Abstracts
BACKGROUND: Leishmaniasis is one of the most important infectious diseases worldwide. Our study can provide more knowledge about angiogenic and hypoxic events in leishmaniasis. We attempted to verify whether the HIF-1 α protein expression may be associated to VEGF-A, VEGFR2 and MMP9 in leishmanial lesions. OBJECTIVES: Besides understanding the pathway, we performed the correlation of VEGF-A, VEGFR2 and MMP9 proteins. METHODS: In this study, we gathered 54 paraffin blocks taken from skin lesions in patients from northern Minas Gerais, Brazil, with confirmed diagnosis of tegumentary leishmaniasis. Immunohistochemistry was used to evaluate the expression of the proteins. The expression of HIF-1α was categorized into two groups according to the median: HIF-1 α lower and HIF-1 α higher. RESULTS: We observed increase of VEGFR2 and MMP9 protein expressions in HIF-1 α higher group of epithelial cells. Spearman analyses in epithelial cells showed correlation between VEGF-A and MMP9, VEGFR2 and MMP9 protein expression. CONCLUSIONS: HIF-1 α higher group showed increase of VEGFR2 and MMP9 proteins. In epithelial cells, VEGF-A was correlated to MMP9 protein. Furthermore, considering leukocyte cells, VEGFR2 was negatively correlated to MMP9 protein levels. This pathway possibly prepares the cells for a higher activity in a hypoxic or an angiogenic microenvironment. Other in vitro and in vivo studies may clarify the activation mechanism and the response from the proteins HIF-1 α, VEGFR2 and MMP-9 in tegumentary leishmaniasis.
Angiogenesis inducing agents; Cell hypoxia; Leishmaniasis
FUNDAMENTOS: A leishmaniose é uma das mais importantes doenças infecciosas em todo o mundo. Em leishmaniose, tem sido sugerido que muitas características da lesão está associado a eventos de hipóxia, podendo este ter um papel fundamental na evolução da doença. OBJETIVO: O presente estudo pode fornecer dados acerca do fenômeno hipóxia e da angiogênese em leishmaniose tegumentar americana. Buscou-se verificar se a expressão da proteína HIF-1 α associa-se à expressão das proteínas VEGF-A, VEGFR2 e MMP9 em lesões de Leishmania sp. MÉTODOS: Neste estudo retrospectivo, foram utilizados 54 blocos de parafina de lesões de leishmaniose tegumentar americana de pacientes do norte de Minas Gerais, Brasil, com diagnóstico confirmado de leishmaniose tegumentar americana. A técnica de imunohistoquimica foi utilizada para avaliação da expressão das proteínas. A expressão da HIF-1α foi categorizada em dois grupos de acordo com a mediana: HIF-1 α abaixo e HIF-1 α acima da mediana. RESULTADOS: Observamos aumento das expressões das proteínas VEGFR2 e MMP9 no grupo HIF-1 α acima da mediana. A análise de Spearman demonstrou correlação entre as proteínas VEGF-A e MMP9, VEGFR2 e MMP9. CONCLUSÃO: Os dados aqui apresentados indicam uma alta expressão da proteína HIF-1 α em LTA. O grupo HIF-1α acima da mediana apresentou maior expressão das proteínas VEGFR2 e MMP9. Foi demonstrada correlação entre as proteínas VEGF-A e MMP9, VEGFR2 e MMP9. Outros estudos in vitro e in vivo devem ser realizados a fim de esclarecer o mecanismo de ativação e resposta das proteínas HIF-1 α, VEGFR2 e MMP-9 em leishmaniose tegumentar americana.
Indutores da angiogênese; Hipóxia celular; Leishmaniose
INVESTIGATION
Immunohistochemical profile of HIF-1α, VEGF-A, VEGFR2 and MMP9 proteins in tegumentary leishmaniasis* * Work performed at the Health Research Laboratory of the Hospital Universitário Clemente de Faria of the Universidade Estadual de Montes Claros (HUCF- UNIMONTES) - Montes Claros (MG), Brazil.
Estudo da expressão imunohistoquímica das proteínas HIF-1 α , VEGF-A, VEGFR2 e MMP9 em leishmaniose tegumentar americana
Carlos Alberto de Carvalho FragaI; Marcos Vinicius Macedo de OliveiraI; Lucas Rodrigues AlvesII; Agostinho Gonçalves VianaIII; Adriana Alkmin de SousaII; Sílvio Fernando Guimarães CarvalhoIV; Alfredo Maurício Batista De PaulaIV; Ana Cristina de Carvalho BotelhoIV; André Luiz Sena GuimarãesIV
IMaster - PhD Candidate in Health Sciences by the Universidade Estadual de Montes Claros (UNIMONTES) - Montes Claros (MG), Brazil
IIUndergraduate studies - Undergraduate student of Dentistry, Universidade Estadual de Montes Claros (UNIMONTES) - Montes Claros (MG), Brazil
IIIMaster - Master in Health Sciences by the Universidade Estadual de Montes Claros (UNIMONTES) - Montes Claros (MG), Brazil
IVPhD - Professor at the Post-Graduation Program in Health Sciences at the Universidade Estadual de Montes Claros (UNIMONTES) - Montes Claros (MG), Brazil
Mailing address Mailing address: André Luiz Sena Guimarães Avenida Cula Mangabeira, 562 Santo Expedito 39401-001 Montes Claros, MG E-mail: andreluizguimaraes@gmail.com
ABSTRACT
BACKGROUND: Leishmaniasis is one of the most important infectious diseases worldwide. Our study can provide more knowledge about angiogenic and hypoxic events in leishmaniasis. We attempted to verify whether the HIF-1 α protein expression may be associated to VEGF-A, VEGFR2 and MMP9 in leishmanial lesions. OBJECTIVES: Besides understanding the pathway, we performed the correlation of VEGF-A, VEGFR2 and MMP9 proteins.
METHODS: In this study, we gathered 54 paraffin blocks taken from skin lesions in patients from northern Minas Gerais, Brazil, with confirmed diagnosis of tegumentary leishmaniasis. Immunohistochemistry was used to evaluate the expression of the proteins. The expression of HIF-1α was categorized into two groups according to the median: HIF-1 α lower and HIF-1 α higher.
RESULTS: We observed increase of VEGFR2 and MMP9 protein expressions in HIF-1 α higher group of epithelial cells. Spearman analyses in epithelial cells showed correlation between VEGF-A and MMP9, VEGFR2 and MMP9 protein expression.
CONCLUSIONS: HIF-1 α higher group showed increase of VEGFR2 and MMP9 proteins. In epithelial cells, VEGF-A was correlated to MMP9 protein. Furthermore, considering leukocyte cells, VEGFR2 was negatively correlated to MMP9 protein levels. This pathway possibly prepares the cells for a higher activity in a hypoxic or an angiogenic microenvironment. Other in vitro and in vivo studies may clarify the activation mechanism and the response from the proteins HIF-1 α, VEGFR2 and MMP-9 in tegumentary leishmaniasis.
Keywords: Angiogenesis inducing agents; Cell hypoxia; Leishmaniasis
RESUMO
FUNDAMENTOS: A leishmaniose é uma das mais importantes doenças infecciosas em todo o mundo. Em leishmaniose, tem sido sugerido que muitas características da lesão está associado a eventos de hipóxia, podendo este ter um papel fundamental na evolução da doença.
OBJETIVO: O presente estudo pode fornecer dados acerca do fenômeno hipóxia e da angiogênese em leishmaniose tegumentar americana. Buscou-se verificar se a expressão da proteína HIF-1 α associa-se à expressão das proteínas VEGF-A, VEGFR2 e MMP9 em lesões de Leishmania sp.
MÉTODOS: Neste estudo retrospectivo, foram utilizados 54 blocos de parafina de lesões de leishmaniose tegumentar americana de pacientes do norte de Minas Gerais, Brasil, com diagnóstico confirmado de leishmaniose tegumentar americana. A técnica de imunohistoquimica foi utilizada para avaliação da expressão das proteínas. A expressão da HIF-1α foi categorizada em dois grupos de acordo com a mediana: HIF-1 α abaixo e HIF-1 α acima da mediana.
RESULTADOS: Observamos aumento das expressões das proteínas VEGFR2 e MMP9 no grupo HIF-1 α acima da mediana. A análise de Spearman demonstrou correlação entre as proteínas VEGF-A e MMP9, VEGFR2 e MMP9.
CONCLUSÃO: Os dados aqui apresentados indicam uma alta expressão da proteína HIF-1 α em LTA. O grupo HIF-1α acima da mediana apresentou maior expressão das proteínas VEGFR2 e MMP9. Foi demonstrada correlação entre as proteínas VEGF-A e MMP9, VEGFR2 e MMP9. Outros estudos in vitro e in vivo devem ser realizados a fim de esclarecer o mecanismo de ativação e resposta das proteínas HIF-1 α, VEGFR2 e MMP-9 em leishmaniose tegumentar americana.
Palavras-chave: Indutores da angiogênese; Hipóxia celular; Leishmaniose
INTRODUCTION
Leishmaniasis is one of the most important infectious diseases worldwide and unfortunately, it causes 60.000 deaths annually. Additionally, the number of cases of human leishmaniasis is increasing worldwide at a disturbing rate, estimated by the World Health Organization in 1998.1,2 Leishmania infections might result in a wide spectrum of clinical manifestations and its outcome is determined by complex hostparasite interactions.3 Leishmaniasis is an infectious disease caused by protozoa of the genus Leishmania that affect humans and different animal species and could manifest in different clinical forms.4 In Brazil, tegumentary leishmaniasis represents a public health problem and its incidence has increased significantly.5
In leishmaniasis, it has been suggested that many characteristics of this lesions is associated to hypoxic events and it could have a role in the disease outcome.3,6-10 Furthermore, hypoxic events could affect cytokine secretion, expression of migration and adhesion cell surface markers of macrophages.3,11 Hypoxia still enhances angiogenic events, being highly depen dent on the function of hypoxia inducible factor-1alpha (HIF-1 α). HIF-1 α associates with the HIF-1β subunit and this complex acts as a transcription factor of hypoxia-inducible genes such as vascular endothelial growth factor A (VEGF-A) and consequently its receptors, erythropoietin (Epo) and matrix metalloproteinases (MMPs), mainly MMP9. These genes code for proteins involved in several events such as embryogenesis, angiogenesis, cell proliferation, metastasis and during the cellular response to hypoxia.12-16
Few studies about proteins associated to hypoxia have been performed in leishmaniasis and hypoxic areas formed in leishmanial lesions during the lesion development are unknown. Our study can provide more knowledge about angiogenic and hypoxic events in leishmaniasis by analyzing proteins involved in both phenomena. We attempted to verify whether the HIF-1 α protein expression may be associated to VEGF-A, VEGFR2 and MMP9 in leishmanial lesions. Besides understanding the pathway, we performed correlation of the VEGF-A, VEGFR2 and MMP9 proteins.
PATIENTS AND METHODS
In this study, we gathered 54 paraffin blocks taken from skin lesions in patients from northern Minas Gerais, Brazil, with confirmed diagnosis of tegumentary leishmaniasis. The diagnosis was confirmed in all cases through biopsy, direct parasitological examination and reaction of Montenegro. Although we did not have access to clinical data of lesions, all of them were cutaneous. All cases were of primary manifestations and visceral infections were discarded. These samples were collected from 54 patients aged between 6 and 90 years. Of them, 17 (31.5%) were females and 37 (68.5%) males. Referring to skin color, 33 (61.2%) were non-caucasian and 14 (25.9%) caucasian. The qualitative analyses can be shown as follows: in the epidermis, there was a presence of accentuated acanthosis, papillomatosis and hyperkeratosis; in the dermis, we observed predominance of intense exudative cellular reactions and the presence of mononuclear cells. All were treated with pentavalent antimonial and were clinically healed. Ethical approval for this study was obtained from the local ethics committees (Unimontes, CEP 1930/2010).
Immunohistochemistry staining
Each resected tissue specimen was fixed in formalin, embedded in paraffin, cut into 3-µm serial sections and mounted on organosilane-coated slides. The following primary mouse monoclonal antibodies were used: anti-HIF-1α (clone HIF-1α 67, Sigma-Aldrich, St. Louis, USA), anti-vascular endothelial growth factor A (clone 26503, Sigma-Aldrich, St. Louis, USA), anti-Flk-1 (VEGFR2) (clone A-3, Santa Cruz Biotechnology, CA, USA) and anti-MMP9 (clone 2C3, Santa Cruz Biotechnology, CA, USA) 11. All monoclonal antibodies were incubated for 18 h at 4ºC. Endogenous peroxidase was blocked by incubation with 0.03% H2O2 in ethanol for 30 min. For antigen retrieval, sections were heated in a steam cooker for 5 min at 125ºC in Tris-EDTA buffer (1 mM Tris base, 1 mM EDTA and 0.05% Tween 20, pH 9.0). The primary antibodies against HIF-1α, VEGFA, VEGFR2 and MMP9 were detected using the LSAB kit (LSAB-Kit Plus Peroxidase, Dako, California, USA). Signals were developed with 3'3-diaminobenzidine-tetrahydrochloride for 5 min and counter-stained with Mayer's haematoxylin for 30 sec. Negative controls were performed by replacing the primary antibody with universal negative control mouse (Dako, Carpinteria, California, USA). In addition, in all the reactions of immunohistochemistry staining we took care to also verify the presence of internal positive control within each slide, ensuring thus the quality of the technique used.
Counting of immunostained samples
The immunohistochemical expression of biomarkers was evaluated using an Olympus® BH2 microscope (10× ocular and 40× objective lenses), and an ocular lattice (area 0.092 mm2) with 100 points composed of 10 horizontal and 10 vertical test lines was superimposed on the test field to be measured. A total area of 1.84 mm2 was evaluated for each sample. Immunohistochemical analyses of all antigens investigated were performed by determining the percentage of positively stained cells (epithelial and leukocyte cells) in all fields counted (10 fields for each specimen). Immunohistochemical expression data are expressed as mean ± standard error (mean ± sd) values.
Statistical analysis
Results of protein expressions are displayed as mean ± s.d. Data were analyzed by Mann-Whitney test. For analyses average duration of lesions, Kaplan-Meier test was performed and the variables were compared using the log-rank test. All statistical analyses were performed with the statistical pack SPSS® (SPSS Inc, Chicago, IL, USA), version 13.0 for Windows®. P values < 0.05 were considered significant.
RESULTS
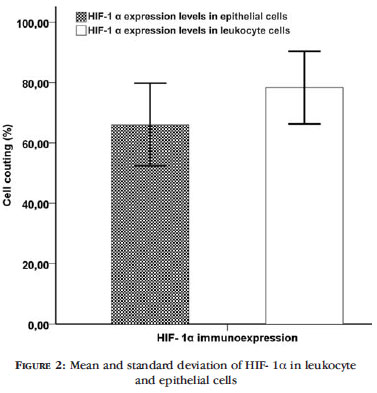
The pattern expression of HIF-1 α, VEGF-A, VEGFR2 and MMP9 proteins by immunohistochemistry is demonstrated in Figure 1. HIF-1 α and VEGF-A presented nuclear and cytoplasmic localization whereas VEGFR2 and MMP9 presented cytoplasmic localization. To determine whether increased levels of HIF1 α protein in leishmaniasis promoted increase of the other proteins, the patients were divided into two groups according to median values (94.70% in epithelial cells and 98.00% in leukocyte cells): HIF-1 α lower and higher. Figure 2 shows mean and standard deviation of HIF- 1α in leukocyte and epithelial cells. We observed increase of VEGFR2 and MMP9 protein expressions in HIF-1 α higher group of epithelial cells (p=0.002 and 0.009, respectively) (Figure 3A). Considering leukocyte cells, we did not find any association between HIF-1 α groups and protein expres-sions (Figure 3B). To understand the pathway, we then performed correlation of the VEGF-A, VEGFR2 and MMP9 proteins. Spearman analyses in epithelial cells showed correlation between VEGF-A and MMP9 protein expressions (r=+0.408 p=0.009) and VEGFR2 and MMP9 protein expression (r=+0.313 p=0.049). According to leukocyte cells, VEGFR2 and MMP9 was correlated (r= -0.440 p=0.004). The average duration was 217.7 ± 194.7 days. There was no association between protein expressions and average duration (data not shown).
DISCUSSION
Angiogenesis is the process of vascular growth via pre-existing vessels. The process of vascularization occurs during embryonic development, growth and development of tissues, formation of corpus luteum and endometrium, regeneration and healing process of wounds.17-20 Abnormal angiogenesis is involved in many pathological processes including tumor growth, metastasis, diabetes and arthritis. Extracellular signals involved in this process are primarily paracrine secretion of factors and extracellular matrix components that usually carries specialized receptors and integrins.18-21 Angiogenesis is rapidly initiated in response to hypoxic, inflammation or ischemic conditions.16,22-26
Hypoxia inducible transcription factor-1 (HIF1) consists of a heterodimer of HIF-1 α, the oxygen responsive component, and HIF-1β.16,22-26 HIF-1 α is held in response to hypoxia and activates expression of genes involved in erythropoiesis, glycolysis, modulation of vascular tone, and angiogenesis.14 In humans and animal models, many characteristics of leishmanial lesions such as microcirculation impairment, metabolic demand for leukocytes, parasite proliferation, and secondary bacterial infection are indicators of a hypoxic event in these lesions.3,6-10 It has been shown that HIF-1 α accumulates in inflamed tissue. In these sites, the number of inflammatory cells increases and their activation induces and produces a variety of cytokines. Similarly, in our study, we found high levels of HIF-1α protein expression in epithelial and leukocyte cells in tegumentary leishmaniasis samples.
In a previous study, HIF-1 α was not detected in the nucleus of macrophages in leishmanial lesions. The authors suggest that HIF-1 α binds to parasite factors, altering or blocking their transport to the nucleus.3 Furthermore, HIF-1 α reduces Staphylococcus aureus infections in mice and HIF-1 α -/-cells, which reduce nitric oxide (NO) production, affect macrophage TNF-α production and bacterial killing. Besides, under hypoxia, the capacity of macrophages to phagocytose is HIF-1 α -dependent. Although we found high expression of HIF-1 α protein in both epithelial and leukocyte cells, in our study, we did not evaluate macrophage cells separately.
In angiogenesis event, it has been suggested that VEGF-A is a major player. VEGF-A induces vasodilation via endothelial NO production and its production is under control of HIF complex. Cell surface receptor tyrosine kinases for VEGF, constituting the VEGFR family, have been identified.27 VEGFR2 is known to be the most essential receptor in angiogenesis, mainly in regulation of capillary tube formation.28,29 Matrix metalloproteinases (MMPs) are secreted as zymogens and selectively degrade components of the extracellular matrix (ECM) and a variety of cells, including epithelial cells, fibroblasts, inflammatory cells, and endothelial cells can produce these endopeptidase.30 In particular, MMP9 protein acts downstream of Th2 cytokine signaling. We observed that only 11 patients presented MMP9 protein expression. However, MMP9 was increased in HIF-1 α higher group. In our study, VEGF-A protein level correlated to MMP9. We demonstrated that VEGFR2 protein level was increased in HIF-1 α higher group and correlated to MMP9 protein. These data support previous findings that show the expression of protein related to hypoxia can induce necessary elements to provide the establishment of angiogenesis in inflammation response.16,22-26 Taken together, HIF-1 α protein could be involved in high expressions of VEGFR2 and MMP9 proteins. These findings together suggest that HIF-1 α can enhance VEGFR2 and MMP9 proteins signals, helping the inflammatory cells response to specific sites in leishmanial lesions.
CONCLUSION
In conclusion, the data presented here indicate a deregulation in angiogenesis pathway and it is might be associated to increase of HIF-1 α protein expression. HIF-1 α higher group showed increase of VEGFR2 and MMP9 proteins. In epithelial cells, VEGF-A was correlated to MMP9 protein. Furthermore, considering leukocyte cells, VEGFR2 was negatively correlated to MMP9 protein levels. This pathway possibly prepares the cells for a higher activity in a hypoxic or an angiogenic microenvironment. The limitations of this study include lack of information about lesion clinical data as well as lack of information about the time of lesion evolution. In order to confirm our findings, in vitro and in vivo studies may clarify the mechanism underlying HIF-1 α induction and activity in tegumentary leishmaniasis. 
Received on 26.08.2011
Approved by the Advisory Board and accepted for publication on 22.01.2012.
Conflict of interest: None
Financial Support: This study was supported by the following Brazilian agencies: the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG). AMB De-Paula is a research fellow of FAPEMIG and ALS Guimarães is a research fellow of CNPq.
- 1. Kenner JR, Aronson NE, Bratthauer GL, Turnicky RP, Jackson JE, Tang DB, et al. Immunohistochemistry to identify Leishmania parasites in fixed tissues. J Cutan Pathol. 1999;26:130-6.
- 2. Scott P, Artis D, Uzonna J, Zaph C. The development of effector and memory T cells in cutaneous leishmaniasis: the implications for vaccine development. Immunol Rev. 2004;201:318-38.
- 3. Arrais-Silva WW, Paffaro VA Jr, Yamada AT, Giorgio S. Expression of hypoxia-inducible factor-1alpha in the cutaneous lesions of BALB/c mice infected with Leishmania amazonensis. Exp Mol Pathol. 2005;78:49-54.
- 4. Carrion J, Folgueira C, Alonso C. Transitory or long-lasting immunity to Leishmania major infection: the result of immunogenicity and multicomponent properties of histone DNA vaccines. Vaccine. 2008;26:1155-65.
- 5. Murback NDN, Nascimento,RAF, Dorval MEMC, Hans-Filho G, Nakazato KRO. Leishmaniose tegumentar americana: estudo clínico, epidemiológico e laboratorial no Hospital Universitário de Campo Grande, Mato Grosso do Sul, Brasil. An Bras Dermatol. 2011;86:55-63.
- 6. El-On J, Sneier R, Elias E. Leishmania major: bacterial contamination of cutaneous lesions in experimental animals. Isr J Med Sci. 1992;28:847-51.
- 7. Giorgio S, Linares E, Ischiropoulos H, Von Zuben FJ, Yamada A, Augusto O. In vivo formation of electron paramagnetic resonance-detectable nitric oxide and of nitrotyrosine is not impaired during murine leishmaniasis. Infect Immun. 1998;66:807-14.
- 8. Grimaldi G Jr, Tesh RB. Leishmaniases of the New World: current concepts and implications for future research. Clin Microbiol Rev. 1993;6:230-50.
- 9. Kanan MW. Mucocutaneous leishmaniasis in guinea-pigs inoculated intravenously with Leishmania enriettii. Preliminary report. Br J Dermatol. 1975;92:663-73.
- 10. McElrath MJ, Kaplan G, Nusrat A, Cohn ZA. Cutaneous leishmaniasis. The defect in T cell influx in BALB/c mice. J Exp Med. 1987;165:546-59.
- 11. Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol. 1999;66:889-900.
- 12. Brennan PA, Umar T, Wilson AW, Mellor TK. Expression of type 2 nitric oxide synthase and vascular endothelial growth factor in oral dysplasia. J Oral Maxillofac Surg. 2002;60:1455-60.
- 13. Brennan PA, Umar T, Smith GI, Lo CH, Tant S. Expression of nitric oxide synthase-2 in cutaneous squamous cell carcinoma of the head and neck. Br J Oral Maxillofac Surg. 2002;40:191-4.
- 14. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721-32.
- 15. Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda). 2004;19:176-82.
- 16. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basichelix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci. 1995;92:5510-4.
- 17. Keck PJ, Hauser SD, Krivi G, Sanzo K, Warren T, Feder J, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309-12.
- 18. Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841-4.
- 19. Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317-26.
- 20. Yan Z, Neulen J, Raczek S, Weich HA, Keck C, Grunwald K, et al. Vascular endothelial growth factor (VEGF)/vascular permeability factor (VPF) production by luteinized human granulosa cells in vitro; a paracrine signal in corpus luteum formation. Gynecol Endocrinol. 1998;12:149-53.
- 21. Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251-75.
- 22. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27-31.
- 23. Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67:563-72.
- 24. Luo Y, He DL, Ning L, Shen SL, Li L, Li X. Hypoxia-inducible factor-1alpha induces the epithelial-mesenchymal transition of human prostatecancer cells. Chin Med J (Engl ). 2006;119:713-8.
- 25. Tanimoto K, Yoshiga K, Eguchi H, Kaneyasu M, Ukon K, Kumazaki T, et al. Hypoxiainducible factor-1alpha polymorphisms associated with enhanced transactivation capacity, implying clinical significance. Carcinogenesis. 2003;24:1779-83.
- 26. Zarember KA, Malech HL. HIF-1alpha: a master regulator of innate host defenses? J Clin Invest. 2005;115:1702-4.
- 27. de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989-91.
- 28. Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604-13.
- 29. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820-7.
- 30. Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237-41.
Publication Dates
-
Publication in this collection
01 Oct 2012 -
Date of issue
Oct 2012
History
-
Received
26 Aug 2011 -
Accepted
22 Jan 2012