacouture M. Management of skin rash during egfr-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:16-26.
- 11. Guhl G, González-de Arriba A, Daudén E. Epidermal growth factor receptor inhibitors side effects. Actas Dermosifiliogr. 2006;97:296-310.
- 12. Varela P, Gonçalo M, Moura C, Barroso A. Reacções Cutâneas Adversas aos Inibidores do EGFR- Receptor do Factor de Crescimento Epidérmico. Soc Port Dermatol Venereol. 2007;65:451-462.
- 13. Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, et al. Cutaneous sideeffects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491-500.
- 14. Dewitt CA, Siroy AE, Stone SP. Acneiform eruptions associated with epidermal growth factor receptor-targeted chemotherapy. J Am Acad Dermatol. 2007;56:500-5.
- 15. Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC. Cutaneous sideeffects in cancer patients treated with the antiepidermal growth factor receptor anti body C225. Br J Dermatol. 2001;144:1169-76.
- 16. Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107-19.
- 17. Lee MW, Seo CW, Kim SW, Yang HJ, Lee HW, Choi JH, et al. Cutaneous side effects in nonsmall cell lung cancer patients treated with Iressa (ZD1839), an inhibitor of epidermal growth factor. Acta Derm Venereol. 2004;84:23-6.
- 18. Fox LP. Nail toxicity associated with epidermal growth factor receptor inhibitor therapy. J Am Acad Dermatol. 2007;56:460-5.
- 19. Osio A, Mateus C, Soria JC, Massard C, Malka D, Boige V, et al. Cutaneous sideeffects in patients on long-term treatment with epidermal growth factor receptor inhibitors. Br J Dermatol. 2009;161:515-21.
- 20. Bennani-Lahlou M, Mateus C, Escudier B, Massard C, Soria JC, Spatz A, et al. Eruptive nevi associated with sorafenib treatment. Ann Dermatol Venereol. 2008;135:672-4.
- 21. Robert C, Mateus C, Spatz A, Wechsler J, Escudier B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60:299-305.
- 22. Cowen EW. Epidermal growth factor receptor inhibitors: a new era of drug reactions in a new era of cancer therapy. J Am Acad Dermatol. 2007;56:514-7.
- 23. Segaert S, Van Cutsem E. Clinical management of EGFRI dermatologic toxicities: the European perspective. Oncology (Williston Park). 2007;21:22-6.
- 24. de Noronha e Menezes NM, Lima R, Moreira A, Varela P, Barroso A, Baptista A, et al. Description and management of cutaneous side effects during erlotinib and cetuximab treatment in lung and colorectal cancer patients: a prospective and descriptive study of 19 patients. Eur J Dermatol. 2009;19:248-51.
- 25. Lynch TJ JR, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610-21.
Publication Dates
-
Publication in this collection
21 June 2011 -
Date of issue
June 2011
History
-
Accepted
31 July 2010 -
Received
14 July 2010