Abstracts
Dermatitis herpetiformis (DH) or Duhring-Brocq disease is a chronic bullous disease characterized by intense itching and burning sensation in the erythematous papules and urticarial plaques, grouped vesicles with centrifuge growth, and tense blisters. There is an association with the genotypes HLA DR3, HLA DQw2, found in 80-90% of cases. It is an IgA-mediated cutaneous disease, with immunoglobulin A deposits appearing in a granular pattern at the top of the dermal papilla in the sublamina densa area of the basement membrane, which is present both in affected skin and healthy skin. The same protein IgA1 with J chain is found in the small intestinal mucosa in patients with adult celiac disease, suggesting a strong association with DH. Specific antibodies such as antiendomysium, antireticulina, antigliadin and, recently identified, the epidermal and tissue transglutaminase subtypes, as well as increased zonulin production, are common to both conditions, along with gluten-sensitive enteropathy and DH. Autoimmune diseases present higher levels of prevalence, such as thyroid (5-11%), pernicious anemia (1-3%), type 1 diabetes (1-2%) and collagen tissue disease. The chosen treatment is dapsone and a gluten-free diet.
Celiac disease; Dermatitis herpetiformis; Skin diseases, Vesiculobullous
Dermatite herpetiforme é uma doença bolhosa crônica caracterizada por intenso prurido e sensação de queimação em pápulas eritematosas e placas urticariformes, vesículas agrupadas com crescimento centrífugo e bolhas tensas. Apresenta associação com genótipos de HLA DR3, HLA DQW2 encontrados em 80 a 90% dos casos. É uma doença cutânea mediada por IgA com depósito de imunoglobulina A em padrão granular no topo da papila dérmica na área da sublâmina densa na zona da membrana basal, presente tanto na pele lesada com em área de pele sã. A mesma cadeia J da proteína IgA1 é encontrada na mucosa do intestino delgado em pacientes com doença celíaca do adulto, sugerindo forte associação com a dermatite herpetiforme. Anticorpos específicos com anti-endomísio, anti-reculina, anti-gliadina, e recentemente identificado, o subtipo transglutaminase epidérmica e tecidual, assim como a produção aumentada da zonulina, são descritas em ambas as afecções enteropatia sensível ao glúten e a deramtite herpetiforme. Exibe depósitos de IgA em padrão granular na papila dérmica. Doenças auto-imunes exibem maior prevalência como tireoidopatia em 5 a 11%, anemia perniciosa em 1 a 3%, diabetes tipo 1 em 1 a 2% e doença do colágeno. O tratamento de escolha é a dapsona e dieta isenta de glútem.
Dermatite herpetiforme; Dermatopatias vesiculobolhosas; Doença celíaca
INTRODUCTION
Bullous diseases constitute one of the most extraordinary chapters of dermatology. Pathophysio logical mechanisms diversity subordinated to its varied etiology, extensive range of clinical manifestations with often systemic disease involvement, require a well-conducted medical evaluation method; therefore, translate into surprising difficulties that require specialized treatment and habilitation in overcoming the diagnostic and therapeutic challenge.
The bubbles are efflorescence filling with liquid composed of plasma and inflammatory cells, resulting from the change of cell structures and intercellular junctions structures responsible for the adhesion of epithelial tissue. 11. Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.
2. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.
3. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.-44. Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119:797-806. Vesicles are known as the diameter of the cavity less than 0.5 cm, and bubble is greater than 0.5 cm, intraepidermal if the lesion is present in the basal layer to the stratum corneum. 11. Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.-22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4. Through knowledge of the pathophysiology of the cleavage plane, the characteristics of the inflammatory infiltrate and especially the mechanism of blistering, it is possible to distinguish bullous dermatoses. 11. Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.,22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,55. Masunaga R. Epidermal basement membrane: its molecular organization and blistering disorders. Connect Tissue Res. 2006;47:55-66.
DERMATITIS HERPETIFORMIS
Dermatitis herpetiformis (DH) was described in 1884 by dermatologist Louis Duhring, who placed it in the same clinical category as pemphigus and pemphigoid, thus composing the class of bullous diseases. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,66. Sampaio SAP. Erupções vésico-bolhosas. In: Sampaio SAP, Rivitti EA. Dermatologia. 3 ed. São Paulo: Artes Médicas; 2008. p.301-30.,77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31. In 1888 Brocq described similar skin lesions diagnosed as "polymorphic pruritic dermatitis" and after examining Duhring's report, admitted that it was the same pathology. Therefore, Duhring-Brocq's disease is now used as a synonym for DH. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.
In 1943, through distinction of vesiculation mechanism, Civatte differentiated pemphigus (intraepidermal bullae), pemphigoid and DH (blistering of the basement membrane zone). 77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31.
The association with celiac disease, a glutensensitive enteropathy, and DH was observed in the sixties by Mards et al., Fry et al. and Shuster et al. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31.
Epidemiologically, DH is a rare disease. 33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.,88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.,99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.,1010. Kosan MK. Dermatitis herpetiformis. Dermatol Online J. 2003;9:8. It affects mainly young adults, although it had been diagnosed in infants aged eight months as well as in elderly people aged ninety years. 33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.,66. Sampaio SAP. Erupções vésico-bolhosas. In: Sampaio SAP, Rivitti EA. Dermatologia. 3 ed. São Paulo: Artes Médicas; 2008. p.301-30.,77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31.,1111. Lemberg D, Day AS, Bohane T. Coeliac disease presenting as dermatitis herpetiformis in infancy. J Paediatr Child Health. 2005;41:294-6.,1212. Lourdes S, Bajanca R, Cabral J, Feadeiro T. Dermatitis herpetiformes: should direct immunofluorescence be the only diagnostic criterion? Pediatr Dermatol. 2002;19:336-9. Males are more affected, with a ratio of 2:1, but in patients under 20, the ratio is 12 females for every 8 males. 33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.,1313. Alonso-Llamazares J, Gibson LE, Rogers RS 3rd. Clinical, pathologic and immunopathologic features of dermatitis herpetiformes: review of the Mayo Clicic experience. Int J Dermatol. 2007;46:910-9.,1414. Rose C, Brocker EB, Zillikens D. Clinical, histological and immunpathological findings in 32 patients with dermatitis herpetiformis Duhring. J Dtsch Dermatol Ges. 2010;8:265-70. Prevalence of DH varies across different countries, with 1:1,000,000 new cases / year in Germany, 11 per 100,000 in Scotland; 20-39 per 100,000 in Sweden and 58.8 per 100,000 in Ireland. 77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31.,99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.,1515. Caproni M, Antiga E, Melani L, Fabbri P; Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-8.,1616. Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, et al. A New Model for Dermatitis Herpetiformis that uses HLA-DQ8 transgenic NOD mice. Minnesota-USA. J Clin Invest. 2004;114:1090-7.
There are reports of disease in other members of the same family, either DH or adult celiac disease, in 2.3 to 10.5% of cases. 77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31.,1111. Lemberg D, Day AS, Bohane T. Coeliac disease presenting as dermatitis herpetiformis in infancy. J Paediatr Child Health. 2005;41:294-6.,1414. Rose C, Brocker EB, Zillikens D. Clinical, histological and immunpathological findings in 32 patients with dermatitis herpetiformis Duhring. J Dtsch Dermatol Ges. 2010;8:265-70.
Ethiopathogenesis has an immunological cause but is not fully understood. It is known that there is a higher incidence of genotypes HLA DR3, HLA DQw2 in 80-90% of patients, HLA B8 and HLA DQ8 in 1020% of cases, as well as adult celiac disease. 11. Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.
2. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.-33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.,88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.,99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.,1313. Alonso-Llamazares J, Gibson LE, Rogers RS 3rd. Clinical, pathologic and immunopathologic features of dermatitis herpetiformes: review of the Mayo Clicic experience. Int J Dermatol. 2007;46:910-9.,1616. Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, et al. A New Model for Dermatitis Herpetiformis that uses HLA-DQ8 transgenic NOD mice. Minnesota-USA. J Clin Invest. 2004;114:1090-7.
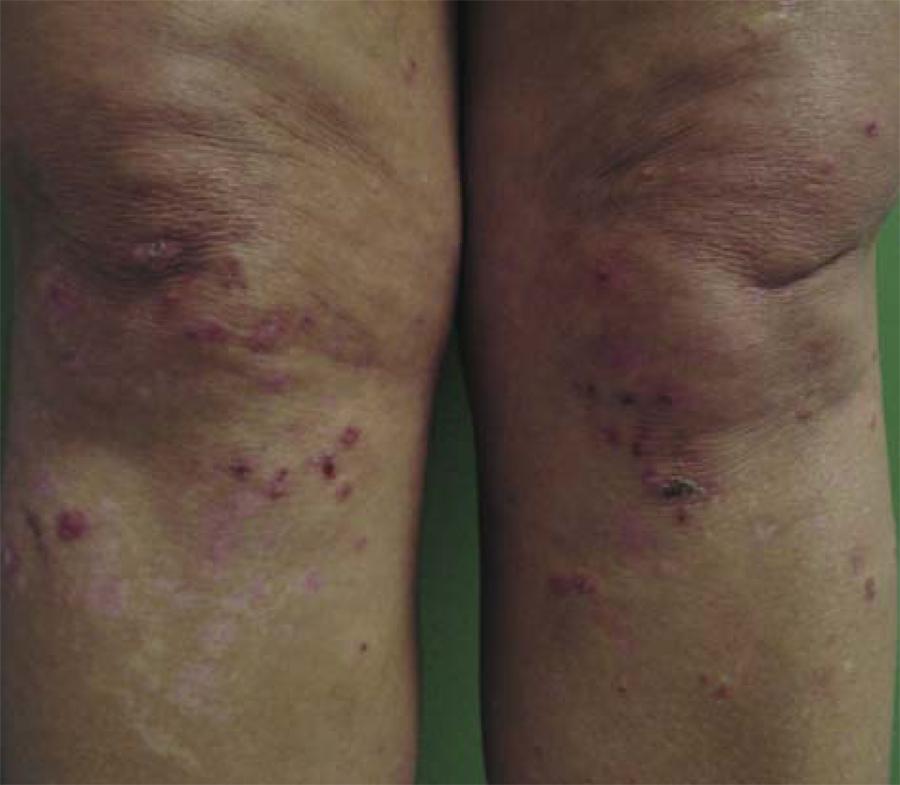
Cutaneous lesions begin with itching or a burning sensation in erythematous papules and urticarial plaques. There are grouped vesicles and tense blisters with centrifugal growth, whose contents may be serous or hemorrhagic, with symmetrical distribution (Figures 1, 2 and 3). 33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.,66. Sampaio SAP. Erupções vésico-bolhosas. In: Sampaio SAP, Rivitti EA. Dermatologia. 3 ed. São Paulo: Artes Médicas; 2008. p.301-30.,88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.,1717. Smith AD, Streilein RD, Hall RP 3rd. Neutrophil CD11b, L-selectin and Fc IgA receptors in patients with dermatitis hepetiformis. Br J Dermatol. 2002;147:1109-17.,1818. Sárdy M, Kárpáti S, Merkl B, Paulsson, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002;195:747-57. Bullous elements rupture, culminating in denuded areas of exulcerated skin and crust. Subsequently, there is residual hypopigmentation or hyperpigmentation. 88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.,1616. Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, et al. A New Model for Dermatitis Herpetiformis that uses HLA-DQ8 transgenic NOD mice. Minnesota-USA. J Clin Invest. 2004;114:1090-7.
Dermatitis herpetiformis: erythematous plaques, grouped vesicles, exulcerations and blood crust in lower limbs
Dermatitis herpetiformis. Vesicles dissected with crusts and residual hypopigmented macules on the knees
Dermatitis herpetiformis. Erythematous papules and urticarial plaques, vesicles with hemorrhagic content, denuded, exulcerated areas and scabs, with residual hypopigmentation on the back and upper limb
The topography usually affected are the extensor areas: lower limbs (anterior thigh and knee), elbows, buttocks and sacral region, although the shoulder, scapular region and scalp may also be affected. 11. Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.,88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39. Lesions in the oral mucosa are unusual. 88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.,99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.
Gastrointestinal clinical manifestation of gluten-sensitive enteropathy may occur at any age, either in childhood when cereals are introduced into the oral diet, or in adulthood without any prior food intolerance reaction. Symptoms include diarrhea, steatorrhea, malabsorption with resulting anemia, metabolic bone disease, weight loss and malnutrition. However some patients have no gastrointestinal signs or symptoms at all, since the majority of DH patients are asymptomatic, as only 20% of them develop intestinal symptoms. 1919. Holtmeier W, Pfänder M, Zollner TM, Kaufmann R, Caspary WF. Distinct TCR delta repertoires are present in the cutaneous lesions and inflamed duodenum of patients with dermatitis herpetiformis. Exp Dermatol. 2002:11:527-31.
Gluten is an amorphous protein composed of gliadin and glutenin amino acids, which are found in cereal seeds from the Grameneas family, such as wheat, barley, oat, malt and rye. They are cereals that contain starch, lipids and proteins (gliadin, glutenin, albumin and globumin). Wheat protein specifically is composed of 68% gliadin and 32% glutamin, therefore it is commonly referred to as wheat gluten. Examples of food products that contain this protein are flour, chocolate milk, which contains malt, processed cheese, beer, whiskey, vodka, mustard, ketchup, mayonnaise and salami. 2020. Biagi F, Bassi E, Ardigó M, Vignini MA, Caravaggi M, Borroni G, et al. In patients with dermatitis herpetiformis distribuition of transglutaminase in cutaneous tissue does not differ from controls. Dig Liver Dis. 2003;35:41-5.,2121. Lacerda, Liziane D. Avaliação das propriedades físico-químicas de proteína isolada de soja, amido e glúten e suas misturas. [dissertação]. Porto Alegre (RS): Universidade Federal do Rio Grande do Sul; 2008.
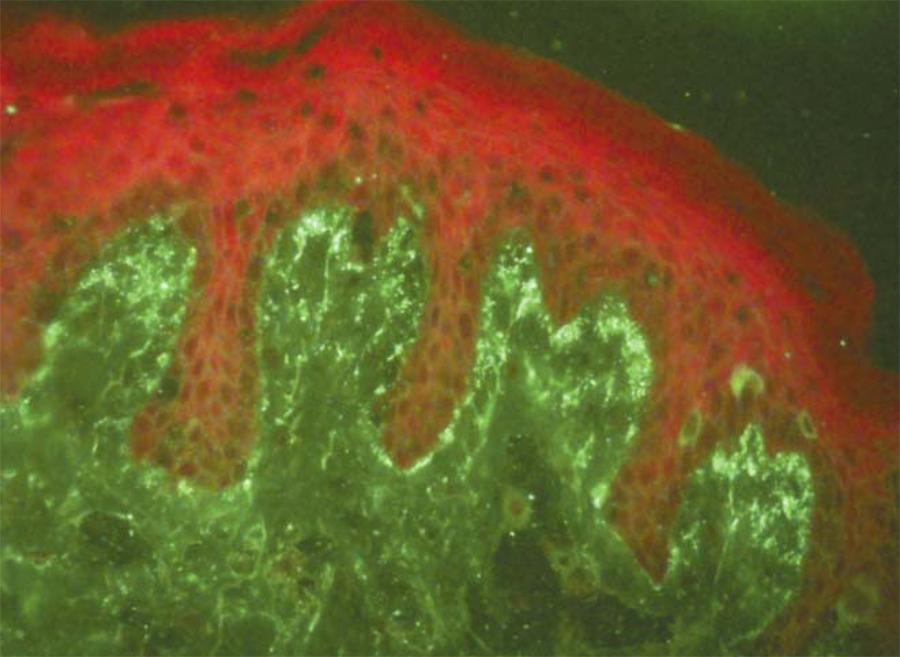
The typical finding regarding DH is the deposition of IgA immunoglobulin in a granular pattern at the top of the dermal papilla in the area of the sublamina densa of the basement membrane, which is present both in affected skin areas and in healthy skin. 88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.,99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.,1919. Holtmeier W, Pfänder M, Zollner TM, Kaufmann R, Caspary WF. Distinct TCR delta repertoires are present in the cutaneous lesions and inflamed duodenum of patients with dermatitis herpetiformis. Exp Dermatol. 2002:11:527-31. This can only be irradicated through adopting a gluten-free diet for several years, because even drug therapy does not alter this pattern. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.
Patients present gluten-sensitive enteropathy diagnosed by villi atrophy in a small intestine biopsy, in addition to specific antibodies in the serum, although most of them are asymptomatic. 88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.,99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.,1212. Lourdes S, Bajanca R, Cabral J, Feadeiro T. Dermatitis herpetiformes: should direct immunofluorescence be the only diagnostic criterion? Pediatr Dermatol. 2002;19:336-9.,2222. Gondim AJC. Glúten e a doença Celíaca. Conselho Regional de Química XIX Região, Dez 2009.
23. Preisz K, Sárdy M, Horváth A, Kárpati S. Immunoglobulin, complement and epidermal transglutaminase deposition in the cutaneous vessels in dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2005;19:74-9.-2424. Powell GR, Bruckner AL, Weston WL. Dermatitis herpetiformis presenting as chronic urticária. Pediatr Dermatol. 2004;21:564-7.
The same protein IgA1 with J chain and secretory component found in the small intestinal mucosa in adult celiac disease, is also present in the skin of patients with DH, suggesting a strong association between both diseases. 11. Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.,88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39. Other antibodies such as specific endomysium, antireticulina, gliadin and, recently identified, the epidermal and tissue transglutaminase subtypes, as well as overproduction of zonulin, are found in both conditions, along with enteropathic sensitivity to gluten and DH. 2525. Smecuol E, Sugai E, Niveloni S, Vázquez H, Pedreira S, Mazure R, et al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol. 2005:3:335-41.
26. Sitaru C, Zilikens D. Mechanisms of blister induction by autoantibodies. Exp Dermatol. 2005;14:861-75.
27. Barta Z, Miltenyl Z, Toth L, Illes A. Hypokalemic myopathy in a patient with glutensensitive enteropathy and dermatitis herpetiformis Duhring: a case report. Hungary. World J Gastroenterol. 2005;11:2039-40.
28. Wankiewicz A, Iwan-Zietek I, Kotschy M, Gwieździński Z. Select parameters of fibrinolysisi system in patients with dermatitis herpetiformis. Med Sci Monit. 2002:8:CR189-92.-2929. Sami N, Yeh SW, Ahmed R. Blistering diseases in the elderly: diagnosis and treatment. Dermatol Clin. 2004;22:73-86. IgM, IgG and C3 may also be present.
Studies have shown that cross-reaction occurs between some substances because they present common epitopes, and are therefore recognized as selfantigens triggering the disease. 2424. Powell GR, Bruckner AL, Weston WL. Dermatitis herpetiformis presenting as chronic urticária. Pediatr Dermatol. 2004;21:564-7.
High association autoimmune diseases include thyroid (5-11%) , pernicious anemia (1-3%), type 1 diabetes (1-2%), and collagen tissue disease. 88. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.,99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.,1414. Rose C, Brocker EB, Zillikens D. Clinical, histological and immunpathological findings in 32 patients with dermatitis herpetiformis Duhring. J Dtsch Dermatol Ges. 2010;8:265-70.,1616. Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, et al. A New Model for Dermatitis Herpetiformis that uses HLA-DQ8 transgenic NOD mice. Minnesota-USA. J Clin Invest. 2004;114:1090-7.,3030. Harrison TR, Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, et al. Medicina Interna. 15 ed. Rio de Janeiro: Mc Graw Hill; 2002. p. 1764-79;2778. Thus, Caproni et al. suggest screening for autoimmune diseases in patients with DH, as determination of antiperoxidase antibodies (present in 20% of cases), TSH, T4 and T3, anti-gastric parietal cells (10-25% positive), ANF, anti -Ro/SSA and glucose. 1616. Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, et al. A New Model for Dermatitis Herpetiformis that uses HLA-DQ8 transgenic NOD mice. Minnesota-USA. J Clin Invest. 2004;114:1090-7.
In 1970, Gjone & Nordoy were the first to report increased incidence of lymphoma in DH patients, which was confirmed for decades, especially T-cell lymphoma. 1414. Rose C, Brocker EB, Zillikens D. Clinical, histological and immunpathological findings in 32 patients with dermatitis herpetiformis Duhring. J Dtsch Dermatol Ges. 2010;8:265-70.,1616. Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, et al. A New Model for Dermatitis Herpetiformis that uses HLA-DQ8 transgenic NOD mice. Minnesota-USA. J Clin Invest. 2004;114:1090-7.,3131. Hervonen K, Viljamaa M, Collin P, Knip M, Reunala T. The occurrence of type 1 diabetes in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol. 2004;150:136-8.
32. Madalan V, Jamieson LA, Bhogalt BS, Wong CS. Inflamatory epidermolysis bullosa mimicking toxic epidermal necrolusis and dermatitis herpetiformis. Clin Exp Dermatol. 2009;34:e705-8.
33. Hervonen K, Vornanen M, Kautiainen H, Collin P, Reunala T. Lymphoma in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol. 2005;152:82-6.
34. Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformes. Gastroenterology. 2002;123:1428-35.
35. Lewis NR, Logan RFA, Hubbard RB, West J. No increase in risk of fracture, malignancy or mortality in dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2008;27:1140-7.
36. Ko CJ, Colegio OR, Moss JE, McNiff JM. Fibrillar IgA deposition in dermatites herpetiformis - an underreported pattern with potential clinical significance. J Cutan Pathol. 2010;37:475-7.
37. Jaskowski TD, Hamblin T, Wilson AR, Hill HR, Book LS, Meyer LJ, et al. IgA Anti-Epidermal Transglutaminase Antibodies in Dermatitis Herpetiformis and Pediatric Celiac Disease. J Invest Dermatol. 2009;129:2728-30.
38. Bardella MT, Fredella C, Saladino V, Trovato C, Cesana BM, Quatrini M, et al. Gluten intolerance: Gender and age-related differences in symptoms. Scand J Gastroenterol. 2005;40:15-9.
39. Peräaho M, Collin P, Kaukinen K, Kekkonen L, Miettinen S, Mäki M. Oats Can Diversify a gluten-free diet in celiac disease and dermatitis herpetiformis. J Am Diet Assoc. 2004;104:1148-50.
40. Korman NJ. New immunomodulating drugs in autoimmune blistering diseases. Dermatol Clin. 2001;19:637-48, viii.
41. Glied M, Rico MJ. Treatment of autoimmune blistering diseases. Dermatol Clin. 1999;17:431-40, x.
42. Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe Dapsone Hypersensitivity Syndrome. J Investig Allergol Clin Immunol. 2006:16:268-70.
43. Sacchidanand S, Hiremath NC, Natraj HV, Revathi TN, Rani S, Pradeep G, et al. Dexamethasone-cyclophosphamide pulse therapy for autoimmune-vesiculobullous disorders at Victoria hospital, Bangalore. Dermatol Online J. 2003;9:2.
44. Korman NJ. New and emerging therapies in the treatment of blistering diseases. Dermatol Clin. 2000:18:127-37, ix-x.-33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42. However, Lewis et al, in a study of 846 patients published in 2008, contradicted this relationship, such as found no increased risk of mortality in these patients. 3030. Harrison TR, Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, et al. Medicina Interna. 15 ed. Rio de Janeiro: Mc Graw Hill; 2002. p. 1764-79;2778.
Although 100% of patients with DH present sensitivity to gluten enteropathy, only a minority develop symptoms of colic or intestinal malabsorption, it is described the ratio of 1:5. 77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31.,1010. Kosan MK. Dermatitis herpetiformis. Dermatol Online J. 2003;9:8.,2020. Biagi F, Bassi E, Ardigó M, Vignini MA, Caravaggi M, Borroni G, et al. In patients with dermatitis herpetiformis distribuition of transglutaminase in cutaneous tissue does not differ from controls. Dig Liver Dis. 2003;35:41-5. There is evidence that a gluten-free diet alone brings about improvement or even complete remission of intestinal symptoms, and improvement of skin lesions in DH. 77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31.,1111. Lemberg D, Day AS, Bohane T. Coeliac disease presenting as dermatitis herpetiformis in infancy. J Paediatr Child Health. 2005;41:294-6.,1818. Sárdy M, Kárpáti S, Merkl B, Paulsson, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002;195:747-57.
The skin biopsy must come from a new, intact bulla. 14 14. Rose C, Brocker EB, Zillikens D. Clinical, histological and immunpathological findings in 32 patients with dermatitis herpetiformis Duhring. J Dtsch Dermatol Ges. 2010;8:265-70. Histopathological studies have revealed the Piérard microabscess on top of the dermal papilla formed by neutrophils and eosinophils, non-acantholytic blisters which initially exhibit subepidermal multilocular cavities that coalesce, forming unilocular cavities, and a perivascular lymphocytic inflammatory infiltrate in the upper dermis. 11. Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.,22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.,66. Sampaio SAP. Erupções vésico-bolhosas. In: Sampaio SAP, Rivitti EA. Dermatologia. 3 ed. São Paulo: Artes Médicas; 2008. p.301-30. The pathology does not differentiate DH from other autoimmune bullous diseases such as bullous pemphigoid, linear IgA dermatosis, and epidermolysis bullosa acquisita. 1414. Rose C, Brocker EB, Zillikens D. Clinical, histological and immunpathological findings in 32 patients with dermatitis herpetiformis Duhring. J Dtsch Dermatol Ges. 2010;8:265-70.
The direct immunofluorescence of perilesional skin affected is the gold standard to confirm the diagnosis with the deposition of IgA1 in granular pattern in the lamina lucida of the basement membrane zone (Figure 4). 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.,3434. Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformes. Gastroenterology. 2002;123:1428-35.,3535. Lewis NR, Logan RFA, Hubbard RB, West J. No increase in risk of fracture, malignancy or mortality in dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2008;27:1140-7. Less than 5% of cases present deposits of IgA in a linear pattern, which must be distinguished from linear IgA dermatosis. 77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31. A fibrillar IgA standard deposit is reportedly found in 50% of the Japanese population with HD, but several authors have questioned whether it corresponds to a variant of DH, a different disease, or only to an alternating pattern consequent longitudinal and transverse orientation of microfibrils, visualized through an electron microscopy, as suggested by Ko CJ et al. 3636. Ko CJ, Colegio OR, Moss JE, McNiff JM. Fibrillar IgA deposition in dermatites herpetiformis - an underreported pattern with potential clinical significance. J Cutan Pathol. 2010;37:475-7. Sometimes, it is necessary to carry out a second test for the diagnosis because, during the early stages of the disease, this typical feature may not be found. The deposit of immunoglobulin A does not change with dapsone, but approximately two years of gluten-free diet abolishes this finding. 11. Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.
Indirect immunofluorescence may be useful to detect the presence of autoantibodies and circulating anti-endomysial, anti-gliadin, and anti-reticulin IgA, and anti-epidermal transglutaminase antibodies. 11. Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.,22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.
Tissue transglutaminase antibodies (anti-tTG) can be measured by ELISA, showing greater than 90% specificity and sensitivity of 47-95%. It is used to diagnose DH and to assess patients adhesion to glutenfree diets and intestinal damage. Anti-tTG is 64% homologous to anti-epidermal transglutaminase (anti-eTG), which acts against the specific antigen in DH. 1616. Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, et al. A New Model for Dermatitis Herpetiformis that uses HLA-DQ8 transgenic NOD mice. Minnesota-USA. J Clin Invest. 2004;114:1090-7.,3737. Jaskowski TD, Hamblin T, Wilson AR, Hill HR, Book LS, Meyer LJ, et al. IgA Anti-Epidermal Transglutaminase Antibodies in Dermatitis Herpetiformis and Pediatric Celiac Disease. J Invest Dermatol. 2009;129:2728-30. Jaskowski et al. suggest that 20% of patients have anti-tTG negative, but these patients are antieTG positive. The same authors found a higher sensitivity of anti-eTG, which may help in cases of difficult diagnosis. Prevalence of anti-eTG decreases in children compared with adults. 3737. Jaskowski TD, Hamblin T, Wilson AR, Hill HR, Book LS, Meyer LJ, et al. IgA Anti-Epidermal Transglutaminase Antibodies in Dermatitis Herpetiformis and Pediatric Celiac Disease. J Invest Dermatol. 2009;129:2728-30.
IgA1 antibodies against smooth muscle have 100% specificity and 52 to 100% sensitivity in the diagnosis of DH. However anti-tTG may be absent in patients with gluten free diets. 1616. Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, et al. A New Model for Dermatitis Herpetiformis that uses HLA-DQ8 transgenic NOD mice. Minnesota-USA. J Clin Invest. 2004;114:1090-7.
Studies show that 100% of patients with DH exhibit histopathological changes of celiac sprue, ie, villous atrophy characterizing an aspect flat surface cuboidal epithelial cells that lose the orientation of the basal nucleus, increased proliferation of crypt cell causing hyperplasia and loss of their structures with increased lymphocytes and plasmocytes cells in the lamina propria. These findings are not pathognomonic of the sprue, but the return of histological pattern after gluten-free diet confirms diagnosis. 1919. Holtmeier W, Pfänder M, Zollner TM, Kaufmann R, Caspary WF. Distinct TCR delta repertoires are present in the cutaneous lesions and inflamed duodenum of patients with dermatitis herpetiformis. Exp Dermatol. 2002:11:527-31.
Villous atrophy of small bowel mucosa biopsy found in most patients with DH is less severe than in celiac disease or no tropical sprue. 77. Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31.
8. du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.-99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92. The absorption test D-xylose is altered in 10-33% of cases. Finding of iron deficiency anemia or megaloblastic anemia for folate deficiency and steatorrhea is not uncommon. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.
Although the clinical manifestations of D differ strongly in EC, both improve significantly with gluten-free diets. This measure provides relief in the itching and burning sensation of the vesicle-erythematous-papule, disseminated or localized on skin. Sometimes, it causes total regression of the cutaneous manifestations. This behavior seems to be great for the prognosis of the disease. However it is arduous and difficult in our country since gluten is a ubiquitous substance, and not all food products in the market contain explicit information regarding the presence or absence of gluten. Furthermore, the high cost of alternative products offered to patients with gluten sensitivity represents a financial obstacle for most Brazilian citizens. Thus, it is very difficult to adhere to and depends on determination, guidance and investment. 9,38,39 Iodine strict restriction is also suggested by some authors. 1313. Alonso-Llamazares J, Gibson LE, Rogers RS 3rd. Clinical, pathologic and immunopathologic features of dermatitis herpetiformes: review of the Mayo Clicic experience. Int J Dermatol. 2007;46:910-9.
The treatment is successful in patients who tolerate dapsone. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.,66. Sampaio SAP. Erupções vésico-bolhosas. In: Sampaio SAP, Rivitti EA. Dermatologia. 3 ed. São Paulo: Artes Médicas; 2008. p.301-30. The initial dose is generally between 100-200 mg per day and the response occurs within three hours to two days, with no new lesions appearing. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92. The patient should take a minimum dose, sufficient to suppress the disease. Some patients take 25 mg of dapsone per week, but others need 400 mg daily. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.
The side effects of drugs are dose-dependent. 50mg of dapsone can cause some hemolysis. Doses of 150 mg may decrease 2 grams of hemoglobin, which can be asymptomatic in healthy patients or trigger several symptoms and signs in patients with cardiac or lung disease, or even in elderly people. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.
Methemoglobinemia is ferrous iron (Fe +2) oxidation from hemoglobin to ferric iron, (Fe +3) caused by chemical substance. It progresses with erythrocytes precipitation and hemolysis characterized by Heinz bodies and "bitten cells." Methemoglobin causes the deviation of the oxygen dissociation curve to the left, resulting in insufficient oxygen to the tissues. 1919. Holtmeier W, Pfänder M, Zollner TM, Kaufmann R, Caspary WF. Distinct TCR delta repertoires are present in the cutaneous lesions and inflamed duodenum of patients with dermatitis herpetiformis. Exp Dermatol. 2002:11:527-31.
Methemoglobinemia is generally less than 5% and does not exceed 12% in DH. The signs and symptoms occur at methemoglobin 3%, characterized by cyanosis, a greyish colour, weakness, headaches, tachycardia, nausea and abdominal pain. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.
Other adverse effects are peripheral neuropathy (reversible with dose reduction); morbiliform eruption, erythema nodosum, erythema multiforme, exfoliative dermatitis, toxic epidermal necrolysis, severe hypoalbuminemia with anasarca, leukopenia, agranulocytosis, which may be fatal during the first three months, cholestasis and hepatitis. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,4040. Korman NJ. New immunomodulating drugs in autoimmune blistering diseases. Dermatol Clin. 2001;19:637-48, viii. The sulfone syndrome usually occurs within 6 weeks of treatment, regardless of dosage, and is characterized by exfoliative dermatitis, hepatitis, fever, lymphadenopathy, leukocytosis, headaches, vomiting and haemolysis. 4141. Glied M, Rico MJ. Treatment of autoimmune blistering diseases. Dermatol Clin. 1999;17:431-40, x.,4242. Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe Dapsone Hypersensitivity Syndrome. J Investig Allergol Clin Immunol. 2006:16:268-70.
Due to both the side effects and gravity of some cases, patients taking dapsone must be closely and continuously monitored. To avoid disastrous developments before starting treatment, deficiency in glucose6-phosphate dehydrogenase enzyme must be investigated, as it evolves with severe hemolysis because enzyme methemoglobin reductase - (NADPH -Methemoglobin reductase) - depend on NADPH to act. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,1919. Holtmeier W, Pfänder M, Zollner TM, Kaufmann R, Caspary WF. Distinct TCR delta repertoires are present in the cutaneous lesions and inflamed duodenum of patients with dermatitis herpetiformis. Exp Dermatol. 2002:11:527-31. A complete hemogram should be performed weekly during the first month, every two weeks within two months and often during the treatment. Renal and hepatic tests should also be required before starting the drug, with regular monitoring. 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.
Patients who cannot tolerate the use of dapsone may benefit from sulfapyridine (1 to 1.5 g / day, tetracycline 2 g / day, together with nicotinamide 1.5 g / day or cyclosporine for resistant cases). 22. Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.,1111. Lemberg D, Day AS, Bohane T. Coeliac disease presenting as dermatitis herpetiformis in infancy. J Paediatr Child Health. 2005;41:294-6.,4242. Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe Dapsone Hypersensitivity Syndrome. J Investig Allergol Clin Immunol. 2006:16:268-70. Sacchidanand S. et. al revealed satisfactory results with dexamethasone-cyclophosphamide pulse therapy. 4343. Sacchidanand S, Hiremath NC, Natraj HV, Revathi TN, Rani S, Pradeep G, et al. Dexamethasone-cyclophosphamide pulse therapy for autoimmune-vesiculobullous disorders at Victoria hospital, Bangalore. Dermatol Online J. 2003;9:2.
An important observation is that anti-inflammatory drugs usually worsen DH. 4343. Sacchidanand S, Hiremath NC, Natraj HV, Revathi TN, Rani S, Pradeep G, et al. Dexamethasone-cyclophosphamide pulse therapy for autoimmune-vesiculobullous disorders at Victoria hospital, Bangalore. Dermatol Online J. 2003;9:2.
Prognosis courses with periods of remission and exacerbation. 33. Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.,4444. Korman NJ. New and emerging therapies in the treatment of blistering diseases. Dermatol Clin. 2000:18:127-37, ix-x. An emotional event or infection may trigger a new worsening of the disease. 99. Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.
FINAL CONSIDERATIONS
It is extremely important for bullous diseases to be recognized by clinicians to prevent worsening of the symptoms with contraindicated or ineffective drugs, since the peculiar characteristics of bullous disease pose a high risk of serious and systemic consequences arising from electrolyte imbalance. 4343. Sacchidanand S, Hiremath NC, Natraj HV, Revathi TN, Rani S, Pradeep G, et al. Dexamethasone-cyclophosphamide pulse therapy for autoimmune-vesiculobullous disorders at Victoria hospital, Bangalore. Dermatol Online J. 2003;9:2.,4444. Korman NJ. New and emerging therapies in the treatment of blistering diseases. Dermatol Clin. 2000:18:127-37, ix-x.
REFERENCES
-
1Cohen LM, Skopicki DK, Harrist TJ, Clark WHJ. Noninfectious Vesiculobullous and Vesiculopustular Diseases In: Elder D, Elenitsas R, Jarsorsky C, Johnson BJ. Lever's Histopathology of the Skin. 8th ed. Raven: Lippincott; 1997. p.209-52.
-
2Katz SI. Dermatitis Herpetiformis. In: Fitzpatrick TB, Eisen AZ, Woff K, Freedberg IM, Austen KF. Dermatology in General Medicine. 7th ed. New York: McGraw-Hill: 2010. p.500-4.
-
3Azulay-Abulafia L, Azulay DR, Azulay RD. Buloses In: Azulay DR, Azulay RD. Dermatologia. 4 ed. Rio de Janeiro: Guanabara; 2006. p.128-42.
-
4Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119:797-806.
-
5Masunaga R. Epidermal basement membrane: its molecular organization and blistering disorders. Connect Tissue Res. 2006;47:55-66.
-
6Sampaio SAP. Erupções vésico-bolhosas. In: Sampaio SAP, Rivitti EA. Dermatologia. 3 ed. São Paulo: Artes Médicas; 2008. p.301-30.
-
7Lionel FRY. Dermatitis Herpetiformis: problems, progress and prospects. Eur J Dermatol. 2002;12:523-31.
-
8du Vivier A. Distúrbios bolhosos da pele. In: du Vivier A. Atlas de dermatologia clínica. 3. ed. Londres: Elsevier; 2004. p.421-39.
-
9Wojnarouska F, Eady RAJ, Burge S. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, Breathnach SM editors. Textbook of Dermatology. 6th ed. London: Blackwell Science; 1998. p.1817-92.
-
10Kosan MK. Dermatitis herpetiformis. Dermatol Online J. 2003;9:8.
-
11Lemberg D, Day AS, Bohane T. Coeliac disease presenting as dermatitis herpetiformis in infancy. J Paediatr Child Health. 2005;41:294-6.
-
12Lourdes S, Bajanca R, Cabral J, Feadeiro T. Dermatitis herpetiformes: should direct immunofluorescence be the only diagnostic criterion? Pediatr Dermatol. 2002;19:336-9.
-
13Alonso-Llamazares J, Gibson LE, Rogers RS 3rd. Clinical, pathologic and immunopathologic features of dermatitis herpetiformes: review of the Mayo Clicic experience. Int J Dermatol. 2007;46:910-9.
-
14Rose C, Brocker EB, Zillikens D. Clinical, histological and immunpathological findings in 32 patients with dermatitis herpetiformis Duhring. J Dtsch Dermatol Ges. 2010;8:265-70.
-
15Caproni M, Antiga E, Melani L, Fabbri P; Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-8.
-
16Marietta E, Black K, Camilleri M, Krause P, Rogers RS 3rd, David C, et al. A New Model for Dermatitis Herpetiformis that uses HLA-DQ8 transgenic NOD mice. Minnesota-USA. J Clin Invest. 2004;114:1090-7.
-
17Smith AD, Streilein RD, Hall RP 3rd. Neutrophil CD11b, L-selectin and Fc IgA receptors in patients with dermatitis hepetiformis. Br J Dermatol. 2002;147:1109-17.
-
18Sárdy M, Kárpáti S, Merkl B, Paulsson, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002;195:747-57.
-
19Holtmeier W, Pfänder M, Zollner TM, Kaufmann R, Caspary WF. Distinct TCR delta repertoires are present in the cutaneous lesions and inflamed duodenum of patients with dermatitis herpetiformis. Exp Dermatol. 2002:11:527-31.
-
20Biagi F, Bassi E, Ardigó M, Vignini MA, Caravaggi M, Borroni G, et al. In patients with dermatitis herpetiformis distribuition of transglutaminase in cutaneous tissue does not differ from controls. Dig Liver Dis. 2003;35:41-5.
-
21Lacerda, Liziane D. Avaliação das propriedades físico-químicas de proteína isolada de soja, amido e glúten e suas misturas. [dissertação]. Porto Alegre (RS): Universidade Federal do Rio Grande do Sul; 2008.
-
22Gondim AJC. Glúten e a doença Celíaca. Conselho Regional de Química XIX Região, Dez 2009.
-
23Preisz K, Sárdy M, Horváth A, Kárpati S. Immunoglobulin, complement and epidermal transglutaminase deposition in the cutaneous vessels in dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2005;19:74-9.
-
24Powell GR, Bruckner AL, Weston WL. Dermatitis herpetiformis presenting as chronic urticária. Pediatr Dermatol. 2004;21:564-7.
-
25Smecuol E, Sugai E, Niveloni S, Vázquez H, Pedreira S, Mazure R, et al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol. 2005:3:335-41.
-
26Sitaru C, Zilikens D. Mechanisms of blister induction by autoantibodies. Exp Dermatol. 2005;14:861-75.
-
27Barta Z, Miltenyl Z, Toth L, Illes A. Hypokalemic myopathy in a patient with glutensensitive enteropathy and dermatitis herpetiformis Duhring: a case report. Hungary. World J Gastroenterol. 2005;11:2039-40.
-
28Wankiewicz A, Iwan-Zietek I, Kotschy M, Gwieździński Z. Select parameters of fibrinolysisi system in patients with dermatitis herpetiformis. Med Sci Monit. 2002:8:CR189-92.
-
29Sami N, Yeh SW, Ahmed R. Blistering diseases in the elderly: diagnosis and treatment. Dermatol Clin. 2004;22:73-86.
-
30Harrison TR, Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, et al. Medicina Interna. 15 ed. Rio de Janeiro: Mc Graw Hill; 2002. p. 1764-79;2778.
-
31Hervonen K, Viljamaa M, Collin P, Knip M, Reunala T. The occurrence of type 1 diabetes in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol. 2004;150:136-8.
-
32Madalan V, Jamieson LA, Bhogalt BS, Wong CS. Inflamatory epidermolysis bullosa mimicking toxic epidermal necrolusis and dermatitis herpetiformis. Clin Exp Dermatol. 2009;34:e705-8.
-
33Hervonen K, Vornanen M, Kautiainen H, Collin P, Reunala T. Lymphoma in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol. 2005;152:82-6.
-
34Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformes. Gastroenterology. 2002;123:1428-35.
-
35Lewis NR, Logan RFA, Hubbard RB, West J. No increase in risk of fracture, malignancy or mortality in dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2008;27:1140-7.
-
36Ko CJ, Colegio OR, Moss JE, McNiff JM. Fibrillar IgA deposition in dermatites herpetiformis - an underreported pattern with potential clinical significance. J Cutan Pathol. 2010;37:475-7.
-
37Jaskowski TD, Hamblin T, Wilson AR, Hill HR, Book LS, Meyer LJ, et al. IgA Anti-Epidermal Transglutaminase Antibodies in Dermatitis Herpetiformis and Pediatric Celiac Disease. J Invest Dermatol. 2009;129:2728-30.
-
38Bardella MT, Fredella C, Saladino V, Trovato C, Cesana BM, Quatrini M, et al. Gluten intolerance: Gender and age-related differences in symptoms. Scand J Gastroenterol. 2005;40:15-9.
-
39Peräaho M, Collin P, Kaukinen K, Kekkonen L, Miettinen S, Mäki M. Oats Can Diversify a gluten-free diet in celiac disease and dermatitis herpetiformis. J Am Diet Assoc. 2004;104:1148-50.
-
40Korman NJ. New immunomodulating drugs in autoimmune blistering diseases. Dermatol Clin. 2001;19:637-48, viii.
-
41Glied M, Rico MJ. Treatment of autoimmune blistering diseases. Dermatol Clin. 1999;17:431-40, x.
-
42Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe Dapsone Hypersensitivity Syndrome. J Investig Allergol Clin Immunol. 2006:16:268-70.
-
43Sacchidanand S, Hiremath NC, Natraj HV, Revathi TN, Rani S, Pradeep G, et al. Dexamethasone-cyclophosphamide pulse therapy for autoimmune-vesiculobullous disorders at Victoria hospital, Bangalore. Dermatol Online J. 2003;9:2.
-
44Korman NJ. New and emerging therapies in the treatment of blistering diseases. Dermatol Clin. 2000:18:127-37, ix-x.
-
*
Work performed at the Dermatology Department at the Regional Hospital of Presidente Prudente - University of Oeste Paulista (HRPP-UNOESTE) - Presidente Prudente (SP), Brazil.
Publication Dates
-
Publication in this collection
Aug 2013
History
-
Received
25 Mar 2012 -
Accepted
13 Aug 2012