Abstracts
BACKGROUND:
The sum of environmental and genetic factors affects the appearance and function of the skin as it ages. The identification of molecular changes that take place during skin aging provides biomarkers and possible targets for therapeutic intervention. Retinoic acid in different formulations has emerged as an alternative to prevent and repair age-related skin damage.
OBJECTIVES:
To understand the effects of different retinoid formulations on the expression of genes associated with biological processes that undergo changes during skin aging.
METHODS:
Ex-vivo skin samples were treated topically with different retinoid formulations. The modulation of biological processes associated with skin aging was measured by Reverse Transcription quantitative PCR (RT-qPCR).
RESULTS:
A formulation containing microencapsulated retinol and a blend of active ingredients prepared as a triple nanoemulsion provided the best results for the modulation of biological, process-related genes that are usually affected during skin aging.
CONCLUSION:
This association proved to be therapeutically more effective than tretinoin or microencapsulated retinol used singly.
Gene expression; Nanotechnology; Tretinoin
FUNDAMENTOS:
A soma de fatores genéticos e ambientais afeta a aparência e a funcionalidade da pele ao longo do envelhecimento. O conhecimento a respeito das mudanças moleculares durante o envelhecimento fornece biomarcadores e possíveis alvos para intervenções terapêuticas. O ácido retinoico em diferentes formulações surgiu como uma alternativa para prevenir e reparar os danos da pele associados ao envelhecimento.
OBJETIVOS:
Avaliar comparativamente os efeitos de diferentes formulações contendo retinoides na expressão de genes associados a processos biológicos que são alterados com o envelhecimento da pele.
MÉTODOS:
Peles ex vivo foram topicamente tratadas com diferentes retinoides, micro e nanoencapsulados. A modulação dos processos biológicos associados ao envelhecimento da pele foi medida por PCR quantitativa, precedida de transcrição reversa (RT-qPCR).
RESULTADOS:
A formulação contendo uma mistura de princípios ativos incorporados em uma tripla nanoemulsão e retinol microencapsulado apresentou os melhores resultados de modulação de genes relacionados a processos biológicos que são normalmente alterados durante o envelhecimento da pele.
CONCLUSÃO:
Essa associação demonstrou uma maior eficácia terapêutica quando comparada ao uso isolado de tretinoína ou retinol microencapsulado.
Expressão gênica; Nanotecnologia; Tretinoína
INTRODUCTION
Skin aging is a continuous process that affects the appearance and function of the skin.
This process is determined intrinsically by individual genetics and extrinsically by
environmental and lifestyle factors. 11. Guinot C, Malvy DJ, Ambroisine L, Latreille J, Mauger E, Tenenhaus M, et
al. Relative Contribution of Intrinsic vs Extrinsic Factors to Skin Aging as Determined by
a Validated Skin Age Score. Arch Dermatol. 2002;138:1454-60.
,
22. Baumann L. Skin ageing and its treatment. J Pathol.
2007;211:241-51. The sum of genetics and environmental factors is
responsible for the condition of skin during aging. Older skins undergo physiologically
abnormal processes that are absent in younger ones. The changes are caused in part by
cumulative endogenous damage due to the continuous formation of reactive oxygen species
(ROS), which are normally generated by cellular metabolism. 33. Ivié NP. Skin aging. Acta Dermatoven. 2008;17:47-54. Despite a strong antioxidant defense system, the damage generated by ROS affects
cellular constituents such as membranes, enzymes and DNA. 44. Yaar M, Gilchrest BA. Aging of skin. In: Freedberg IM, Eisen AZ, Wolff K,
Austen KF, Goldsmith LA, Katz SI, et al. editors. Dermatology in General Medicine. New
York: McGraw-Hill; 1999. p 1697- 1706. Cellular stress accumulated over time potentially impacts cell metabolism and,
consequently, tissue regeneration and function. The progressive loss of function impairs
tissue physiology, as is frequently observed in older skins. The diminished cutaneous
microvasculature seen in older persons accounts for the progressively lower nutritional
support in aging skin. 55. Kelly RI, Pearse R, Bull RH, Leveque JL, de Rigal J, Mortimer PS. The
effects of aging on the cutaneous microvasculature. J Am Acad Dermatol.
1995;33:749-56. In addition, obliterated
vessels have been associated with disturbances of the normal architecture of the vascular
plexus in the dermis 44. Yaar M, Gilchrest BA. Aging of skin. In: Freedberg IM, Eisen AZ, Wolff K,
Austen KF, Goldsmith LA, Katz SI, et al. editors. Dermatology in General Medicine. New
York: McGraw-Hill; 1999. p 1697- 1706. . Changes in skin
pigmentation, lipid and water content, and response to stress and inflammation, are
frequently observed during aging. 66. Castanet J, Ortonne JP. Pigmentary changes in aged and photoaged skin.
Arch Dermatol. 1997;133:1296-9.
7. Waller JM, Maibach HI. Age and skin structure and function, a
quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and
structure. Skin Res Technol. 2006;12:145-54.
-
88. Castle SC. Clinical relevance of age-related immune dysfunction. Clin
Infect Dis. 2000;31:578-85 The pathophysiological process of skin aging
derives largely from an aberrant regulation of a multitude of finely tuned molecular
mechanisms, which have evolved to preserve the structural integrity of dermal connective
tissue. 99. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al.
Mechanisms of Photoaging and Chronological Skin Aging. Arch Dermatol.
2002;138:1462-70. These molecular mechanisms enable skin
cells to communicate both with each other and their environment. Understanding the relevant
signal transduction pathways and their effectors will provide exciting opportunities for
therapeutic intervention to prevent and repair age-related skin damage. 99. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al.
Mechanisms of Photoaging and Chronological Skin Aging. Arch Dermatol.
2002;138:1462-70.
In recent years, the use of retinoids has become popular as a therapeutic option to prevent and minimize signs of aging. 1010. Reinoso YDR, Mendonça IRSM, Azulay DR, Hofmeister HA, Azulay RD. Envelhecimento cutâneo extrínseco: tratamento com tretinoina. An Bras Dermatol. 1993;68:3-6. Retinoids are a class of compounds that are chemically related to vitamin A - an essential nutrient that plays a key role in eyesight, as well as cell growth and differentiation. In vivo, retinoids must bind with specific proteins to perform their necessary functions. 1111. Zhang YR, Zhao YQ, Huang JF. Retinoid-Binding Proteins: Similar Protein Architectures Bind Similar Ligands via Completely Different Ways. PLoS One. 2012;7:e36772.
The modern history of retinoids began in 1909, when vitamin A, an essential factor for the viability of an embryo, was discovered in the fatty extract of egg yolks. Retinoids were eventually introduced in the treatment of dermatoses, including photoaging, over two decades ago. 1212. Ramos-e-Silva M, Hexsel DM, Rutowitsch MS, Zechmeister M. Hydroxy acids and retinoids in cosmetics. Clin Dermatol. 2001;19:460-6.
Studies have described the rapid metabolization of retinoic acid by 10T1/2 cells and most transformed derivatives, whereas retinol is only marginally metabolized by, but highly concentrated in, these cells. Furthermore, the lack of retinoic acid activity seems unlikely to be due to the lack of appropriate retinoic acid binding protein, since cellular retinoic acid-binding proteins (CRABPs) were detected at concentration levels four times greater than those of cellular retinol-binding proteins (CRBPs). The differential activity of these two retinoids may thus be explicable on pharmacokinetic grounds. 1313. Rundhaug J, Gubler ML, Sherman MI, Blaner WS, Bertram JS. Differential uptake, binding, and metabolism of retinol and retinoic acid by 10T1/2 cells. Cancer Res. 1987;47:5637-43.
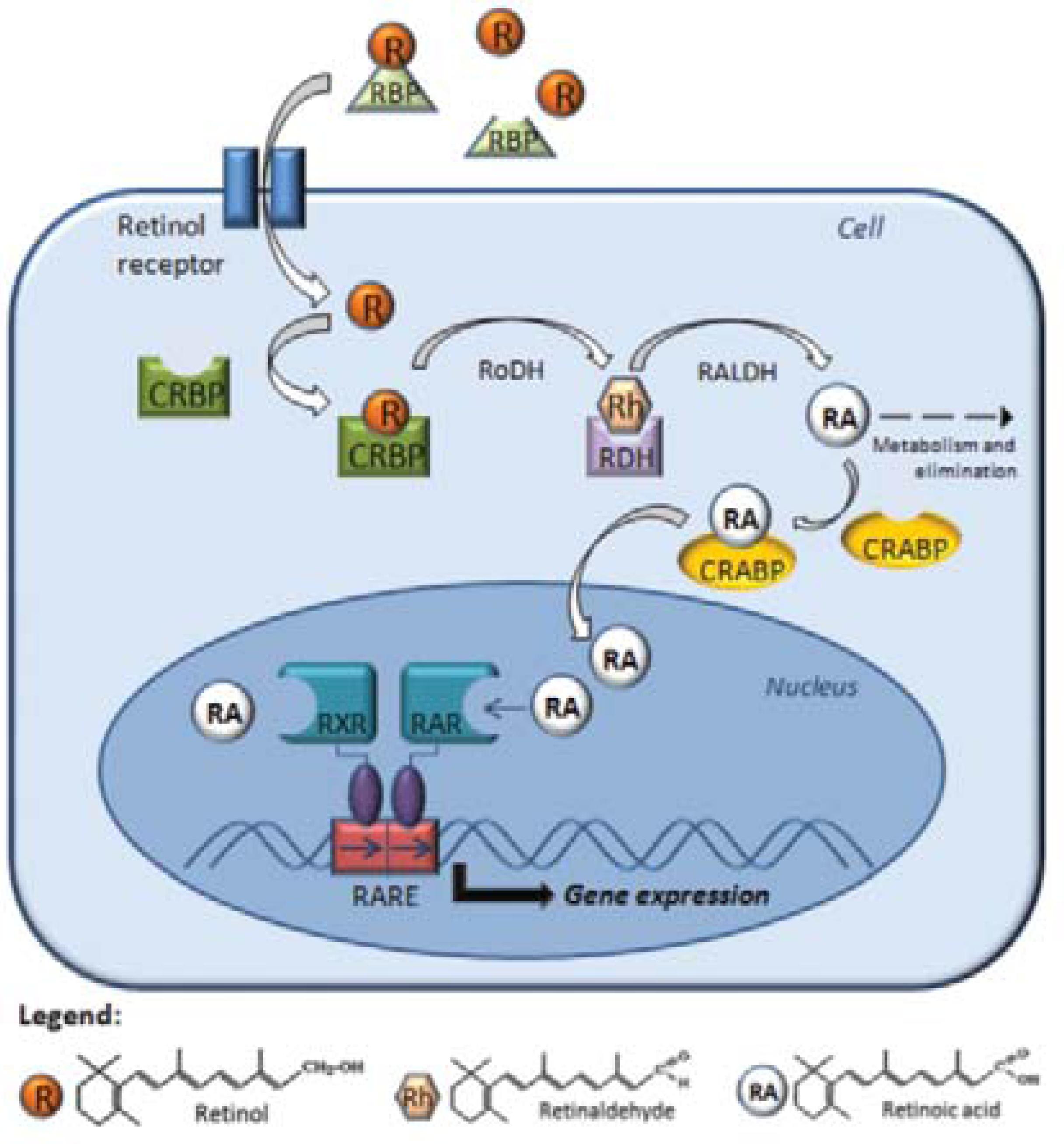
Structurally, all retinoids consist of a β-ionone ring and a polyunsaturated side chain containing an alcohol, aldehyde, a carboxylic acid group, or an ester group. Because of their chemical instability and fairly low solubility in aqueous media, retinoids must be bound by specific proteins found in bodily fluids and within cells. CRBPs and CRABPs carry retinoids inside cells (Figure 1). Interestingly, although all of these transport proteins possess similar structures, the binding modes for the ligands of different retinoids with their carrier proteins are different. 1414. Newcomer ME. Retinoid-binding proteins: structural determinants important for function. FASEB J. 1995;9:229-39.
The cellular mechanism of retinoid action. Retinol interacts with specific proteins called retinol-binding proteins (RBP),which aid intracellular transfer via the receptor protein, binding with CRBP (cellular retinol-binding proteins) in the cytoplasm. The retinol dehydrogenase (RoDH) enzymes metabolize retinol into retinaldehyde (Rh), then retinaldehyde is metabolized into retinoic acid (RA) by the retinaldehyde dehydrogenases (RALDHs). In the cytoplasm, RA is bound by CRABP (cellular RA-binding protein) or transformed into more polar compounds, which are subject to further metabolization and elimination. RA enters the nucleus and binds to the RA receptors (RARs) and retinoid X receptors (RXRs), which they themselves heterodimerize and bind to a sequence of DNA known as RARE (RA-response element), which activates the transcription of target genes
The present study investigated the influence of retinoids on biological processes related to skin aging and compared the role of different retinoids and delivery systems in gene expression by means of Reverse Transcription quantitative PCR (RT-qPCR). RT-qPCR has become a popular method to assess total RNA and mRNA expression because of its sensitivity, accuracy, and ability to replicate mRNA. 1515. Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155-66. Over the past 10 years, the popularity of this method has grown exponentially with the publication of well over 25,000 papers from various fields of science, involving RT-qPCR data in agricultural, environmental, industrial, and medical research. 1616. Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. A practical approach to RTqPCR-Publishing data that conform to the MIQE guidelines. Methods. 2010;50:S1-5.
MATERIALS AND METHODS
Biological samples and experimental conditions
Samples were taken from normal human skin excised during plastic surgery procedures performed on three patients who had agreed to participate in the study. Ex vivo human skin samples were topically treated with tretinoin 0.1%, the leading worldwide commercial product (tretinoin 0.1%), microencapsulated retinol 0.1%, and association with triple nanoemulsion (Triple Nanoemulsion (TNE) + Cosmetic and/or Dermatological Preparation containing TNE - PI 0904697-6, 2009). The ex vivo skin samples treated were cultivated at an air-liquid interphase by using a 24-transwell plate filled with 1 mL culture medium, which consisted of DMEM (GIBCO) containing 10% FBS (SIGMA) and 1% Pen/Strep (GIBCO). The samples were incubated for 24h at 37ºC in a humidified environment containing 5% CO2. Following the incubation period, the samples were subjected to RNA extraction. All the experiments were performed in triplicate. Untreated skin samples were used as control.
RNA extraction and reverse transcription quantitative PCR (RT-qPCR)
RNA extraction was conducted using an RNeasy mini kit (QIAGEN) as per manufacturer's instructions. The purity and concentration of RNA were determined with the aid of a NanoDrop ND2000c (Thermo Scientific). cDNA was synthesized from 1 µg of total RNA using a high-capacity cDNA reverse transcription kit with RNase inhibitor (Life Technologies). Real-time PCR reactions were performed in duplicate, using a StepOnePlus Real-Time PCR system and FAST SYBR Green Master Mix (Applied Biosystems), for the evaluation of the gene expression associated with the following processes involved in skin aging: vasculogenesis, lipid metabolism, negative regulation of inflammatory response, response to oxidative stress, cell proliferation, cell-cell signaling, cell adhesion, melanin biosynthesis process regulation, extracellular matrix, skin barrier development and cell differentiation.
Data analysis
The relative gene expression in the treated and untreated samples was analyzed by means of the ΔΔCt method. A ΔΔCt value was calculated by subtracting the ΔCt for the control samples from the ΔCt for each treatment. The ΔΔCt values were converted into fold differences by raising 2 to the (-ΔΔCt)th power [2(-ΔΔCt)]. 1717. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods. 2001;25:402-8. The data were analyzed by one-way ANOVA with the post hoc Tukey's test for pairwise group comparison using JMP statistical software (SAS Institute). P ≤ 0.05 was considered statistically significant.
RESULTS
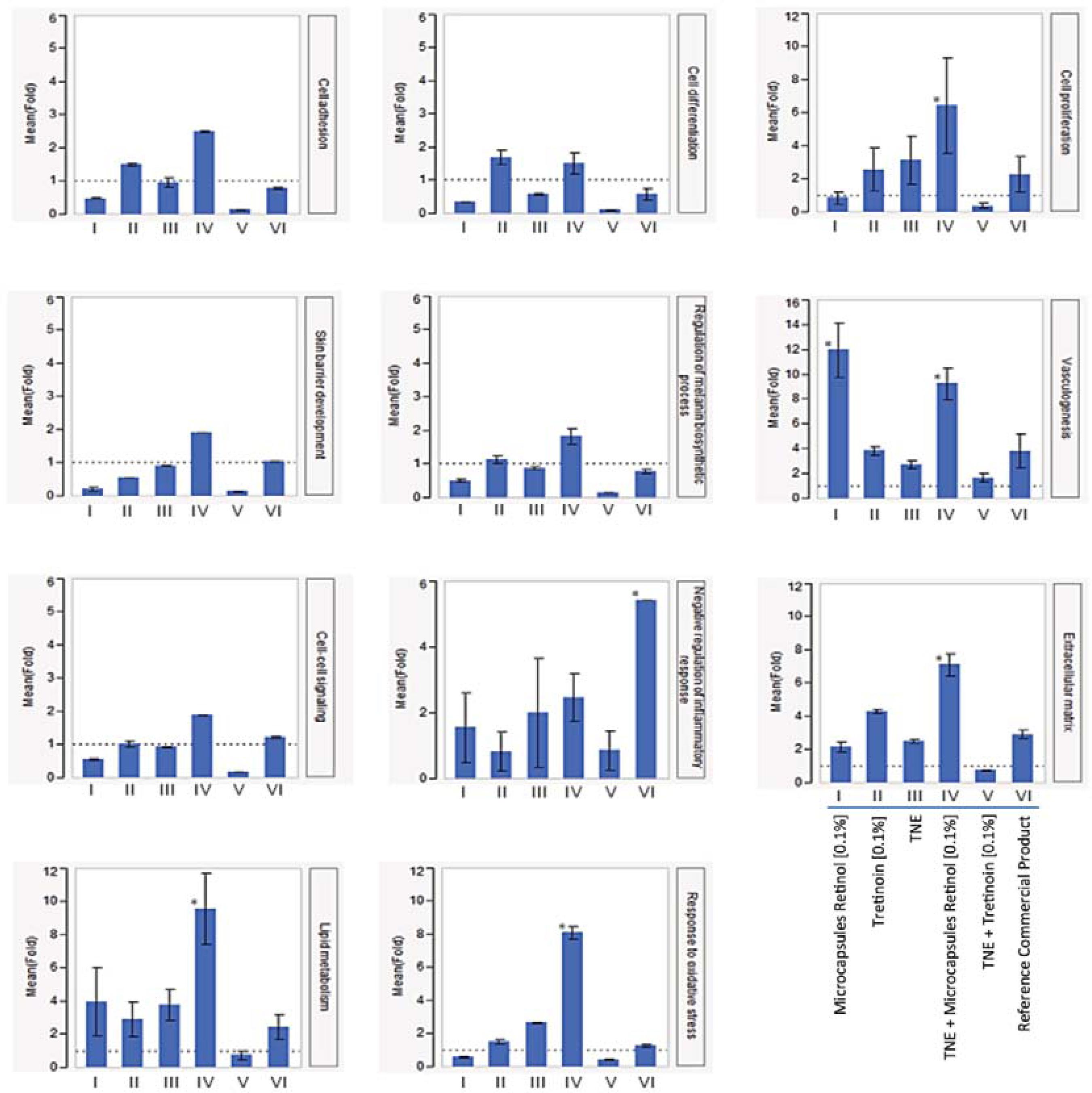
The effect of retinoid formulations on the expression of genes associated with vasculogenesis, lipid metabolism, control of inflammation, response to oxidative stress, cell proliferation, cell-cell signaling, cell adhesion, melanin biosynthesis process regulation, extracellular matrix, skin barrier development, and cell differentiation, was evaluated by RT-qPCR. Results are shown as fold change (FC) in comparison with untreated control samples.
For all processes evaluated, the association between TNE and microencapsulated retinol (0.1%) induced gene expression, and generated a better response than other formulations, including the association of TNE with tretinoin (0.1%), - the most widely used treatment for skin aging and other skin conditions (Figure 2).
Gene expression involved in biological processes related to skin aging in different treatments. Bars represent the mean values of fold; each error bar was constructed using 1 standard error from the mean (y axis – fold values; x axis – treatments); * represents p≤ 0.05 for the Tukey's test in the comparison of treatments
The association of TNE and microencapsulated retinol (0.1%) strongly induced lipid metabolism (9.57 FC), oxidative stress response (8.09 FC), extracellular matrix (7.11 FC), cell proliferation (6.43 FC), and vasculogenesis (9.24 FC). Vasculogenesis was also highly induced after treatment with microencapsulated retinol at 0.1% (11.94 FC). Although the modulation generated by TNE+microencapsulated retinol (0.1%) in cell adhesion (2.49 FC), skin barrier development (1.91 FC), cell-cell signaling (1.87 FC), cell differentiation (1.5 FC), melanin biosynthesis process regulation (1.83 FC) and inflammation (2.46 FC) was not highly significant (p≥0.05) in comparison with other treatments, it is clear that the association of TNE with microencapsulated retinol usually induces a better response than microencapsulated retinol, tretinoin, a leading commercial product, TNE, or TNE+retinoic acid association used singly.
DISCUSSION
For drug molecules to be clinically effective, it is of prime importance that they be administered via a route, which provides them with a proper channel to reach their target. Further, they need to be suitably protected in the biological environment until they are delivered to the required site of action. Drug delivery systems, such as microcapsules and nanoemulsions, have been developed to improve cosmetic and pharmaceutical formulations in order to increase biochemical stability and permeability, and ultimately increase the intracellular bioavailability of the drug. The transdermal delivery system is a successful channel whereby drug-loaded nanoparticles are delivered through the skin. 1818. Fang JY. Viral Nanoparticles: Adsorption and Self-Organization on Surfaces. Encyclopedia of Nanoscience & Nanotechnology. USA: American Scientific Publishers; 2004. p.3953.
Nanoemulsions consist of fine oil-in-water dispersions of usually spherical droplets, with a mean droplet diameter of about 20-200 nm. Additionally, depending on the physicochemical properties of the drugs, nanoemulsions can be structurally engineered to maximize their solubilization according to the required route of delivery, which is heavily dependent on the drug structure. 1919. Prakash RTU, Thiagarajan P. Nanoemulsions for drug delivery through different routes. Res J Biotechnol. 2011;2:1-13. The topical application of nanoemulsion offers many potential therapeutic advantages for suppressing or inducing gene expression in the skin and modulating biological processes involved in skin aging. 2020. Wang Z, Liu H, Yang SH, Wang T, Liu C, Cao YC. Nanoparticle-based artificial RNA silencing machinery for antiviral therapy. Proc Natl Acad Sci U S A. 2012;109:12387-92. Studies have demonstrated that using nanoemulsions as delivery systems can increase drug retention times in the body and consequently lower drug quantity required for the therapeutic action. The use of nanoemulsion technology has been reported to enhance the bioavailability of lipophilic drugs. 2121. Tiwari SB, Shenoy DB, Amiji MM. Nanoemulsion Formulations for improved oral delivery of poorly soluble drugs. Nanotech. 2006;1:475-8.
Our results showed that the most efficient treatment for skin aging-related processes is provided by the association of TNE with microencapsulated retinol (0.1%). This association was shown to increase gene expression in all the processes, even when compared with the classic tretinoin (0.1%) treatment, applied singly or in association with TNE.
Previous studies have described the effects of retinoids on the proliferation and differentiation of normal and neoplastic cells and tissues, including those found in the skin. 2222. Gudas LJ, Sporn MB, Roberts A. Cellular biology and biochemistry of the retinoids. In: Sporn MB, Roberts AA, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. New York: Raven Press; 1994. p. 443-520. , 2323. Lotan R. Retinoids in cancer chemoprevention. FASEB J. 1996;10:1031-9. Vitamin A (retinol), its natural metabolites and synthetic derivatives known as retinoids play important roles in regulating cell proliferation, differentiation, and embryonic development. 2424. Means AL, Gudas LJ. The roles of retinoids in vertebrate development. Annu Rev Biochem. 1995;64:201-33. The physiological activities of retinoids are primarily carried out via nuclear receptors that transcriptionally activate certain retinoid-responsive genes. 2222. Gudas LJ, Sporn MB, Roberts A. Cellular biology and biochemistry of the retinoids. In: Sporn MB, Roberts AA, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. New York: Raven Press; 1994. p. 443-520.
Human skin requires retinol for the regulation of keratinocyte growth and differentiation. 2525. Fuchs E, Green H. Regulation of Terminal Differentiation of Cultured Human Keratinocytes by Vitamin A. Cell. 1981;25:617-25. The biologic activity of retinol is considered to be predominantly exhibited by its natural oxidation product, retinoic acid. 2626. Kurlandsky SB, Xiao JH, Duell EA, Voorhees JJ, Fisher GJ. Biological activity of alltrans-retinol requires metabolic conversion to all-trans-retinoic and is mediated through activation of nuclear receptors in human keratinocytes. J Biol Chem. 1994;269:32821-7. Vitamin A and retinoids are, in certain cases, essential to the normal process of cell differentiation. Squamous keratinocytes shed daily from the surface of the skin, are continuously replaced by differentiating cells moving vectorially outward. These also consist mainly of keratinocytes and their outer root sheath is contiguous with the epidermis. 2727. Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55-61.
A low (0.1%) retinol dose was found to promote keratinocyte proliferation in vivo and improve the clinical treatment of fine lines and skin tone unevenness caused by photoaging in the lateral periorbital regions, when applied for a nine-month period; such treatment is reported to be minimally irritating. 2828. Bellemère G, Stamatas GN, Bruère V, Bertin C, Issachar N, Oddos T. Antiaging action of retinol: from molecular to clinical. Skin Pharmacol Physiol. 2009;22:200-9.
Ligand deprivation and pharmacological studies, conducted both in vivo and in vitro with keratinocytes in culture, have indicated that the active retinoid derivatives of vitamin A (primarily retinoic acid, RA) can play critical regulatory roles in the growth, differentiation, and maintenance of the mammalian epidermis and hair follicles. 2929. Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996;10:1002-13. , 3030. Roos TC, Jugert FK, Merk HF, Bickers DR. Retinoid metabolism in the skin. Pharmacol Rev. 1998;50:315-33.
As illustrated in figure 2, the cell proliferationrelated process was significantly induced by the treatment with TNE, incorporating retinol microcapsules at 0.1%, suggesting that the retinol microcapsules carried within the triple nanoemulsion were producing a synergic effect. Although the microcapsules are necessary to increase the stability of retinol, its retention time in the skin, and its absorption by skin cells, improve the intracellular bioavailability of the drug. TNE increases the permeation of the microcapsules because of their reduced mean particle size, improving the spreadability of the emulsion over skin pores, around hair follicles, and across intercellular gaps.
Topical tretinoin did not affect embryonic development in a number of studies performed in different models. In high doses, however, tretinoin can produce local inflammation, which may, for example, enhance absorption through the skin. Vehicles can cause differences in absorption characteristics. Pharmacokinetic findings offer explanations for the different behavior patterns of retinoids. 3131. Kochhar DM, Christian MS. Tretinoin: A review of the toxicology experience nonclinical developmental toxicology experience. J Am Acad Dermatol. 1997;36:S47-59.
One of the most common side effects of tretinoin therapy is dermatitis. It is characterized by xerosis, peeling and subjective irritation, occurring predominantly on intertriginous sites between the first and tenth weeks of topical tretinoin therapy. The inflammation of presumed subclinical actinic keratosis is a desired effect of the therapy, since it may cause these lesions to disappear. This effect tends to occur after at least 3 months of topical tretinoin therapy. The inflammation may be punctate or reticulate and is accompanied by xerosis and scaling. This reaction may be difficult to distinguish from tretinoin-induced dermatitis except for its usually later onset and discrete pattern. 3232. Weiss JS, Ellis CN, Headington JT, Voorhees JJ. Topical tretinoin in the treatment of aging, J Am Acad Dermatol. 1988;19:169-75
Other studies have shown that using tretinoin brings about a more intense anti-inflammatory activity than adapalene. It should therefore be the preferred option in treating inflammatory lesions. 3333. Akdeniz N, Calka O, Ozbek H, Metin A. Anti-inflammatory effects of tretinoin (all-trans-retinoic acid) 0.1% and adapalene 0.1% in rats. Clin Exp Dermatol. 2005;30:570-2. Corroborating our data, a recent review of the immunomodulating properties of tretinoin suggests that it has five important comedolytic-related properties that can be considered anti-inflammatory in nature. 3434. Schmidt N, Gans EH. Tretinoin: A Review of Its Anti-inflammatory Properties in the Treatment of Acne. J Clin Aesthet Dermatol. 2011;4:22-9.
Retinoic acid is the biologically active retinoid that is involved in producing beneficial effects on the skin. For cosmetic purposes, however, trans-retinol is used more frequently, as this pro-hormone of retinoic acid causes similar qualitative molecular changes but is less irritating. In the skin, trans-retinol can be metabolized in keratinocytes in the upper layers of the viable epidermis. Although the role of trans-retinol in preserving the physiology and function of skin has been intensively investigated, the physiological effect of this substance has been limited by its inefficient penetration through the natural skin barrier. 3535. Mélot M, Pudney PD, Williamson AM, Caspers PJ, Van Der Pol A, Puppels GJ. Studying the effectiveness of penetration enhancers to deliver retinol through the stratum cornum by in vivo confocal Raman spectroscopy. J Control Release. 2009;138:32-9.
Our results show an increased tretinoininduced anti-inflammatory response in ex vivo treatments in comparison with other retinoids. Also, the treatment using TNE associated with retinol microcapsules at 0.1% reduced the inflammation response significantly, compared with treatment using a leading commercial product. These results corroborate the data described in the literature on the potency of inflammation caused by tretinoin treatment. The potentiation effects of the treatment with retinol microcapsules in triple nanoemulsion indicate that retinol should be prescribed instead of tretinoin, since the retinol treatment generally induces a positive modulation of other age-related processes. Retinol microcapsules improve intracellular bioavailability and, with the increased permeation provided by TNE, increases the efficacy of the treatment.
Retinoic acid, a derivative of vitamin A, is widely used to treat acne vulgaris and photoaging, and has a profound effect on cellular differentiation, angiogenesis, new collagen formation, and fibroplasia. When applied topically to a poorly granulating wound bed, retinoic acid may restore and accelerate the formation of granulation tissue. However, retinoic acid is quite irritating to the surrounding skin and this problem has prevented its widespread use.
Since direct topical treatment with RA causes severe side effects, such as skin irritation, cosmetic uses of RA are limited. 3636. Nau H. Embryotoxicity and teratogenicity of topical retinoic acid. Skin Pharmacol. 1993;6 Suppl 1:35-44., 3737. Mills OH Jr, Berger RS. Irritation potential of a new topical tretinoin formulation and a commercially-available tretinoin formulation as measured by patch testing in human subjects. J Am Acad Dermatol. 1998;38:S11-6. Retinol is an alternative for longterm treatment because of its anti-aging activity. The photo-instability of retinol, however, diminishes its utility for general cosmetic and pharmaceutical formulations. 3838. Brisaert M, Plaizier-Vercammen J. Investigation on the photostability of a tretinoin lotion and stabilization with additives. Int J Pharm. 2000;199:49-57. To solve these problems, many research groups have synthesized retinol derivatives, blended retinol with soybean oil, or microencapsulated retinol. 3939. Han HS, Kwon YJ, Park MS, Park SH, Cho SM, Rho YS, et al. Efficacy Validation of Synthesized Retinol Derivatives In Vitro: Stability, Toxicity, and Activity. Bioorg Med Chem. 2003;11:3839-45.
According to Cho et al . (2012) and other authors, micro-encapsulation enhances the skin permeation of retinol and increases the UV/thermal stability of emulsions containing retinol. 3939. Han HS, Kwon YJ, Park MS, Park SH, Cho SM, Rho YS, et al. Efficacy Validation of Synthesized Retinol Derivatives In Vitro: Stability, Toxicity, and Activity. Bioorg Med Chem. 2003;11:3839-45. , 4040. Cho HK, Cho JH, Choi SW, Cheong IW. Topical delivery of retinol emulsions co-stabilized by PEO-PCL-PEO triblock copolymers: effect of PCL block length. J Microencapsul. 2012;29:739-46.
It is important to mention that TNE was able to increase and potentiate the effects of microencapsulated retinol, creating a better, more efficient and stabler treatment for skin aging.
Our data also demosntrated the positive effects of TNE and microencapsulated retinol on
biological processes related to oxidative stress reaction, extracellular matrix lipid
metabolism, and cell proliferation. The activities of superoxide dismutase, catalase and
glutathione peroxidase increased in response to retinol treatment, suggesting that retinol
induces the production of antioxidant enzymes, which demonstrates its role in the body's
response to oxidative stress. 4141. Dal-Pizzol F, Klamt F, Benfato MS, Bernard EA, Moreira JC. Retinol
supplementation induces oxidative stress and modulates antioxidant enzyme activities in
rat sertoli cells. Free Radic Res. 2001;34:395-404.
,
4242. Gimeno A, Zaragozá R, Vivó-Sesé I, Viña JR, Miralles VJ. Retinol, at
concentrations greater than the physiological limit, induces oxidative stress and
apoptosis in human dermal fibroblasts. Exp Dermatol. 2004;13:45-54. Other studies reported that retinoids increase
extracellular matrix proteins and stimulate fibroblast proliferation. 4343. Varani J, Mitra RS, Gibbs D, Phan SH, Dixit VM, Mitra R Jr, et al.
All-trans retinoic acid stimulates growth and extracellular matrix production in
growth-inhibited human skin fibroblasts. J Invest Dermatol.
1990;94:717-23.
44. Demetriou AA, Levenson SM, Rettura G, Seifter E. Vitamin A and retinoic
acid induced fibroblasts differentiation in vitro. Surgery.
1985:98:931-4.
45. Priestley GC. Proliferation and glycosaminoglycans secretion in
fibroblasts from psoriatic skin: differential responses to retinoids. Br J Dermatol.
1987;117:575-83.
-
4646. Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, et
al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix
metalloproteinases and stimulates collagen accumulation in naturally human skin. J Invest
Dermatol. 2000;114:480-6. Recent studies have demonstrated a molecular
mechanism by which retinol-binding protein (RBP) can have an effect on the signaling of
cell-surface receptors; when connected to retinol, this receptor induces the phosphorylation
of a tyrosine residue in the receptor C-terminus, thereby activating a JAK/STAT signaling
cascade. Consequently, in cells such as adipocytes, RBP-Retinol induces the expression of
STAT target genes, including SOCS3 and PPARγ, which enhances lipid accumulation. 4747. Berry DC, Noy N. Signaling by vitamin A and retinol-binding protein in
regulation of insulin responses and lipid homeostasis. Biochim Biophys Acta.
2012;1821:168-76.
CONCLUSION
Considered the gold standard of anti-age treatment by dermatologists specialized in skin aging, retinoic acid is nonetheless losing its therapeutic appeal because of its well-known tendency to irritate the skin and, consequently, cause intolerance in patients. Therefore, both retinoic acid and tretinoin - which in daily practice are used synonymously - are having their excellent therapeutic potential go to waste because of the patients' lack of adherence to continuous use. Moreover, retinoic acid is classified as a medical drug and, as such, may not be included in the formulation of countless products marketed with anti-aging claims.
The results detailed in this study are in agreement with the expected effects of retinoids as previously described in the literature. In addition, our data show that a blend of actives prepared as a triple nanoemulsion in association with microencapsulated retinol is therapeutically more effective than a leading commercial product, tretinoin or microencapsulated retinol, used separately. This association emerges as a new possibility for the treatment and prevention of skin aging.
REFERENCES
-
1Guinot C, Malvy DJ, Ambroisine L, Latreille J, Mauger E, Tenenhaus M, et al. Relative Contribution of Intrinsic vs Extrinsic Factors to Skin Aging as Determined by a Validated Skin Age Score. Arch Dermatol. 2002;138:1454-60.
-
2Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241-51.
-
3Ivié NP. Skin aging. Acta Dermatoven. 2008;17:47-54.
-
4Yaar M, Gilchrest BA. Aging of skin. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, et al. editors. Dermatology in General Medicine. New York: McGraw-Hill; 1999. p 1697- 1706.
-
5Kelly RI, Pearse R, Bull RH, Leveque JL, de Rigal J, Mortimer PS. The effects of aging on the cutaneous microvasculature. J Am Acad Dermatol. 1995;33:749-56.
-
6Castanet J, Ortonne JP. Pigmentary changes in aged and photoaged skin. Arch Dermatol. 1997;133:1296-9.
-
7Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (II): protein, glycosaminoglycan, water, and lipid content and structure. Skin Res Technol. 2006;12:145-54.
-
8Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578-85
-
9Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of Photoaging and Chronological Skin Aging. Arch Dermatol. 2002;138:1462-70.
-
10Reinoso YDR, Mendonça IRSM, Azulay DR, Hofmeister HA, Azulay RD. Envelhecimento cutâneo extrínseco: tratamento com tretinoina. An Bras Dermatol. 1993;68:3-6.
-
11Zhang YR, Zhao YQ, Huang JF. Retinoid-Binding Proteins: Similar Protein Architectures Bind Similar Ligands via Completely Different Ways. PLoS One. 2012;7:e36772.
-
12Ramos-e-Silva M, Hexsel DM, Rutowitsch MS, Zechmeister M. Hydroxy acids and retinoids in cosmetics. Clin Dermatol. 2001;19:460-6.
-
13Rundhaug J, Gubler ML, Sherman MI, Blaner WS, Bertram JS. Differential uptake, binding, and metabolism of retinol and retinoic acid by 10T1/2 cells. Cancer Res. 1987;47:5637-43.
-
14Newcomer ME. Retinoid-binding proteins: structural determinants important for function. FASEB J. 1995;9:229-39.
-
15Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155-66.
-
16Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. A practical approach to RTqPCR-Publishing data that conform to the MIQE guidelines. Methods. 2010;50:S1-5.
-
17Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods. 2001;25:402-8.
-
18Fang JY. Viral Nanoparticles: Adsorption and Self-Organization on Surfaces. Encyclopedia of Nanoscience & Nanotechnology. USA: American Scientific Publishers; 2004. p.3953.
-
19Prakash RTU, Thiagarajan P. Nanoemulsions for drug delivery through different routes. Res J Biotechnol. 2011;2:1-13.
-
20Wang Z, Liu H, Yang SH, Wang T, Liu C, Cao YC. Nanoparticle-based artificial RNA silencing machinery for antiviral therapy. Proc Natl Acad Sci U S A. 2012;109:12387-92.
-
21Tiwari SB, Shenoy DB, Amiji MM. Nanoemulsion Formulations for improved oral delivery of poorly soluble drugs. Nanotech. 2006;1:475-8.
-
22Gudas LJ, Sporn MB, Roberts A. Cellular biology and biochemistry of the retinoids. In: Sporn MB, Roberts AA, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. New York: Raven Press; 1994. p. 443-520.
-
23Lotan R. Retinoids in cancer chemoprevention. FASEB J. 1996;10:1031-9.
-
24Means AL, Gudas LJ. The roles of retinoids in vertebrate development. Annu Rev Biochem. 1995;64:201-33.
-
25Fuchs E, Green H. Regulation of Terminal Differentiation of Cultured Human Keratinocytes by Vitamin A. Cell. 1981;25:617-25.
-
26Kurlandsky SB, Xiao JH, Duell EA, Voorhees JJ, Fisher GJ. Biological activity of alltrans-retinol requires metabolic conversion to all-trans-retinoic and is mediated through activation of nuclear receptors in human keratinocytes. J Biol Chem. 1994;269:32821-7.
-
27Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55-61.
-
28Bellemère G, Stamatas GN, Bruère V, Bertin C, Issachar N, Oddos T. Antiaging action of retinol: from molecular to clinical. Skin Pharmacol Physiol. 2009;22:200-9.
-
29Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. FASEB J. 1996;10:1002-13.
-
30Roos TC, Jugert FK, Merk HF, Bickers DR. Retinoid metabolism in the skin. Pharmacol Rev. 1998;50:315-33.
-
31Kochhar DM, Christian MS. Tretinoin: A review of the toxicology experience nonclinical developmental toxicology experience. J Am Acad Dermatol. 1997;36:S47-59.
-
32Weiss JS, Ellis CN, Headington JT, Voorhees JJ. Topical tretinoin in the treatment of aging, J Am Acad Dermatol. 1988;19:169-75
-
33Akdeniz N, Calka O, Ozbek H, Metin A. Anti-inflammatory effects of tretinoin (all-trans-retinoic acid) 0.1% and adapalene 0.1% in rats. Clin Exp Dermatol. 2005;30:570-2.
-
34Schmidt N, Gans EH. Tretinoin: A Review of Its Anti-inflammatory Properties in the Treatment of Acne. J Clin Aesthet Dermatol. 2011;4:22-9.
-
35Mélot M, Pudney PD, Williamson AM, Caspers PJ, Van Der Pol A, Puppels GJ. Studying the effectiveness of penetration enhancers to deliver retinol through the stratum cornum by in vivo confocal Raman spectroscopy. J Control Release. 2009;138:32-9.
-
36Nau H. Embryotoxicity and teratogenicity of topical retinoic acid. Skin Pharmacol. 1993;6 Suppl 1:35-44.
-
37Mills OH Jr, Berger RS. Irritation potential of a new topical tretinoin formulation and a commercially-available tretinoin formulation as measured by patch testing in human subjects. J Am Acad Dermatol. 1998;38:S11-6.
-
38Brisaert M, Plaizier-Vercammen J. Investigation on the photostability of a tretinoin lotion and stabilization with additives. Int J Pharm. 2000;199:49-57.
-
39Han HS, Kwon YJ, Park MS, Park SH, Cho SM, Rho YS, et al. Efficacy Validation of Synthesized Retinol Derivatives In Vitro: Stability, Toxicity, and Activity. Bioorg Med Chem. 2003;11:3839-45.
-
40Cho HK, Cho JH, Choi SW, Cheong IW. Topical delivery of retinol emulsions co-stabilized by PEO-PCL-PEO triblock copolymers: effect of PCL block length. J Microencapsul. 2012;29:739-46.
-
41Dal-Pizzol F, Klamt F, Benfato MS, Bernard EA, Moreira JC. Retinol supplementation induces oxidative stress and modulates antioxidant enzyme activities in rat sertoli cells. Free Radic Res. 2001;34:395-404.
-
42Gimeno A, Zaragozá R, Vivó-Sesé I, Viña JR, Miralles VJ. Retinol, at concentrations greater than the physiological limit, induces oxidative stress and apoptosis in human dermal fibroblasts. Exp Dermatol. 2004;13:45-54.
-
43Varani J, Mitra RS, Gibbs D, Phan SH, Dixit VM, Mitra R Jr, et al. All-trans retinoic acid stimulates growth and extracellular matrix production in growth-inhibited human skin fibroblasts. J Invest Dermatol. 1990;94:717-23.
-
44Demetriou AA, Levenson SM, Rettura G, Seifter E. Vitamin A and retinoic acid induced fibroblasts differentiation in vitro. Surgery. 1985:98:931-4.
-
45Priestley GC. Proliferation and glycosaminoglycans secretion in fibroblasts from psoriatic skin: differential responses to retinoids. Br J Dermatol. 1987;117:575-83.
-
46Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally human skin. J Invest Dermatol. 2000;114:480-6.
-
47Berry DC, Noy N. Signaling by vitamin A and retinol-binding protein in regulation of insulin responses and lipid homeostasis. Biochim Biophys Acta. 2012;1821:168-76.
-
*
Work performed at the Laboratório de Biologia Molecular (LABIM) Grupo Boticário - Curitiba (PR), Brazil.
-
Financial Support: Skingen - Inteligência Genética, Grupo Boticário.
Publication Dates
-
Publication in this collection
Nov-Dec 2013
History
-
Received
17 Oct 2012 -
Accepted
07 Jan 2013