Abstract
Chronic urticaria has been explored in several investigative aspects in the new millennium, either as to its pathogenesis, its stand as an autoimmune or auto-reactive disease, the correlation with HLA-linked genetic factors, especially with class II or its interrelation with the coagulation and fibrinolysis systems. New second-generation antihistamines, which act as good symptomatic drugs, emerged and were commercialized over the last decade. Old and new drugs that may interfere with the pathophysiology of the disease, such as cyclosporine and omalizumab have been developed and used as treatments. The purpose of this article is to describe the current state of knowledge on aspects of chronic urticaria such as, pathophysiology, diagnosis and the current therapeutic approach proposed in the literature.
Angioedema; Biological factors; Colchicine; Cyclosporine; Dapsone; Immunosuppressive agents; Urticaria
INTRODUCTION
Acute urticaria and angioedema can be part of the clinical spectrum of anaphylaxis and thus present a lethal risk if left untreated.11 Cousin F, Philips K, Favier B, Bienvenu J, Nicolas JF. Drug-induced urticaria. Eur J Dermatol. 2001;11:181-7. Chronic urticaria (CU) on the other hand is a disease with major negative impact on the patients' daily activities and can therefore worsen their quality of life.
Over the last decade, European consensus regulated the classification, diagnosis and treatment of this group of diseases, based on critical analysis of Evidence-Based Medicine (EBM). 22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.
This review will approach relevant aspects of the "Position Paper of the Fourth International Consensus Meeting on Urticaria, Urticaria 2012", etiologic factors and pathophysiologic mechanisms associated with CU in the literature that were deemed relevant in the new millennium.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.
Physiopathologic mechanisms / Etiologic factors
Urticaria is a cutaneous reaction characterized by a sudden pruriginous rash accompanied by erythema and edema, defined borders, location, size and shapes that lasts for a few hours and is linked to the release of chemical mediators, mainly histamine, from mast cells in the dermis.11 Cousin F, Philips K, Favier B, Bienvenu J, Nicolas JF. Drug-induced urticaria. Eur J Dermatol. 2001;11:181-7. So, it is a heterogeneous group of diseases caused by or related to various factors, marked by the pattern of response with skin wheals/or angioedema. According to its evolution over time, it can be classified as acute (<6 weeks) or chronic (> 6 weeks).22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.
Several etiologic factors have been associated with CU throughout history: thyroid diseases, pseudo-allergens, actual allergens, Helicobacter pylori infection, other infections/infestations and autoimmunity/autoreactivity.
Helicobacter pylori infection and its relation to CU: Hizalet al demonstrated the positivity of autologous serum skin test (ASST) and high levels of IgG against Helicobacter pylori among patients with CU and concluded that the link between autoimmunity and infection by Helicobacter pylori warranted further studies.33 Hizal M, Tuzun B, Wolf R, Tuzun Y. The relationship between Helicobacter pylori IgG antibody and autologous serum test in chronic urticaria. Int J Dermatol. 2000;39:443-5.
Federman et al.44 Federman DG, Kirsner RS, Moriarty JP, Concato J. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. J Am Acad Dermatol. 2003;49:861-4. in an attempt to try and resolve this controversy, performed a literature review and selected ten relevant studies published in English that fulfilled the following criteria: (i) patients with CU only, (ii) exclusion of other known causes of urticaria through specific tests, (iii) initial diagnosis of H. pylori infection established by serology, urea test or endoscopy, and (iv) complete treatment of H. pylori with antibiotics.44 Federman DG, Kirsner RS, Moriarty JP, Concato J. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. J Am Acad Dermatol. 2003;49:861-4. The authors observed that the resolution of CU was more likely after the H. pylori treatment had been completed, than if the patogen was not eradicated. About 50% of the population has serologic evidence of past or present H. pylori infections and at least 30% of CU patients are infected with this agent, but in general, the treatment of this bacterium does not influence the course of CU.44 Federman DG, Kirsner RS, Moriarty JP, Concato J. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. J Am Acad Dermatol. 2003;49:861-4.
Greaves55 Burova KP, Mallet AI, Greaves MW. Is Helicobacter pylori a cause of urticaria? Br J Dermatol. 1999;51:42. suggested that H. pylori infection might have an indirect role in CU pathogenesis. Because of the immunogenicity of the patogen's cell envelope, it could be linked to the production of autoantibodies against Lewis X and Y blood group polysaccharide antigens, similar to that which occurs through molecular mimetism in Campylobacter jejunii infections and during Guillain-Barre syndrome. Therefore, H. pylori can have an indirect involvement in the etiology of CU, by reducing the immune tolerance and inducing the formation of autoantibodies, including the production of autoantibodies to antiFcεRIα.66 Greaves MW. Chronic idiopathic urticaria and H pylori: not directly causative but could there be a link? ACI Int. 2001;13:23-26.
Based on these data, there is still no overall consensus that the investigation of H. pylori should be performed as a routine or, that when it is present, the treatment might influence the course of CU.
Urticaria: food as a cause of pseudo-allergic reactions
Tharp et al77 Tharp MD, Thirlby R, Sullivan TJ. Gastrin induces histamine release from human cutaneous mast cells. J Allergy ClinImmunol. 1984;74:159-65. suggested that gastrin, a 17-aminoacid peptide released by G cells in the gastric antrum and proximal duodenum immediately after feeding, may be involved in anaphylactic reactions and urticaria reported after the ingestion of certain foods. This is corroborated by the observation that, it is not always possible to establish a direct correlation between clinical symptoms and the detection of antigen-specific IgE antibodies in cases of suspected food allergy.77 Tharp MD, Thirlby R, Sullivan TJ. Gastrin induces histamine release from human cutaneous mast cells. J Allergy ClinImmunol. 1984;74:159-65.
In recurring CU, it is assumed that there might be histamine intolerance caused by an excessive dose of histamine in the diet and/or by abnormal histamine metabolism (diamine oxidase deficiency).88 Lessof MH, Gant V, Hinuma K, Murphy GM, Dowling RH. Recurrent urticaria and reduced diamine oxidase activity. Clin Exp Allergy. 1990;20:373-6.
Diamine oxidase is the main enzyme involved in the degradation of histamine, acting predominantly in the intestinal mucosa. Alcohol and some medications may decrease the activity of this enzyme and determine a higher sensitivity to histamine-rich or histamine-producing foods. Several experiments have demonstrated deficiency of diamine oxidase in enterocytes of patients with recurrent CU.88 Lessof MH, Gant V, Hinuma K, Murphy GM, Dowling RH. Recurrent urticaria and reduced diamine oxidase activity. Clin Exp Allergy. 1990;20:373-6.
Certain fishes (tuna, sardines, anchovies), cheeses (Emmenthal, Gouda), salami, sausage, certain fruits and vegetables (tomatoes), wine and beer are histamine-rich foods.99 Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185-96. Drugs that may inhibit the intestinal activity of diamine oxidase and determine a higher concentration of histamine in general are: imipenem, dobutamine, pancuronium, pentamidine, salazosulfapyridine, verapamil, isoniazid, clavulanic acid, dihydralazine, chloroquine, cycloserine, acetylcysteine, metoclopramide and cefuroxime.99 Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185-96.,1010 Asero R. Multiple intolerance to food additives. J Allergy ClinImmunol. 2002;110:531-2.
Food additives such as preservatives, dyes and natural salicylates may trigger or aggravate urticaria through pseudo-allergic non-IgE-dependent mechanisms. These additives are: sodium metabisulfite, sodium benzoate, monosodium glutamate (MSG), sodium nitrate, tartrazine, erythrosine, sorbic acid and butylated hydroxyanisole.1010 Asero R. Multiple intolerance to food additives. J Allergy ClinImmunol. 2002;110:531-2. Regarding MSG, there is still no definitive conclusion about its causal relation to chronic urticaria, despite the existence of controlled studies.1111 Simon RA. Additive-induced urticaria: experience with monosodium glutamate (MSG). J Nutr. 2000;130:1063S-6S.
Di Lorenzo et al1212 Di Lorenzo G, Pacor ML, Mansueto P, Martinelli N, Esposito-Pellitteri M, Lo Bianco C, et al. Food-additive-induced urticaria: a survey of 838 patients with recurrent chronic idiopathic urticaria. Int Arch Allergy Immunol. 2005;138:235-42. studied pseudo-food allergy in 838 patients with chronic/recurrent idiopathic urticaria and found it present in about 1.0 to 3.0% of their population. The provocation tests with double-blind placebo-controlled technique were performed using the following substances: tartrazine (E102), erythrosine (E127), monosodium benzoate (E211), phydroxybenzoate (E218), metabisulfite (E223) and monosodium glutamate (E620). The authors recommend considering the possibility of exclusion diets and provocation tests with food additives in cases of CU/refractory angioedema that fail to fully respond to H1-antihistamine treatment. The general consensus is that, regarding CU, food additives can aggravate the disease but they are rarely its sole cause.
Dental infections and urticaria
The connection between dental infections and CU remains unclear.1313 Goga D, Vaillant L, Pandraud L, Mateu J, Ballon G, Beutter P. The elimination of dental and sinusal infectious foci in dermatologic pathology. A double-blind study in 27 cases confined to chronic urticaria. Rev Stomatol Chir Maxillofac. 1988;89:273-5. There have been reports of transient urticaria with high fever outbreaks after dental treatment, suggesting that bacteremia and/or toxemia arising from treatment would induce urticaria through immune and non-immune mechanisms. Histamine release by mast cells, secondary to lipopolysaccharides from oral flora Gram-negative bacteria such as Veilonellasp, could be relevant as a pathogenic factor in urticaria outbreaks in patients with odontogenic infection; furthermore, these ana-phylotoxins can have an acute direct vasodilator effect that determines urticaria outbreaks.1313 Goga D, Vaillant L, Pandraud L, Mateu J, Ballon G, Beutter P. The elimination of dental and sinusal infectious foci in dermatologic pathology. A double-blind study in 27 cases confined to chronic urticaria. Rev Stomatol Chir Maxillofac. 1988;89:273-5. We believe that patients with urticaria should have their dental condition assessed.
Hepatitis B and C infection
The fact that hepatitis B may be the cause of wheals or hives, particularly acute urticaria or CU is already well established.1414 Vaida GA, Goldman MA, Bloch KJ. Testing for hepatitis B virus in patients with chronic urticaria and angioedema. J Allergy Clin Immunol. 1983;72:193-8. It has been widely debated that hepatitis C (HC) can cause hives. We concur with the opinion of Siddique et al, that patients with urticaria living in areas of high prevalence of HC infection should be screened for it.1515 Siddique N, Pereira BN, Hasan Arshad S. Hepatitis C and urticaria: cause and effect? Allergy. 2004;59:668.
Helminthic parasites and infestations
The association of acute urticaria and angioedema or CU with infestations by
parasitic, protozoa, ectoparasites and helminthes has been postulated for many
decades. In fact, the literature is replete with case reports or case series, but
there are few case-control studies or meta-analyses on this subject. The association
of urticaria with the following parasites have been reported: Giardia
lamblia, Fasciola hepatica, Toxocara canis, Echinococcus granulosus, Strongyloides
stercoralis, Hymenolepis nana, Blastocystis hominis, Ascaris lumbricoides,
Anisakis simplex, Cimexlectularius (bedbug), Argas
reflexus (bird tick).1616 Nenoff P, Domula E, Willing U, Herrmann J.[Giardia lamblia--cause of
urticaria and pruritus or accidental association?]. Hautarzt. 2006;57:518-20,
521-2.
17 Demirci M, Yildirim M, Aridogan BC, Baysal V, Korkmaz M. Tissue
parasites in patients with chronic urticaria. J Dermatol.
2003;30:777-81.
18 Ismail MA, Khalafallah O. Toxocaracanis and chronic urticaria in
Egyptian patients. J Egypt Soc Parasitol. 2005;35:833-40.
19 Gulalp B, Koseoglu Z, Toprak N, Satar S, Sebe A, GokelY, et al. Ruptured
hydatid cyst following minimal trauma and few signs on presentation. Neth J Med.
2007;65:117-8.
20 Pattison DA, Speare R. Strongyloidiasis in personnel of the Regional
Assistance Mission to Solomon Islands (RAMSI). Med J Aust.
2008;189:203-6.
21 Marseglia GL, Marseglia A, Licari A, Castellazzi AM, Ciprandi G. Chronic
urticaria caused by Hymenoleptis nana in an adopted girl. Allergy.
2007;62:821-2.
22 Zuel-Fakkar NM, Abdel Hameed DM, Hassanin OM. Study of
Blastocystishominis isolates in urticaria: a case-control study. Clin Exp Dermatol.
2011;36:908-10.
23 Nenoff P, Domula E, Willing U, Herrmann J. [Giardia lamblia--cause of
urticaria and pruritus or accidental association?]. Hautarzt. 2006;57:518-20,
521-2.
24 Kaji K, Yoshiji H, Yoshikawa M, Yamazaki M, Ikenaka Y, Noguchi R, et al.
Eosinophilic cholecystitis along with pericarditis caused by Ascarislumbricoides: a
case report. World J Gastroenterol. 2007;13:3760-2.
25 Criado PR, Belda Junior W, Criado RF, Vasconcelos e Silva R,
Vasconcellos C. Bedbugs (Cimicidae infestation): the worldwide renaissance of an old
partner of human kind. Braz J Infect Dis. 2011;15:74-80.-2626 Spiewak R, Lundberg M, Johansson G, Buczek A. Allergy to pigeon tick
(Argasreflexus) in Upper Silesia, Poland. Ann Agric Environ Med.
2006;13:107-12.
The association between parasitism and urticaria has been better established with Anisakis simplex and recently with Blastocystis hominis. Anisakis simplex, also known as Pseudoterranova decipiens, Terranova decipiens or Phocanema decipiens belongs to the Anisakidae family.2727 Armentia A, Martin-Gil FJ, Pascual C, Martin-Esteban M, Callejo A, Martinez C. Anisakis simplex allergy after eating chicken meat. J Investig Allergol Clin Immunol. 2006;16:258-63. These nematodes have been described in infestations affecting humans after the ingestion of raw or not fully cooked seafood.2727 Armentia A, Martin-Gil FJ, Pascual C, Martin-Esteban M, Callejo A, Martinez C. Anisakis simplex allergy after eating chicken meat. J Investig Allergol Clin Immunol. 2006;16:258-63. Anisakiasis is the term used to describe the acute form of the disease in humans. Seafood is the main source of larval infection. Aside from urticaria and anaphylaxis, other manifestations such as rheumatic symptoms, contact dermatitis, Crohn's disease, eosinophilic gastroenteritis, conjunctivitis, and asthma have been reported.2727 Armentia A, Martin-Gil FJ, Pascual C, Martin-Esteban M, Callejo A, Martinez C. Anisakis simplex allergy after eating chicken meat. J Investig Allergol Clin Immunol. 2006;16:258-63. Sensitization to Anisakis simplex can be investigated through specific RAST test in peripheral blood.
The prevalence of Blastocystis hominis ranges from 10% in developed countries to 50% in those in process of development.2828 Daschner A, De Frutos C, Valls A, Vega F. Anisakis simplex sensitization-associated urticaria: short-lived immediate type or prolonged acute urticaria. Arch Dermatol Res. 2010;302:625-9. Several authors have correlated different Blastocystis hominis genetic subtypes especially subtype 3 - with cases of CU and acute urticaria, a fact that was not confirmed by other researchers.2828 Daschner A, De Frutos C, Valls A, Vega F. Anisakis simplex sensitization-associated urticaria: short-lived immediate type or prolonged acute urticaria. Arch Dermatol Res. 2010;302:625-9. Apparently the subtype identified may vary according to the different regions of the world, climate or seasonal changes, and source of infection.2828 Daschner A, De Frutos C, Valls A, Vega F. Anisakis simplex sensitization-associated urticaria: short-lived immediate type or prolonged acute urticaria. Arch Dermatol Res. 2010;302:625-9. Therefore, cases of CU in highly endemic geographic areas should be investigated for Blastocystis hominis in the stool and if the diagnosis is confirmed, treatment should be prescribed with metronidazole.
Chronic urticaria and thyroid
Hashimoto's thyroiditis and Graves' disease are associated with idiopathic CU.2929 Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. 1983;119:636-40.,3030 Leznoff A, Sussman GL. Syndrome of idiopathic urticaria and angioedema with thyroid autoimmunity: a study of 90 patients. J Allergy Clin Immunol. 1989;84:66-71. Antithyroid antibodies are found in 27% of patients with idiopathic CU and 19% of patients have abnormal thyroid function.3131 Zauli D, Grassi A, Ballardini G, Contestabile S, Zucchini S, Bianchi FB. Thyroid autoimmunity in chronic idiopathic urticaria. Am J Clin Dermatol. 2002;3:525-8. In such CU cases, high titers of antithyroid antibodies (antithyroglobulin and antiperoxidase) can be detected, while that occurs in only about 3% to 4% in the general population without thyroid diseases.3232 Turktas I, Gokcora N, Demirsoy S, Cakir N, Onal E. The association of chronic urticaria and angioedema with autoimmune thyroiditis. Int J Dermatol. 1997;36:187-90.
The simultaneous occurrence of antithyroid antibodies and anti-FcεRIα in some patients with so-called "idiopathic" urticaria seems to indicate the existence of a disease or a "state" secondary to an underlying autoimmune process and/or a disruption of immune regulation. Rottem (2003) reinforces this concept, suggesting that there are no data to support a connection between the presence of antithyroid antibodies and CU's pathogenesis, and that these are most likely parallel events, as occurs with autoimmune diseases.3333 Rottem M. Chronic urticaria and autoimmune thyroid disease: is there a link? Autoimmun Rev. 2003;2:69-72. In conclusion, tests for antithyroid antibodies and thyroid function should be performed in all CU cases in order to detect early those thyroid dysfunctions that require follow-up and treatment.3333 Rottem M. Chronic urticaria and autoimmune thyroid disease: is there a link? Autoimmun Rev. 2003;2:69-72.
Autoimmune / auto-reactive chronic urticaria
About 50% of the cases of chronic urticaria are considered autoimmune diseases, due
to the presence of circulating histamine-releasing autoantibodies, especially
directed against IgE high-affinity receptors (FcεRIα) present in the cytoplasmic
membrane of mast cells and basophils or anti-IgE autoantibodies.3434 Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P,
Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin
test in urticaria. Allergy. 2009;64:1256-68. There is clear evidence for a genetic predisposition to
develop CU, i.e., there is a strong association with HLA and familial inheritance or
autoimmune etiology in some cases, especially those with a positive autologous serum
skin test (ASST).3434 Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P,
Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin
test in urticaria. Allergy. 2009;64:1256-68. There are reports
suggesting a connection between CU with a positive ASST result and autoimmune
diseases, such as autoimmune thyroiditis, celiac disease, rheumatoid arthritis,
Grave's disease and type 1 diabetes mellitus; also, a higher frequency of
autoimmunity serum markers like rheumatoid factor, antinuclear and antithyroid
antibodies.3434 Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P,
Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin
test in urticaria. Allergy. 2009;64:1256-68. Patients with CU and positive
ASST have a high frequency of HLA-DRB1 * 04 and its associated allele DQB1 * 0302
when compared to the healthy population and patients with CU and negative ASST.3434 Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P,
Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin
test in urticaria. Allergy. 2009;64:1256-68.
35 Tedeschi A, Cottini M, Asero R. Simultaneous occurrence of chronic
autoimmune urticaria and non-allergic asthma: a common mechanism? Eur Ann Allergy
Clin Immunol. 2009;41:56-9.-3636 Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A.
Chronic urticaria and autoimmunity: associations found in a large population study. J
Allergy Clin Immunol. 2012;129:1307-13.
Unfortunately, we still lack a routine laboratory test to detect anti-FcεRIα and / or circulating and functionally active anti-IgE. ASST autoantibodies have been considered an "in vivo" test to confirm both the presence of these autoantibodies and histamine release in basophils.3434 Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin test in urticaria. Allergy. 2009;64:1256-68.,3636 Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012;129:1307-13.
In a retrospective study performed in Israel, Confino-Cohen et
al3636 Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A.
Chronic urticaria and autoimmunity: associations found in a large population study. J
Allergy Clin Immunol. 2012;129:1307-13. analyzed data from 12,778
patients with CU during a period of 17 years, and compared clinical and laboratory
data with 10,714 control patients without CU. There was a definite increase in the
odds ratio of hypo-or hyperthyroidism and the presence of
antithyroid antibodies among patients with CU. Female patients with CU had a higher
incidence of rheumatoid arthritis, Sjögren's syndrome, celiac disease, type I
diabetes mellitus and systemic lupus erythematosus during their lifetime and those
illnesses were diagnosed mainly in the 10 years following the diagnosis of CU.
Increase in mean platelet volume, positivity for rheumatoid factor and antinuclear
antibodies were more prevalent and significant among patients with CU. Probably the
presence of a chronic inflammatory process, implied by the increased mean platelet
volume, shares a common pathogenic pathway with autoantibody formation in patients
with CU. However, 50-60% of CU cases remain idiopathic, the so-called "spontaneous"
CU.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica
GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification,
diagnosis, and management ofurticaria: the 2013 revision and update. Allergy.
2014;69:868-87.,3636 Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A.
Chronic urticaria and autoimmunity: associations found in a large population study. J
Allergy Clin Immunol. 2012;129:1307-13. Recently, some authors demonstrated the activation of the
coagulation system in patients with CU via thrombin generation, initiated by the
increased expression of coagulation tissue factor on eosinophils.3737 Cugno M, Marzano AV, Asero R, Tedeschi A. Activation of blood
coagulation in chronic urticaria: pathophysiological and clinical implications.
Intern Emerg Med. 2010;5:97-101.
38 Asero R, Tedeschi A, Riboldi P, Griffini S, Bonanni E, Cugno M.Severe
chronic urticaria is associated with elevated plasma levels of D-dimer. Allergy.
2008;63:176-80.-3939 Takahagi S, Mihara S, Iwamoto K, Morioke S, Okabe T, Kameyoshi Y, et al.
Coagulation/fibrinolysis and inflammation markers are associated with disease
activity in patients with chronic urticaria. Allergy.
2010;65:649-56. This determines a potential contribution to the increase in capillary
permeability. These patients often have elevated coagulation and fibrinolysis serum
markers, such as fragment 1 +2 prothrombin and D-dimer, whose levels seem to
correlate with the severity of CU.3737 Cugno M, Marzano AV, Asero R, Tedeschi A. Activation of blood
coagulation in chronic urticaria: pathophysiological and clinical implications.
Intern Emerg Med. 2010;5:97-101.
38 Asero R, Tedeschi A, Riboldi P, Griffini S, Bonanni E, Cugno M.Severe
chronic urticaria is associated with elevated plasma levels of D-dimer. Allergy.
2008;63:176-80.-3939 Takahagi S, Mihara S, Iwamoto K, Morioke S, Okabe T, Kameyoshi Y, et al.
Coagulation/fibrinolysis and inflammation markers are associated with disease
activity in patients with chronic urticaria. Allergy.
2010;65:649-56. In animal models,
thrombin shows increased capillary permeability by direct action on the endothelium
and indirectly by inducing the release of pro-inflammatory mediators by mast cells,
increasing C5a in the absence of C3, and bypassing the first part of the complement
cascade.3737 Cugno M, Marzano AV, Asero R, Tedeschi A. Activation of blood
coagulation in chronic urticaria: pathophysiological and clinical implications.
Intern Emerg Med. 2010;5:97-101.
38 Asero R, Tedeschi A, Riboldi P, Griffini S, Bonanni E, Cugno M.Severe
chronic urticaria is associated with elevated plasma levels of D-dimer. Allergy.
2008;63:176-80.-3939 Takahagi S, Mihara S, Iwamoto K, Morioke S, Okabe T, Kameyoshi Y, et al.
Coagulation/fibrinolysis and inflammation markers are associated with disease
activity in patients with chronic urticaria. Allergy.
2010;65:649-56. It is possible that, a synergy between the action of
autoantibodies and the coagulation cascade exists in some patients with chronic
urticaria.3737 Cugno M, Marzano AV, Asero R, Tedeschi A. Activation of blood
coagulation in chronic urticaria: pathophysiological and clinical implications.
Intern Emerg Med. 2010;5:97-101.
An article by the "European Academy of Allergy and Clinical Immunology" was published in 2013, defining and proposing criteria for the diagnosis of autoimmune urticaria.4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36. The following criteria were proposed as the gold standard for autoimmune chronic urticaria:4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36. (a) presence of a positive in vitro biological test to demonstrate the autoantibodies' functionality (basophil histamine release test or the expression of a basophil activation marker such as CD63 or better still, CD203c during flow cytometry) AND (b) positive autoreactivity (positive ASST) in order to demonstrate the in vivo relevance of mast cell degranulation and the increase in capillary permeability, AND (c) a positive immunological assay for autoantibodies against FcεRIα receptors (Western blot or ELISA), to demonstrate the autoantibodies' specificity.
In daily practice, however, most physicians have only the autologous serum skin test available and its positivity in a patient with CU can only suggest "autoreactivity". Unfortunately, we do not have the other tests indicated for the diagnosis of autoimmune CU in our country.
Chronic urticaria and allergic etiology
The link between CU, IgE sensitization, aeroallergens and allergy has been much discussed but seldom studied.4141 Augey F, Gunera-Saad N, Bensaid B, Nosbaum A, Berard F, Nicolas JF. Chronic spontaneous urticaria is not an allergic disease. Eur J Dermatol. 2011;21:349-53.
Between 2006 and 2008, in France, Augeyet al4141 Augey F, Gunera-Saad N, Bensaid B, Nosbaum A, Berard F, Nicolas JF. Chronic spontaneous urticaria is not an allergic disease. Eur J Dermatol. 2011;21:349-53. studied 128 adult patients with CU under the aspects of IgE sensitization and allergy. These authors considered CU an allergic disease if: i) there was a high correlation of positive prick tests (skin puncture tests) to an allergen that was clinically relevant in the patient's medical history, and ii) a complete remission of urticaria occurred within the first two months after the allergen was removed. Among 105 patients with interpretable puncture tests, 46.7% were sensitized by IgE. Two patients had clinically relevant puncture tests, however their CU had many other triggering factors and there was no remission after withdrawal from allergen exposure. The authors concluded that the rate of IgE sensitization is higher among patients with CU, compared to the general adult population.4141 Augey F, Gunera-Saad N, Bensaid B, Nosbaum A, Berard F, Nicolas JF. Chronic spontaneous urticaria is not an allergic disease. Eur J Dermatol. 2011;21:349-53. However, these CU cases cannot be considered as an expression of IgE-mediated allergies, but as a chronic inflammatory disease that is more common in IgE sensitized subjects and induced by many factors, amongst which IgE-mediated allergy is the least frequent one.4141 Augey F, Gunera-Saad N, Bensaid B, Nosbaum A, Berard F, Nicolas JF. Chronic spontaneous urticaria is not an allergic disease. Eur J Dermatol. 2011;21:349-53.
The relevance of allergic sensitization to dust mites in the etiology and treatment of CU is still unknown. In Turkey, Caliskaner et al4242 Caliskaner Z, Ozturk S, Turan M, Karaayvaz M.Skin Test positivity to aeroallergens in the patients with chronic urticaria without respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50-4. studied 259 patients with chronic urticaria and angioedema, without respiratory allergic diseases (rhinitis and/or asthma) and compared their results with 300 healthy controls and 300 atopic patients. Immediate cutaneous reactivity to one or more allergens was detected in 71 cases of CU and angioedema (27.4%). The most commonly detected allergen was residential house dust (24.7%).4242 Caliskaner Z, Ozturk S, Turan M, Karaayvaz M.Skin Test positivity to aeroallergens in the patients with chronic urticaria without respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50-4. Positive prick tests (skin puncture tests) were correlated to other aeroallergens, including pollen, mold and cockroaches in 7.7%, 0.4% and 0.8% of patients respectively. In the healthy control group, 7% of cases were considered atopic regarding the prick test results.4242 Caliskaner Z, Ozturk S, Turan M, Karaayvaz M.Skin Test positivity to aeroallergens in the patients with chronic urticaria without respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50-4. Pollen (6%) and house dust (4.7%) were the most commonly found allergens in the healthy control group. In the atopic control group, pollen and dust mites were the allergens most commonly detected in the prick test (62% and 50.3%, respectively).4242 Caliskaner Z, Ozturk S, Turan M, Karaayvaz M.Skin Test positivity to aeroallergens in the patients with chronic urticaria without respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50-4. The difference between patients with CU and the control group was statistically significant, regarding the presence of atopy and sensitivity to mites (p <0.001).4242 Caliskaner Z, Ozturk S, Turan M, Karaayvaz M.Skin Test positivity to aeroallergens in the patients with chronic urticaria without respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50-4. The proportion of skin test that were positive to house dust was higher in the CU group than among healthy cases, but not as high as in atopic patients.4242 Caliskaner Z, Ozturk S, Turan M, Karaayvaz M.Skin Test positivity to aeroallergens in the patients with chronic urticaria without respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50-4. Furthermore, the rate of cutaneous reactivity to other aeroallergens did not differ from the healthy group. The authors concluded that urticaria, as an isolated clinical manifestation in patients who are sensitive to dust, was not common in this study.
Therefore, it is well established that CU is not included in the indications for immunotherapy with allergy desensitization.4242 Caliskaner Z, Ozturk S, Turan M, Karaayvaz M.Skin Test positivity to aeroallergens in the patients with chronic urticaria without respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50-4. So, in our opinion, the systematic practice of skin prick test reading should not be routinely performed during the investigation of CU, unless there is a definite correlation between episodes of CU worsening and data from the patient's medical history.
Definition, classification and course of the disease
Urticaria is characterized by the sudden appearance of wheals, which may be accompanied by angioedema.4343 Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-91.,4444 Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777-80. Superficial dermal edema is called wheals or hives, while edema in the deep dermis, hypodermis and gastrointestinal tract is known as angioedema.4444 Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777-80.,4545 Grattan CEH. The urticaria spectrum: recognition of clinical patterns can help management. Clin Exp Dermatol. 2004;29:217-21. Diagnosis is mainly clinical.11 Cousin F, Philips K, Favier B, Bienvenu J, Nicolas JF. Drug-induced urticaria. Eur J Dermatol. 2001;11:181-7.
Wheals have three typical features: (i) central edema of variable size, almost always surrounded by a reflex erythema, (ii) association with pruritus and sometimes a burning sensation, (iii) ephemeral nature, with the skin returning to its regular appearance in usually 1 to 24 hours. Angioedema is characterized by: (i) a sudden, pronounced edema of the deep dermis and subcutaneous tissue: (i) pain more often than pruritus (iii) frequent involvement of mucous membranes, and (iv) resolution of symptoms in about 72 hours, more protracted than in the case of wheals.11 Cousin F, Philips K, Favier B, Bienvenu J, Nicolas JF. Drug-induced urticaria. Eur J Dermatol. 2001;11:181-7.
It is well known that, the spectrum of clinical manifestations of the different subtypes of urticaria is very broad. Moreover, two or more subtypes of urticaria can coexist in the same patient. During the "4th International Consensus Meeting on Urticaria 2012", the adoption of a clinical classification (shown in chart 1) was proposed.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.
Undoubtedly, this classification is not perfect since there are still some inconsistencies, e.g., physical urticaria also has a chronic nature.4242 Caliskaner Z, Ozturk S, Turan M, Karaayvaz M.Skin Test positivity to aeroallergens in the patients with chronic urticaria without respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50-4. However, physical urticarias are grouped as a result of the special nature of their causal factors, while in acute and chronic urticarias, lesions appear spontaneously without external physical stimuli.33 Hizal M, Tuzun B, Wolf R, Tuzun Y. The relationship between Helicobacter pylori IgG antibody and autologous serum test in chronic urticaria. Int J Dermatol. 2000;39:443-5.
In 2001, Kozelet al4343 Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-91. published a study conducted in the Netherlands, with 220 adults diagnosed with urticaria. Of these, 72 cases (33.2%) had physical urticaria, 24 (10.9%) had a combination of physical urticaria and idiopathic CU, 78 (36%) had idiopathic CU, 20 cases (9.0%) were induced by drugs, 15 (6.8%) by food, four (1.8%) by infections, three (1.4%) by internal diseases, and two (0.9%) were urticaria due to contact. Causality was established in 53.1% of the cases. Thirty-five percent of the cases were free of symptoms after one year and in 28.9% there was an improvement of symptoms. Spontaneous remission occurred in 47.4% of patients in whom no causal agent was identified and only in 16.4% of cases with physical urticaria. In this study, patients with physical urticaria had the worst prognosis regarding disease duration, as 84% of them still had symptoms after one year.4343 Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-91. Another important factor in urticaria is to classify the intensity and activity of the disease. Młyneket al4444 Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777-80. proposed a classification, later validated in 2008, which was ease to adhere to by both physicians and patients in their daily activities (Chart 2).44 Federman DG, Kirsner RS, Moriarty JP, Concato J. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. J Am Acad Dermatol. 2003;49:861-4. Due to the variable intensity pattern of urticaria along the day, sequential dermatological inspections (visualization of the skin) should be made periodically (Urticaria Activity Score - UAS 0-6 points) to increase the accuracy of the score; the sum of points scored over seven days (UAS 7, 0-42 points) is currently being used in clinical studies.4444 Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777-80. In general, larger wheals indicate outbreaks that are more intense and more difficult to treat.4444 Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777-80.
DIAGNOSIS
Spontaneous UC is a disease that causes serious impact on patients and high direct and indirect costs to the health system, as well as extensive socioeconomic implications, since it delays by about 20-30% the return of productive individuals to the work force.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87. Due to the wide heterogeneity of this group of diseases called urticaria, routine investigation should include a good history, a good physical exam, quests for information on possible causal factors and important data on the nature of urticaria.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87. The next step is laboratory testing to exclude significant systemic diseases, provided they are warranted by clinical history and physical examination.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.
Skin biopsy of wheals must be performed if there is suspicion of vasculitis or urticaria vasculitis, which usually persists for more than 24 hours in the same location and can leave hyperchromic or purplish residual lesions, although that does not always occur.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.,4545 Grattan CEH. The urticaria spectrum: recognition of clinical patterns can help management. Clin Exp Dermatol. 2004;29:217-21. Furthermore, burning symptoms isolated or coupled with pruritus may be reported by these patients. Skin biopsy of wheals is also indicated in cases that are refractory to treatment with antihistamines.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.,4545 Grattan CEH. The urticaria spectrum: recognition of clinical patterns can help management. Clin Exp Dermatol. 2004;29:217-21.
Considerations on autologous serum skin test (ASST)
In 1946, Malmros published the first report on the likelihood of autologous serum (from patients with urticaria) triggering positive skin tests, although this hypothesis was disregarded for decades.4646 Malmros H. Autoserumtest. Nordisk Med 1946;29:150-1.
The autologous serum skin test (ASST) is an "in vivo" test that measures the autoreactivity of an individual.4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36.,4747 Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006;154:813-9. This autoreaction is characterized by the formation of wheals and pruritus in response to an intradermal injection of autologous serum (obtained from the patient during the clinical activity of urticaria, or crisis), which acts indirectly through the release of mediators from mast cells/other cells or directly by acting on the skin's microvasculature.4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36.,4747 Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006;154:813-9. It should be stressed that, autoreactivity does not define nor does it imply in the presence of autoimmune urticaria, but it may be an indication of mast cell activation by the autoantibodies present in the autologous serum of patients with CU and positive ASST.4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36.,4747 Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006;154:813-9.
The frequency of positive ASST in adults with CU ranges from 4.1% to 76.5% depending on different criteria for positivity, including confirmation by histamine release test (HRT).4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36. The discrepancy in results can be attributed to patient selection bias, severity of illness, methodology and interpretation of the results or even the actual prevalence of autoimmune urticaria in the population tested.4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36. The frequency of positive ASST is 45.5% (95%CI, 24.7-74.4%) when establishing a 1.5 mm difference in diameter between the response obtained with cases tested with saline (negative control) and 43.5% (95%CI, 34.8-62.1%) when the difference is 2 mm.4747 Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006;154:813-9. A positive ASST result on a CU case could mean an "auto-reactive CU".4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36.
Current recommendations to establish the methodological standardization for ASST are summarized in Figure 1.4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36. In order to perform an ASST, it is necessary to take the patient off any medication with antihistamine activity for a variable period of time before testing, as depicted on chart 3.4040 Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36.
Suppression of urticated response after prick test with histamine by different medications with anti-H1 activity
Considerations on the autologous plasma skin test (APST)
The autologous serum skin test is based on intradermal injection of autologous serum, and merely represents a diagnostic procedure used in autoimmune CU.4848 Yıldız H, Karabudak O, Doğan B, Harmanyeri Y. Evaluation of autologous plasma skin test in patients with chronic idiopathic urticaria. Br J Dermatol. 2011;165:1205-9.,4949 Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes whealand- flare reaction much more frequently than frequently than autologous serum. J Allergy Clin Immunol. 2006;117:1113-7. The plasma used in the APST also contains coagulation factors and sodium citrate. Therefore, the positivity occurring in the APST may be due both to autoantibodies that induce thrombin activity and/or to the sodium citrate.4848 Yıldız H, Karabudak O, Doğan B, Harmanyeri Y. Evaluation of autologous plasma skin test in patients with chronic idiopathic urticaria. Br J Dermatol. 2011;165:1205-9.
In a study with 96 patients with CU, Asero et al4949 Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes whealand- flare reaction much more frequently than frequently than autologous serum. J Allergy Clin Immunol. 2006;117:1113-7. found that 51 of 96 patients (53%) were ASST-positive, and 61 of 71 (86%) were APST-positive. Asero's group in Italy advocates that, the autologous plasma skin test increases the sensitivity of diagnosis in cases of autoreactive CU, since it indicates the activation of the coagulation cascade in the presence of autoantibodies. These autoantibodies, when degranulating mast cells and basophils, activate eosinophils, which in turn release tissue factor thus stimulating the coagulation cascade and compensatory fibrinolysis. The latter will generate thrombin, which will induce the activation of more mast cells and the vascular endothelium; also ddimers are detected at higher levels as a final result of fibrinolysis.
In another multicentre study,5050 Metz M, Gimenez-Arnau A, Borzova E, Grattan CE, Magerl M, Maurer M. Frequency and clinical implications of skin autoreactivity to serum versus plasma in patients with chronic urticaria. J Allergy Clin Immunol. 2009;123:705-6. the positivity rate was 37.5% for ASST and 43% for APST among 200 patients with CU (146 women and 54 men).
Yildizet al.5151 Yıldız H, Karabudak O, Doğan B, HarmanyeriY.Evaluation of autologous plasma skin test in patients with chronic idiopathic urticaria.Br J Dermatol. 2011;165:1205-9. studied 42 patients (19 males, 23 females, mean age: 35.7, range: 28-76 years) and 35 healthy volunteers (19 males, 16 females, mean age: 30.28, range: 20-80 years). The authors performed APST, ASST, negative control with saline and sodium citrate and positive control with histamine. In terms of positivity, no statistically significant difference between APST and ASST was found.5151 Yıldız H, Karabudak O, Doğan B, HarmanyeriY.Evaluation of autologous plasma skin test in patients with chronic idiopathic urticaria.Br J Dermatol. 2011;165:1205-9. Therefore, the authors concluded that APST to assess autoreactivity in clinical practice was not superior to ASST and further studies should be conducted to corroborate these findings.
TREATMENT
The treatment of urticaria includes pharmacological and non-pharmacological interventions.5252 Criado PR, Criado RFJ, Maruta CW, Martins JEC, Rivitti EA. Urticaria. An Bras Dermatol 2005;80;613-30.,5353 Criado PR, Criado RFJ, Maruta CW, Machado CDA. Histamine, histamine receptors and antihistamines: new concepts. An Bras Dermatol. 2010;85:195-210. Non-pharmacological interventions for physical urticaria are limited to reduction of stress levels, and sun exposure and diminished alcohol intake. There is little evidence that reducing drug-intake and the exposure to pseudo-allergens would improve urticaria in these patients, except in cases of spontaneous CU, which is aggravated by nonsteroidal anti-inflammatory drugs (especially aspirin) in 25-30% of patients with urticaria.5252 Criado PR, Criado RFJ, Maruta CW, Martins JEC, Rivitti EA. Urticaria. An Bras Dermatol 2005;80;613-30. Cooling lotions, such as 1% menthol can be soothing to many patients with very active urticaria.5252 Criado PR, Criado RFJ, Maruta CW, Martins JEC, Rivitti EA. Urticaria. An Bras Dermatol 2005;80;613-30.
In our opinion, it is important in the treatment of chronic urticaria to make the patient understand the need for continuous, and not intermittent treatment, in order to achieve the proper control of the disease; also, to explain that this is a chronic condition that requires uninterrupted medication similarly to hypothyroidism or diabetes mellitus, although unlike these two diseases, urticaria tends to go into remission over time.
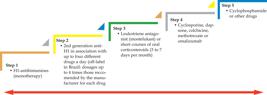
Treatment of wheals outbreaks is sequential or made in steps, but its main cornerstone
is the use of antihistamines (Figure 2).5252 Criado PR, Criado RFJ, Maruta CW, Martins JEC, Rivitti EA. Urticaria. An
Bras Dermatol 2005;80;613-30. Therefore, H1 antihistamines (anti-H1) are
crucial in the treatment of urticaria.5252 Criado PR, Criado RFJ, Maruta CW, Martins JEC, Rivitti EA. Urticaria. An
Bras Dermatol 2005;80;613-30.,5353 Criado PR, Criado RFJ, Maruta CW, Machado CDA. Histamine, histamine
receptors and antihistamines: new concepts. An Bras Dermatol.
2010;85:195-210. Nevertheless, some CU
cases may present a marked inflammatory cell infiltration, which can be somewhat
refractory to anti-H1 and respond satisfactorily to oral corticosteroids or second-line
agents (montelukast, dapsone or colchicine).5252 Criado PR, Criado RFJ, Maruta CW, Martins JEC, Rivitti EA. Urticaria. An
Bras Dermatol 2005;80;613-30.
53 Criado PR, Criado RFJ, Maruta CW, Machado CDA. Histamine, histamine
receptors and antihistamines: new concepts. An Bras Dermatol.
2010;85:195-210.-5454 Pires JS, de Ue APF, Furlani EJ, de Souza PK, Rotta O. Dapsone as an
alternative to the treatment of chronic urticaria non-responsive to antihistamines.
An Bras Dermatol. 2008;83:413-8.
Criado et al5555 Criado RF, Criado PR, Martins JE, Valente NY, Michalany NS, Vasconcellos C. Urticaria unresponsive to antihistaminic treatment: an open study of therapeutic options based on histopathologic features. J Dermatolog Treat. 2008;19:92-6. studied 22 CU patients unresponsive to conventional antihistamine treatment. The patients had uncontrolled urticaria even with multiple combinations of antihistamines at maximum doses and corticosteroids for short cycles (prednisone 20-40 mg, p.o, once a day, 3-7 days per month). Cutaneous biopsies of the wheals were performed. The findings were classified as: (i) dermal perivascular mixed inflammatory infiltrate comprised of lymphocytes, monocytes and neutrophils and/or eosinophils; (ii) inflammatory infiltrate formed mainly by neutrophils; and (iii) inflammatory infiltrate composed mainly of eosinophils. According to histopathologic results, patients were subjected to one of the following regimens: Class A - antihistamine associated with dapsone; Class B - colchicine or dapsone; Class C - montelukast. Four patients in class A, eight in class B and seven in class C showed complete remission of urticaria after 12 weeks of treatment; one patient of class B and two in class C did not respond to therapy. Two years after treatment discontinuation, 16 patients remained urticaria-free. The authors concluded that, dapsone or colchicine might be effective adjuvant drugs in the presence of intense neutrophilic infiltrate, as could montelukast in cases with eosinophilic infiltrate.
H1-antihistamines are used in the treatment of urticaria since 1950.5656 Jauregui I, Ferrer M, Montoro J, Davila I, Bartra J, del Cuvillo A, et al. Antihistamines in the treatment of chronic urticaria. J Investig Allergol Clin Immunol. 2007;17:41-52. Although first-generation drugs have a relatively lower cost than second-generation ones, the former have pronounced anticholinergic effects, with sedative action on the central nervous system (CNS) that lasts 12 hours, while their anti-pruritic effects last between 4 to 6 hours.5656 Jauregui I, Ferrer M, Montoro J, Davila I, Bartra J, del Cuvillo A, et al. Antihistamines in the treatment of chronic urticaria. J Investig Allergol Clin Immunol. 2007;17:41-52. As a consequence, there have been many reports on interactions between first-generation anti-H1 drugs, alcohol and medications (analgesics, hypnotics, sedatives and antidepressant drugs), causing unwanted effects in the CNS.5656 Jauregui I, Ferrer M, Montoro J, Davila I, Bartra J, del Cuvillo A, et al. Antihistamines in the treatment of chronic urticaria. J Investig Allergol Clin Immunol. 2007;17:41-52.,5757 Kalivas J, Breneman D, Tharp M, Bruce S, Bigby M. Urticaria: clinical efficacy of cetirizine in comparison with hydroxyzine and placebo. J Allergy ClinImmunol. 1990;86:1014-8. In addition, monoamine oxidase inhibitors may prolong and intensify the anticholinergic effects of first generation H1-antihistamines.5454 Pires JS, de Ue APF, Furlani EJ, de Souza PK, Rotta O. Dapsone as an alternative to the treatment of chronic urticaria non-responsive to antihistamines. An Bras Dermatol. 2008;83:413-8.
The most used first-generation, anti-H1 drugs to treat CU belong to the groups of ethanolamines (diphenhydramine, clemastine), piperazines (hydroxyzine, dexchlorpheniramine) and piperidines (cyproheptadine and ketotifen).5656 Jauregui I, Ferrer M, Montoro J, Davila I, Bartra J, del Cuvillo A, et al. Antihistamines in the treatment of chronic urticaria. J Investig Allergol Clin Immunol. 2007;17:41-52. Some randomized studies compared the effects of cetirizine versus hydroxyzine and loratadine in the treatment of CU and demonstrated similar clinical efficacy but superior safety profile for cetirizine in comparison to hydroxyzine. The main differences between first and second-generation anti-H1 are listed in chart 4.5757 Kalivas J, Breneman D, Tharp M, Bruce S, Bigby M. Urticaria: clinical efficacy of cetirizine in comparison with hydroxyzine and placebo. J Allergy ClinImmunol. 1990;86:1014-8.,5858 Zuberbier T, Bindslev-Jensen C, Canonica W, Grattan CE, Greaves MW, Henz BM, et al. EAACI/GA2LEN/EDF. EAACI/GA2LEN/EDF guideline: management of urticaria. Allergy. 2006;61:321-31.
Second-generation H1-antihistamines are the only drugs for the treatment of chronic urticaria, which are supported by high levels of scientific evidence, from the perspective of evidence-based medicine, as there are prospective, randomized and double-blinded controlled studies published.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.,5757 Kalivas J, Breneman D, Tharp M, Bruce S, Bigby M. Urticaria: clinical efficacy of cetirizine in comparison with hydroxyzine and placebo. J Allergy ClinImmunol. 1990;86:1014-8. Therefore, these medications are indicated as first-line treatment for symptomatic CU.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87. Second-generation antihistamines provide moderate-to-good control for 44-91% of all types of urticaria and 55% of patients with CU.5959 Asero R. Chronic unremitting urticaria: is the use of antihistamines above the licensed dose effective? A preliminary study of cetirizine at licensed and above-licensed doses. Clin Exp Dermatol. 2007;32:34-8. In general, all H1-antihistamines are effective in reducing pruritus in urticaria, although less often affecting the number and size of wheals.6060 Black AK, Greaves MW. Antihistamines in urticaria and angioedema. Clin Allergy Immunol. 2002;17:249-86. So, there is a sizable group of patients with CU for whom H1antihistamines in doses usually recommended on label are not able to control disease symptoms.
Some authors propose that, in young adults without associated disease, the doses of second generation anti-H1 should be increased up to four times those recommended by manufacturers on label, before substituting the drug or adding another medication in the treatment of CU (not approved by the National Health Surveillance Agency - ANVISA in Brazil).22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.,5353 Criado PR, Criado RFJ, Maruta CW, Machado CDA. Histamine, histamine receptors and antihistamines: new concepts. An Bras Dermatol. 2010;85:195-210.,5353 Criado PR, Criado RFJ, Maruta CW, Machado CDA. Histamine, histamine receptors and antihistamines: new concepts. An Bras Dermatol. 2010;85:195-210.,6161 Kozel MM, Sabroe RA. Chronic urticaria: aetiology, management and current and future treatment options. Drugs. 2004;64:2515-36.
A study using cetirizine in 22 patients with CU confronted these recommendations, seen as no improvement was observed after two weeks of treatment with 30 mg of cetirizine, though this may be due to the limited observation period during the study.5959 Asero R. Chronic unremitting urticaria: is the use of antihistamines above the licensed dose effective? A preliminary study of cetirizine at licensed and above-licensed doses. Clin Exp Dermatol. 2007;32:34-8.
Weller et al6262 Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H1- Antihistamine Up-Dosing in Chronic Spontaneous Urticaria: Patients' Perspective of Effectiveness and Side Effects - A Retrospective Survey Study. PLoS One. 2011;6:e23931. conducted a retrospective questionnaire survey on 319 patients diagnosed with spontaneous CU. The aim of this study was to establish the patient's perception on the effectiveness and adverse events of treatment with H1-antihistamines, both in standard and higher doses. Of the total population, 121 patients received questionnaires from their doctors or hospitals and 198 were informed about the research over the Internet, at the Allergie-Centrum-Charitè home-page or at the Urticaria Network (www.urtikaria.net) webpage. The latter group completed the questionnaire online.6262 Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H1- Antihistamine Up-Dosing in Chronic Spontaneous Urticaria: Patients' Perspective of Effectiveness and Side Effects - A Retrospective Survey Study. PLoS One. 2011;6:e23931. Both questionnaires were identical. All surveys were completed anonymously and no Internet Protocol (IP) was retrieved. The only pre-requisite for participation was that subjects had spontaneous CU (spontaneous wheals recurring for more than six weeks) and that they were adults, over 18 years of age. Participants agreed that the usual doses of second-generation antihistamines (recommended by the manufacturer) were significantly more effective than those of the first-generation drugs (p <0.005).6262 Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H1- Antihistamine Up-Dosing in Chronic Spontaneous Urticaria: Patients' Perspective of Effectiveness and Side Effects - A Retrospective Survey Study. PLoS One. 2011;6:e23931. Furthermore, they found that second-generation drugs caused significantly fewer side effects (p <0.001) and less sedation than first-generation antihistamine medications (p <0.001).6262 Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H1- Antihistamine Up-Dosing in Chronic Spontaneous Urticaria: Patients' Perspective of Effectiveness and Side Effects - A Retrospective Survey Study. PLoS One. 2011;6:e23931. Three quarters of the patients reported that they had increased the doses of second-generation antihistamines, and 40%, 42% and 54% of the subjects reported significantly higher benefits by taking 2, 3 or 4 tablets per day, respectively.6262 Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H1- Antihistamine Up-Dosing in Chronic Spontaneous Urticaria: Patients' Perspective of Effectiveness and Side Effects - A Retrospective Survey Study. PLoS One. 2011;6:e23931. The number of reports on adverse events and sedation with higher doses was not significantly different from those reported with usual doses.6262 Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H1- Antihistamine Up-Dosing in Chronic Spontaneous Urticaria: Patients' Perspective of Effectiveness and Side Effects - A Retrospective Survey Study. PLoS One. 2011;6:e23931.
In a literature review study, Sánchez-Borges et al6363 Sanchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A. Treatment of recalcitrant chronic urticaria with nonsedating antihistamines: is there evidence for updosing? J Investig Allergol Clin Immunol. 2013;23:141-4. concluded that the use of higher second-generation anti-H1 doses increased the proportion of patients who achieved the control of urticaria symptoms, without producing higher rates of adverse events, including somnolence.6363 Sanchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A. Treatment of recalcitrant chronic urticaria with nonsedating antihistamines: is there evidence for updosing? J Investig Allergol Clin Immunol. 2013;23:141-4.
Despite the small number of studies, the best results seem to be those obtained with cetirizine, levocetirizine and desloratadine, and regarding dosage increase, there were mixed results with rupatadine and one study with fexofenadine failed to show greater improvement with higher doses.6363 Sanchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A. Treatment of recalcitrant chronic urticaria with nonsedating antihistamines: is there evidence for updosing? J Investig Allergol Clin Immunol. 2013;23:141-4.
Overall, studies comparing various second-generation anti-H1 drugs in the treatment of CU, observed no significant difference regarding symptom control, quality-of-life and safety profile, and all of them are designated as first-line agents in the treatment of CU.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.,5858 Zuberbier T, Bindslev-Jensen C, Canonica W, Grattan CE, Greaves MW, Henz BM, et al. EAACI/GA2LEN/EDF. EAACI/GA2LEN/EDF guideline: management of urticaria. Allergy. 2006;61:321-31. Chart 5 summarizes the recommendations for the treatment of urticaria, according to the "4th Consensus Meeting, Urticaria 2012" held in Berlin. An ample variety of H1-antihistamines available on the market, their dosage and recommended doses can be seen on chart 6.
Management of urticaria: recommendation guide proposed by the "4th International Meeting on Urticaria Consensus" in 2012
A recent review performed by Fedorowiczet al6464 Fedorowicz Z, van Zuuren EJ, Hu N. Histamine H2-receptor antagonists for urticaria. Cochrane Database Syst Rev. 2012;3:CD008596. for the "Cochrane Skin Group Specialized Register" concluded that the presence of few studies and small case series in the literature regarding the use of antiH2 (cimetidine and ranitidine) in the treatment of urticaria, preclude any conclusion on their effectiveness in treating the disease.
Other pharmacological interventions in chronic urticaria (third-line agents)
While anti-histaminic treatments with high-dose second-generation anti-H1 (up to four times the recommended doses) can control the symptoms in most patients, alternative treatments may be required for the remaining unresponsive population.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87. Since the intensity of chronic urticaria fluctuates, and spontaneous remission may occur in 50% of patients within 6 months of diagnosis, it is advisable to re-evaluate the need for continued treatment or alternative therapies every 3 to six months, although in our opinion, patients should be assessed at interval periods no longer than two months.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87. There are numerous studies on alternative drugs for the treatment of chronic urticaria, either in combination with H1-antihistamines or as monotherapy, however with low levels of scientific evidence.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87. Examples for this strategy include: ketotifen, montelukast, warfarin, nifedipine, tranexamic acid, colchicine, dapsone, sulfasalazine, methotrexate, plasmapheresis, intravenous immunoglobulin, hydroxychloroquine, biological agents, danazol / stanozolol and cyclophosphamide, among others.44 Federman DG, Kirsner RS, Moriarty JP, Concato J. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. J Am Acad Dermatol. 2003;49:861-4. Even with low levels of scientific evidence, many of these drugs are used in antihistamine-resistant patients with chronic urticaria, therefore we will discuss them further along.
Cyclosporine
Most studies on cyclosporine used mg/kg/day doses for periods ranging from 8-16 weeks
with good results, varying from 64% to 95% approximately. Some studies used smaller
doses (2 to 5 mg/kg/day).6565 Grattan CE, O'Donnell BF, Francis DM, Niimi N, Barlow RJ, Seed PT, et
al. Randomized double-blind study of cyclosporine in chronic 'idiopathic' urticaria.
Br J Dermatol. 2000;143:365-72.
66 Hollander SM, Joo SS, Wedner HJ. Factors that predict the success of
cyclosporine treatment for chronic urticaria. Ann Allergy Asthma Immunol.
2011;107:523-8.
67 Di Leo E, Nettis E, Aloia AM, Moschetta M, Carbonara M, Dammacco F, et
al. Cyclosporin-A efficacy in chronic idiopathic urticaria. Int J Immunopathol
Pharmacol. 2011;24:195-200.
68 Vena GA, Cassano N, Colombo D, Peruzzi E, Pigatto P; Neo-I-30 Study
Group.Neo-I-30 Study Group. Cyclosporine in chronic idiopathic urticaria: a
double-blind, randomized, placebo-controlled trial. J Am Acad Dermatol.
2006;55:705-9.-6969 Hollander SM, Joo SS, Wedner HJ. Factors that predict the success of
cyclosporine treatment for chronic urticaria. Ann Allergy Asthma Immunol.
2011;107:523-8. At these doses, side effects occur less
frequently than at higher ones. In these cases, particularly, there have been reports
on infections, hypertension, nephrotoxicity and increased malignancy.7070 Neverman L, Weinberger M. Treatment of chronic urticaria in children
with antihistamines and cyclosporine. J Allergy Clin Immunol Pract.
2014;2:434-8.,7171 Kessel A, Toubi E. Cyclosporine-A in severe chronic urticaria: the
option for longterm therapy. Allergy. 2010;65:1478-82. Other less severe side effects include hirsutism, headache, nausea,
paresthesias, abdominal pain and hypertension. For this reason, it is necessary to
monitor blood pressure, renal function and cyclosporine levels and even glycemia and
lipidemia.7070 Neverman L, Weinberger M. Treatment of chronic urticaria in children
with antihistamines and cyclosporine. J Allergy Clin Immunol Pract.
2014;2:434-8.,7171 Kessel A, Toubi E. Cyclosporine-A in severe chronic urticaria: the
option for longterm therapy. Allergy. 2010;65:1478-82.
Methotrexate
This drug has anti-inflammatory and immunosuppressive activities, although much of its mechanisms of action are unknown. It is known that, methotrexate increases adenosine levels, inducing the apoptosis of CD4 lymphocytes and inhibiting neutrophil chemotaxis. It has been used in doses of 10 to 15 mg per week in cases that were resistant to antihistamines and systemic corticosteroids, with results achieved after 3 to 6 weeks of treatment.7272 Sagi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91:303-6.,7373 Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-4. It is considered as a corticosteroid-sparing alternative. Gastrointestinal side effects, stomatitis, headache, fatigue, and hematological alterations may occur at low doses.7272 Sagi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91:303-6.,7373 Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-4. Since there is the risk of hepatotoxicity and myelosuppression, laboratory surveillance is mandatory, especially of hepatic function.7272 Sagi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91:303-6.,7373 Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-4.
Cyclophosphamide
Prescribed usually in cases of extreme treatment resistance, it has been used orally at 100 mg doses (equivalent to 1.5 mg/kg for 5 days, every week) associated with 600 mg/day acetylcysteine to reduce bladder toxicity, with good results.7474 Asero R. Oral cyclophosphamide in a case of cyclosporine and steroid-resistant chronic urticaria showing autoreactivity on autologous serum skin testing. Clin Exp Dermatol. 2005;30:582-3. It should be used sparingly, due to its many potential adverse events.7474 Asero R. Oral cyclophosphamide in a case of cyclosporine and steroid-resistant chronic urticaria showing autoreactivity on autologous serum skin testing. Clin Exp Dermatol. 2005;30:582-3.
Biologic agents
a. Intravenous immunoglobulin
It has immunomodulatory activities, including complement and cell adhesion
modulation and acts at the level of cytokines and autoantibodies. This drug has
been used with good results in CU, at a dose of 0.4 mg/kg/day for 5 consecutive
days, or in other schemes, both intravenously and subcutaneously.7575 O'Donnell BF, Barr RM, Black AK, Francis DM, Kermani F, Niimi N, et al.
Intravenous immunoglobulin in autoimmune chronic urticaria. Br J Dermatol.
1998;138:101-6.
76 Pereira C, Tavares B, Carrapatoso I, Loureiro G, Faria E, Machado D, et
al. Lowdose intravenous gammaglobulin in the treatment of severe autoimmune
urticaria. Eur Ann Allergy Clin Immunol. 2007;39:237-42.-7777 Mitzel-Kaoukhov H, Staubach P, Muller-BrenneT. Effect of high-dose
intravenous immunoglobulin treatment in therapy-resistant chronic spontaneous
urticaria. Ann Allergy Asthma Immunol. 2010;104:253-8. Anaphylactoid reactions, aseptic meningitis and renal failure are
reported as rare adverse events and despite being relatively safe, this is a very
high-cost treatment.
b. Omalizumab
This is an anti-IgE monoclonal antibody approved for the treatment of moderate to severe asthma. It decreases free IgE and inhibits the expression of high affinity IgE receptors on mast cells and basophils. Applied subcutaneously, it was one of the first biopharmaceuticals on the market indicated to treat severe allergic asthma. When treating CU, one must take in consideration the presence of IgE autoantibodies.7878 Maurer M, Altrichter S, Bieber T, Biedermann T, Brautigam M, Seyfried S, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202-209.e5.,7979 Khan DA. Alternative Agents in Refractory Chronic Urticaria: Evidence and Considerations on Their Selection and Use. J Allergy Clin Immunol Pract. 2013;1:433-440.e1. Good results have been reported in idiopathic and cholinergic CU, urticaria secondary to sun or cold exposure, dermographism and angioedema.7878 Maurer M, Altrichter S, Bieber T, Biedermann T, Brautigam M, Seyfried S, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202-209.e5.,7979 Khan DA. Alternative Agents in Refractory Chronic Urticaria: Evidence and Considerations on Their Selection and Use. J Allergy Clin Immunol Pract. 2013;1:433-440.e1.
A randomized multicenter, double-blinded study evaluated 90 patients with chronic spontaneous urticaria unresponsive to antihistamines, aged 12-75 years, and good control of the disease was achieved with one dose of omalizumab.8080 Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy ClinImmunol. 2011;128:567-73.e1. The improvement obtained with the medication versus placebo was statistically significant at 300 and 600 mg doses, but not at 75 mg, complete resolution of urticaria was achieved in the first two weeks in 36% of patients in the 300mg group, 28.6% in the 600 mg and 4.4% in the 75 mg and 0% in the placebo group.8080 Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy ClinImmunol. 2011;128:567-73.e1.
Recently, a randomized multicenter study with 323 patients treated every 4 weeks observed, at week 16, a 44% improvement in the 300mg group, 22% at 150mg, 16% in the 75mg group and 5% in the placebo group.8181 Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924-35.
Therefore, the use of omalizumab at 300 mg or 600 mg doses seems to be a rapid and effective treatment for patients with CU who are persistently symptomatic despite the use of anti-H1 medications.8181 Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924-35.,8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16.
c. Anti-CD20 drugs (Rituximab and other)
It is a chimeric monoclonal antibody agai D20 protein, which is expressed mainly
on B cells (lymphocytes).8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol
Allergy Clin North Am. 2014;34:105-16. Rituximab
destroys B-lymphocytes and decreases the production of autoantibodies. In three
case reports, the drug was effective in two patients with H1-antihistamine
resistant CU and in another case it was ineffective.8383 Chakravarty SD, Yee AF, Paget SA. Rituximab successfully treats
refractory chronic autoimmune urticaria caused by IgE receptor autoantibodies. J
Allergy ClinImmunol. 2011;128:1354-5.
84 Arkwright PD.Anti-CD20 or anti-IgE therapy for severe chronic autoimmune
urticaria. J Allergy Clin Immunol. 2009;123:510-1.-8585 Mallipeddi R, Grattan CE. Lack of response of severe steroid-dependent
chronic urticaria torituximab. Clin Exp Dermatol. 2007;32:333-4.
d. Anti-TNF-alpha agents
Wilson et al8686 Wilson LH, Eliason MJ, Leiferman KM, Hull CM, Powell DL. Treatment of refractory chronic urticaria with tumor necrosis factor-alfainhibitors. J Am Acad Dermatol. 2011;64:1221-2. treated 6 patients with CU, who had unsuccessfully received a combination of H1antihistamines and immunosuppressants, with different anti-TNF agents (4 with etanercept, 1 infliximab and 1 adalimumab) based on previous studies that had shown elevated serum and cutaneous TNFα in patients with CU compared to controls.8787 Piconi S, Trabattoni D, Iemoli E, Fusi ML, Villa ML, Milazzo F, et al. Immune profiles of patients with chronic idiopathic urticaria. Int Arch Allergy Immunol. 2002;128:59-66.,8888 Hermes B, Prochazka AK, Haas N, Jurgovsky K, Sticherling M, Henz BM. Upregulation of TNF-alpha and IL-3 expression in lesional and uninvolved skin in different types of urticaria. J Allergy Clin Immunol. 1999;103:307-14. The authors reported improvement on the urticaria, with length of remission varying from months to years, and three patients continuing in long-lasting remissions even after all medications were withdrawn.8686 Wilson LH, Eliason MJ, Leiferman KM, Hull CM, Powell DL. Treatment of refractory chronic urticaria with tumor necrosis factor-alfainhibitors. J Am Acad Dermatol. 2011;64:1221-2.
Corticosteroids
Corticosteroids are known to be effective in the treatment of CU in patients who are unresponsive to anti-H1 medications.8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16. Nonetheless, controlled studies on the subject are still lacking. Clinical effects may be evident at 25 mg doses in the early days of treatment, however, given the potential adverse events associated with the chronic use of corticosteroids (diabetes mellitus, hypertension, osteoporosis and gastrointestinal bleeding), oral corticosteroids should be used for short periods of time (3-7 days per month) and at the smallest effective dose.22 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.,8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16.
Drugs with anti-inflammatory effects
These medications are generally safe and affordable to the majority of the population, have still limited evidence of effectiveness in the literature, but can be used in the treatment of CU before more expensive or toxic drugs are considered.8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16.
a. Hydroxychloroquine
Reeves et al8989 Reeves GE, Boyle MJ, Bonfield J, Dobson P, Loewenthal M. Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J. 2004;34:182-6. studied 18 patients with CU who were treated with hydroxychloroquine for 12 weeks. The treatment significantly improved the quality of life of patients, although the levels of urticaria activity suffered little influence. This drug is relatively safe, though it can cause retinopathy usually after 5 years of continuous use.8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16.
b. Colchicine
There are two studies published in the indexed literature, a prospective one by Criado et al5555 Criado RF, Criado PR, Martins JE, Valente NY, Michalany NS, Vasconcellos C. Urticaria unresponsive to antihistaminic treatment: an open study of therapeutic options based on histopathologic features. J Dermatolog Treat. 2008;19:92-6. and a retrospective analysis by Pho et al9090 Pho LN, Eliason MJ, Regruto M, Hull CM, Powell DL. Treatment of chronic urticaria with colchicine. J Drugs Dermatol. 2011;10:1423-8., evaluating the use of colchicine in the treatment of CU. The drug acts by precluding the formation of the apparatus that extrudes granules from mast cells and basophils. Both studies found partial or complete response in at least half the patients. Adverse events are bearable, including diarrhea, leukopenia, and hematuria; furthermore it is a low-cost medication. Colchicine should not be used in pregnant women.
c. Dapsone
Dapsone has been reported to be effective in the treatment of CU/angioedema at doses of 25 to 50 mg per day.5454 Pires JS, de Ue APF, Furlani EJ, de Souza PK, Rotta O. Dapsone as an alternative to the treatment of chronic urticaria non-responsive to antihistamines. An Bras Dermatol. 2008;83:413-8.,5555 Criado RF, Criado PR, Martins JE, Valente NY, Michalany NS, Vasconcellos C. Urticaria unresponsive to antihistaminic treatment: an open study of therapeutic options based on histopathologic features. J Dermatolog Treat. 2008;19:92-6.,8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16.,9191 Noda S, Asano Y, Sato S. Long-term complete resolution of severe chronic idiopathic urticaria after dapsone treatment. J Dermatol. 2012;39:496-7. In a study, in association with desloratadine and as monotherapy, it showed higher rates of remission, although urticaria activity scores were not reduced.9292 Engin B, Ozdemir M. Prospective randomized non-blinded clinical trial on the use of dapsone plus antihistamine vs. antihistamine in patients with chronic idiopathic urticaria. J Eur Acad Dermatol Venereol. 2008;22:481-6. According to Noda et al, dapsone's anti-inflammatory activity occurs by inhibiting the chemotactic migration of neutrophils, protecting cells from neutrophil and eosinophil-mediated damage, reducing the release of prostaglandins and leukotrienes and reducing the integrin-mediated neutrophil adherence, which inhibits the migration of these cells into the extravascular compartment.9191 Noda S, Asano Y, Sato S. Long-term complete resolution of severe chronic idiopathic urticaria after dapsone treatment. J Dermatol. 2012;39:496-7. Although generally well tolerated, dapsone can induce dose-dependent anemia and less frequently peripheral neuropathy, exanthema, DRESS syndrome, gastrointestinal symptoms, hepatotoxicity and methemoglobinemia. Monitoring serum levels of glucose-6-phosphate dehydrogenase (G6PD) is necessary, due to the risk of severe hemolysis in patients lacking this enzyme.8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16.
d. Sulfasalazine
The recommended dose is 2g, p.o daily, with results usually seen within a month.8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16. Adverse events such as nausea, dyspepsia, headache, proteinuria, hepatotoxicity and hematologic abnormalities have been reported.8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16.
Leukotriene receptor antagonist (Montelukast)
Montelukast is used at 10mg per day in adults, as an adjuvant drug to the anti-histaminic treatment, with excellent safety profile.5555 Criado RF, Criado PR, Martins JE, Valente NY, Michalany NS, Vasconcellos C. Urticaria unresponsive to antihistaminic treatment: an open study of therapeutic options based on histopathologic features. J Dermatolog Treat. 2008;19:92-6.,8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16. Leukotrienes C4, D4 and E4 play an important role in the process of allergic inflammation, warranting this drug as an adjunct in the treatment of CU. Khan & Lynch9393 Khan S, Lynch N. Efficacy of montelukast as added therapy in patients with chronic idiopathic urticaria.Inflamm Allergy Drug Targets. 2012;11:235-43. achieved CU control in 48% of 25 patients treated with montelukast associated to anti-H1 and anti-H2, 11 had no improvement and in two patients the urticaria worsened after the drug was introduced. Adverse events included upper respiratory tract symptoms, diarrhea, nausea, vomiting, exanthema, elevated transaminases and exceptionally, psychiatric disorders.9393 Khan S, Lynch N. Efficacy of montelukast as added therapy in patients with chronic idiopathic urticaria.Inflamm Allergy Drug Targets. 2012;11:235-43.
Anticoagulants
Frequently, patients with CU show an increase in plasma markers of thrombin
generation and fibrinolysis during periods of disease exacerbation, perhaps as a
consequence of tissue factor expression on the plasma membrane of activated
eosinophils.8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol
Allergy Clin North Am. 2014;34:105-16.,9494 Criado PR, Antinori LC, Maruta CW, Reis VM. Evaluation of D-dimer serum
levels among patients with chronic urticaria, psoriasis and urticarial vasculitis. An
Bras Dermatol. 2013;88:355-60. The activation of coagulation and fibrinolysis
decreases as the disease enters remission.8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol
Allergy Clin North Am. 2014;34:105-16.
The exact role of this phenomenon (activation of coagulation and fibrinolysis) acting
as the centerpiece of the disease's pathophysiology or epiphenomenon acting as an
amplifier of inflammation, is not yet elucidated. According to Asero et
al8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol
Allergy Clin North Am. 2014;34:105-16. it seems reasonable to use
anticoagulant or antifibrinolytic agents in patients with CU, since the disease's
activity runs parallel to this phenomenon. Reports on the use of oral
anticoagulation, warfarin (keeping INR over 2) and also heparin have been published
for the last decade.9595 Asero R, Tedeschi A, Cugno M. Heparin and tranexamic Acid therapy may be
effective in treatment-resistant chronic urticaria with elevated d-dimer: a pilot
study. Int Arch Allergy Immunol. 2010;152:384-9.
96 Samarasinghe V, Marsland AM. Class action of oral coumarins in the
treatment of a patient with chronic spontaneous urticaria and delayed-pressure
urticaria. Clin Exp Dermatol. 2012;37:741-3.
97 Chua SL, Gibbs S. Chronic urticaria responding to subcutaneous heparin
sodium. Br J Dermatol. 2005;153:216-7.-9898 Parslew R, Pryce D, Ashworth J, Friedmann PS. Warfarin treatment of
chronic idiopathic urticaria and angio-oedema. Clin Exp Allergy.
2000;30:1161-5. However, the use of these drugs is still not
routinely recommended in the treatment of CU.8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol
Allergy Clin North Am. 2014;34:105-16.
Levothyroxine
The association between thyroid autoimmunity and CU is well established. Karaayvazet al9999 Karaayvaz M, Calişkaner Z, Turan M, Akar A, Ozturk S, Ozanguc N. Levothyroxine versus ketotifen in the treatment of patients with chronic urticaria and thyroid autoimmunity. J Dermatolog Treat. 2002;13:165-72. conducted a study with 60 patients with UC, divided into two groups matched for sex and age, using ketotifen or levocetirizine. The group using ketotifen achieved symptom relief while taking the medication, but relapsed after drug withdrawal. Eighteen patients in the group using levothyroxine achieved complete remission and 3 had partial improvements; furthermore, symptoms did not recur in those with complete response. Treatment with levothyroxine takes at least 10 days to demonstrate effect; 0.1 mg/kg/day for four weeks is usually appropriate and does not cause alterations in thyroid function.9999 Karaayvaz M, Calişkaner Z, Turan M, Akar A, Ozturk S, Ozanguc N. Levothyroxine versus ketotifen in the treatment of patients with chronic urticaria and thyroid autoimmunity. J Dermatolog Treat. 2002;13:165-72. It is advisable to approach these cases in conjunction with an endocrinologist, especially in situations where no control of chronic urticaria associated to thyroid autoimmunity was obtained with other pharmacological interventions and provided that there are no contraindications to thyroid hormone replacement therapy.9999 Karaayvaz M, Calişkaner Z, Turan M, Akar A, Ozturk S, Ozanguc N. Levothyroxine versus ketotifen in the treatment of patients with chronic urticaria and thyroid autoimmunity. J Dermatolog Treat. 2002;13:165-72. It is hypothesized that patients with autoimmune thyroiditis have autoantibodies against thyroid proteins (antithyroglobulin and antiperoxidase), which induce inflammation and cytokine release that will subsequently bind to C4, activate the complement system and stimulate the release of histamine by basophils and mast cells.100100 Temboury Molina C, AlinsSahun Y, Cerecedo Carballo I. [Recurrent urticaria and autoimmune thyroiditis: the influence of thyroxin treatment on the outcome of the urticaria]. An Pediatr (Barc). 2012;77:66-7. Thus, antithyroid and anti-FcεRIα antibodies activate the complement in a synergic manner, increasing the release of vasoactive amines, leading to urticaria.
Therapeutic strategies in clinical practice
In our understanding, the treatment of chronic urticaria should be customized according to the patients' lifestyles (profession, social interactions and recreational activities), their socioeconomic level and understanding of disease and treatment. A basic principle is to avoid known aggravating or triggering agents, such as alcohol and indiscriminate use of COX inhibitors and nonsteroidal anti-inflammatory drugs. Each therapeutic intervention cannot be assessed as to its effectiveness before 2 to 4 weeks, at the earliest. If disease control is not achieved with a certain drug, as long as there is no contraindication for an association, it should be maintained at least until the illness is controlled.
Patients with CU tend to develop psychological/psychiatric problems, some even
preceding the initial event of urticaria, such as post-traumatic stress disorder,
alexithymia (marked difficulty in verbaliz-ing emotions, describing feelings or
bodily sensations), anxiety (particularly phobias) and depression, which may affect
up to 48% of patients with spontaneous CU.101101 Gupta MA, Gupta AK. Chronic idiopathic urticaria and post-traumatic
stress disorder (PTSD): an under-recognized comorbidity. Clin Dermatol.
2012;30:351-4.
102 Hunkin V, Chung MC. Chronic idiopathic urticaria, psychological
co-morbidity and posttraumatic stress: the impact of alexithymia and repression.
Psychiatr Q. 2012;83:431-47.-103103 Staubach P, Dechene M, Metz M, Magerl M, Siebenhaar F, Weller K, et al.
High prevalence of mental disorders and emotional distress in patients with chronic
spontaneous urticaria. Acta Derm Venereol. 2011;91:557-61. Thus,
psychological and/or psychiatric support, beside positive attitudes from the health
care team can always be helpful to the treatment.
The quality-of-life of patients with CU is greatly affected, as patients suffer with pruritus, wheals and present fatigue caused by sleep disorders and adverse events of medications.104104 O'Donnell BF. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014;34:89-104. The disease affects many realms of the patient's life, having also an economic impact for the patient and the health system.104104 O'Donnell BF. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014;34:89-104. In tertiary referral centers, the impact in quality of life for these patients is comparable to that experienced by older subjects with severe ischemic heart disease and overall, on various dimensions, to the impact suffered by patients with psoriasis and atopic dermatitis.104104 O'Donnell BF. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014;34:89-104.
So, face to the reality of medical practice in Brazil, we adopted a strategy of sequential treatment (in steps), ranging from the use of anti-H1 as standard initial drugs, to second-line agents (leukotrienes and corticosteroids in short courses), third-line medications (omalizumab, cyclosporine, methotrexate; anti-inflammatory drugs, such as dapsone, hydroxychloroquine and colchicine) and fourth-line drugs (immunosuppressants that are more toxic), considering that in most cases, progression to the next level of therapy also implies increase in direct or indirect costs, as well as greater risks of adverse events (Figure 2).8282 Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16.
Chronic urticaria: severity markers and disease prognosis
Rabelo-Filardiet al105105 Rabelo-Filardi R, Daltro-Oliveira R, Campos RA.Parameters associated with chronic spontaneous urticaria duration and severity: a systematic review. Int Arch Allergy Immunol. 2013;161:197-204. in a systematic review of 34 studies published on spontaneous chronic urticaria, concluded that the clinical severity of CU can forecast disease duration, and laboratory parameters such as elevation of serum levels of fragments 1+2 prothrombin, d-dimer and C-reactive protein (CRP) may reflect the gravity of the disease, and perhaps its resistance to conventional treatment. Patients with more severe symptoms may have more persistent disease courses.106106 Sanchez-Borges M, Asero R, Ansotegui IJ, Baiardini I, Bernstein JA, Canonica GW, et al. Diagnosis and treatment of urticaria and angioedema: a worldwide perspective. World Allergy Organ J. 2012;5:125-47. Spontaneous remissions occur in 30-50% of patients within a year of disease evolution, and another 20% within 5 years.106106 Sanchez-Borges M, Asero R, Ansotegui IJ, Baiardini I, Bernstein JA, Canonica GW, et al. Diagnosis and treatment of urticaria and angioedema: a worldwide perspective. World Allergy Organ J. 2012;5:125-47. About 20% of patients with CU remain ill after five years of evolution.106106 Sanchez-Borges M, Asero R, Ansotegui IJ, Baiardini I, Bernstein JA, Canonica GW, et al. Diagnosis and treatment of urticaria and angioedema: a worldwide perspective. World Allergy Organ J. 2012;5:125-47. Nearly half of patients with CU lasting six months, will probably still have the disease 10 years later.106106 Sanchez-Borges M, Asero R, Ansotegui IJ, Baiardini I, Bernstein JA, Canonica GW, et al. Diagnosis and treatment of urticaria and angioedema: a worldwide perspective. World Allergy Organ J. 2012;5:125-47.
A Korean study with 131 patients with CU evaluated the presence of signs of metabolic syndrome, disease activity score and serum markers for inflammatory activity.107107 Ye YM, Jin HJ, Hwang EK, Nam YH, Kim JH, Shin YS, Co-existence of chronic urticaria and metabolic syndrome: clinical implications. Acta Derm Venereol. 2013;93:156-60. Thirty-nine patients (28.9%) had metabolic syndrome (MetS) compared to 17.8% of subjects in the matched control group (P = 0.001). Patients with CU and metabolic syndrome were older, had higher mean scores of disease activity, higher levels of serum eosinophil cationic protein (ECP), TNFα and complement system factors, besides higher frequency of negative TCSA when compared to patients with CU without MetS. Logistic regression showed that an urticaria activity score ≥ 13 (p=0.025) and the presence of MetS (p=0.036) were independent predictors of a likely difficult-to-treat CU.107107 Ye YM, Jin HJ, Hwang EK, Nam YH, Kim JH, Shin YS, Co-existence of chronic urticaria and metabolic syndrome: clinical implications. Acta Derm Venereol. 2013;93:156-60. Therefore, both CU and MetS may share low-grade chronic inflammation, involving TNFα, ECP and C3, which may be mutually triggering or exacerbating the disease.107107 Ye YM, Jin HJ, Hwang EK, Nam YH, Kim JH, Shin YS, Co-existence of chronic urticaria and metabolic syndrome: clinical implications. Acta Derm Venereol. 2013;93:156-60. Future studies will better elucidate subsequent disorders in patients with CU, since the disease may course with coexisting MetS, activation of coagulation/fibrinolysis and chronic inflammation, which may represent potential cardiovascular diseases and diabetes mellitus during the life of these patients.
CONCLUSIONS
-
-CU is currently classified as spontaneous or induced by physical stimuli. Convention determined that the term "idiopathic" should be avoided;
-Studies with larger samples should be conducted in the future, to assess the value of screening and treatment of infectious and parasitic agents in the course of CU, especially in endemic areas;
-The presence of autoreactivity in patients with spontaneous CU can be demonstrated in 50% of cases, in a simple manner by the autologous serum skin test;
-The vast majority of CUs are not IgE-mediated allergic diseases.
-The diagnosis of autoimmune CU should be based on strict criteria established by the European consensus;
-The link between inflammation and coagulation, boosting the mediators release cascade was demonstrated in a group of patients with CU and high levels of eosinophilic activation;
-First-line treatment of CU in the first decade of this century is based on second-generation antihistamines, however in some patients it is necessary to associate anti-inflammatory or immunosuppressive drugs, besides the promising use of immunobiological agents such as omalizumab;
-Similar to some cases of psoriasis, spontaneous CU can have a major impact on patients' qualityof-life, and also in the various realms of the psyche. Further studies will confirm or deny, the progressive course of metabolic syndrome and cardiovascular complications among patients with long lasting spontaneous CU.
-
Financial Support: None
-
How to cite this article: Criado PR, Criado RFJ, Maruta CW, Reis VMS. Chronic urticaria in adults: state-of-the-art in the new millennium. An Bras Dermatol. 2015;90(1):74-89.
-
*
Work performed at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (FMUSP) – São Paulo (SP), Brazil.
REFERENCES
-
1Cousin F, Philips K, Favier B, Bienvenu J, Nicolas JF. Drug-induced urticaria. Eur J Dermatol. 2001;11:181-7.
-
2Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management ofurticaria: the 2013 revision and update. Allergy. 2014;69:868-87.
-
3Hizal M, Tuzun B, Wolf R, Tuzun Y. The relationship between Helicobacter pylori IgG antibody and autologous serum test in chronic urticaria. Int J Dermatol. 2000;39:443-5.
-
4Federman DG, Kirsner RS, Moriarty JP, Concato J. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. J Am Acad Dermatol. 2003;49:861-4.
-
5Burova KP, Mallet AI, Greaves MW. Is Helicobacter pylori a cause of urticaria? Br J Dermatol. 1999;51:42.
-
6Greaves MW. Chronic idiopathic urticaria and H pylori: not directly causative but could there be a link? ACI Int. 2001;13:23-26.
-
7Tharp MD, Thirlby R, Sullivan TJ. Gastrin induces histamine release from human cutaneous mast cells. J Allergy ClinImmunol. 1984;74:159-65.
-
8Lessof MH, Gant V, Hinuma K, Murphy GM, Dowling RH. Recurrent urticaria and reduced diamine oxidase activity. Clin Exp Allergy. 1990;20:373-6.
-
9Maintz L, Novak N. Histamine and histamine intolerance. Am J Clin Nutr. 2007;85:1185-96.
-
10Asero R. Multiple intolerance to food additives. J Allergy ClinImmunol. 2002;110:531-2.
-
11Simon RA. Additive-induced urticaria: experience with monosodium glutamate (MSG). J Nutr. 2000;130:1063S-6S.
-
12Di Lorenzo G, Pacor ML, Mansueto P, Martinelli N, Esposito-Pellitteri M, Lo Bianco C, et al. Food-additive-induced urticaria: a survey of 838 patients with recurrent chronic idiopathic urticaria. Int Arch Allergy Immunol. 2005;138:235-42.
-
13Goga D, Vaillant L, Pandraud L, Mateu J, Ballon G, Beutter P. The elimination of dental and sinusal infectious foci in dermatologic pathology. A double-blind study in 27 cases confined to chronic urticaria. Rev Stomatol Chir Maxillofac. 1988;89:273-5.
-
14Vaida GA, Goldman MA, Bloch KJ. Testing for hepatitis B virus in patients with chronic urticaria and angioedema. J Allergy Clin Immunol. 1983;72:193-8.
-
15Siddique N, Pereira BN, Hasan Arshad S. Hepatitis C and urticaria: cause and effect? Allergy. 2004;59:668.
-
16Nenoff P, Domula E, Willing U, Herrmann J.[Giardia lamblia--cause of urticaria and pruritus or accidental association?]. Hautarzt. 2006;57:518-20, 521-2.
-
17Demirci M, Yildirim M, Aridogan BC, Baysal V, Korkmaz M. Tissue parasites in patients with chronic urticaria. J Dermatol. 2003;30:777-81.
-
18Ismail MA, Khalafallah O. Toxocaracanis and chronic urticaria in Egyptian patients. J Egypt Soc Parasitol. 2005;35:833-40.
-
19Gulalp B, Koseoglu Z, Toprak N, Satar S, Sebe A, GokelY, et al. Ruptured hydatid cyst following minimal trauma and few signs on presentation. Neth J Med. 2007;65:117-8.
-
20Pattison DA, Speare R. Strongyloidiasis in personnel of the Regional Assistance Mission to Solomon Islands (RAMSI). Med J Aust. 2008;189:203-6.
-
21Marseglia GL, Marseglia A, Licari A, Castellazzi AM, Ciprandi G. Chronic urticaria caused by Hymenoleptis nana in an adopted girl. Allergy. 2007;62:821-2.
-
22Zuel-Fakkar NM, Abdel Hameed DM, Hassanin OM. Study of Blastocystishominis isolates in urticaria: a case-control study. Clin Exp Dermatol. 2011;36:908-10.
-
23Nenoff P, Domula E, Willing U, Herrmann J. [Giardia lamblia--cause of urticaria and pruritus or accidental association?]. Hautarzt. 2006;57:518-20, 521-2.
-
24Kaji K, Yoshiji H, Yoshikawa M, Yamazaki M, Ikenaka Y, Noguchi R, et al. Eosinophilic cholecystitis along with pericarditis caused by Ascarislumbricoides: a case report. World J Gastroenterol. 2007;13:3760-2.
-
25Criado PR, Belda Junior W, Criado RF, Vasconcelos e Silva R, Vasconcellos C. Bedbugs (Cimicidae infestation): the worldwide renaissance of an old partner of human kind. Braz J Infect Dis. 2011;15:74-80.
-
26Spiewak R, Lundberg M, Johansson G, Buczek A. Allergy to pigeon tick (Argasreflexus) in Upper Silesia, Poland. Ann Agric Environ Med. 2006;13:107-12.
-
27Armentia A, Martin-Gil FJ, Pascual C, Martin-Esteban M, Callejo A, Martinez C. Anisakis simplex allergy after eating chicken meat. J Investig Allergol Clin Immunol. 2006;16:258-63.
-
28Daschner A, De Frutos C, Valls A, Vega F. Anisakis simplex sensitization-associated urticaria: short-lived immediate type or prolonged acute urticaria. Arch Dermatol Res. 2010;302:625-9.
-
29Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. 1983;119:636-40.
-
30Leznoff A, Sussman GL. Syndrome of idiopathic urticaria and angioedema with thyroid autoimmunity: a study of 90 patients. J Allergy Clin Immunol. 1989;84:66-71.
-
31Zauli D, Grassi A, Ballardini G, Contestabile S, Zucchini S, Bianchi FB. Thyroid autoimmunity in chronic idiopathic urticaria. Am J Clin Dermatol. 2002;3:525-8.
-
32Turktas I, Gokcora N, Demirsoy S, Cakir N, Onal E. The association of chronic urticaria and angioedema with autoimmune thyroiditis. Int J Dermatol. 1997;36:187-90.
-
33Rottem M. Chronic urticaria and autoimmune thyroid disease: is there a link? Autoimmun Rev. 2003;2:69-72.
-
34Konstantinou GN, Asero R, Maurer M, Sabroe RA, Schmid-Grendelmeier P, Grattan CE. EAACI/GA(2)LEN task force consensus report: the autologous serum skin test in urticaria. Allergy. 2009;64:1256-68.
-
35Tedeschi A, Cottini M, Asero R. Simultaneous occurrence of chronic autoimmune urticaria and non-allergic asthma: a common mechanism? Eur Ann Allergy Clin Immunol. 2009;41:56-9.
-
36Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012;129:1307-13.
-
37Cugno M, Marzano AV, Asero R, Tedeschi A. Activation of blood coagulation in chronic urticaria: pathophysiological and clinical implications. Intern Emerg Med. 2010;5:97-101.
-
38Asero R, Tedeschi A, Riboldi P, Griffini S, Bonanni E, Cugno M.Severe chronic urticaria is associated with elevated plasma levels of D-dimer. Allergy. 2008;63:176-80.
-
39Takahagi S, Mihara S, Iwamoto K, Morioke S, Okabe T, Kameyoshi Y, et al. Coagulation/fibrinolysis and inflammation markers are associated with disease activity in patients with chronic urticaria. Allergy. 2010;65:649-56.
-
40Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27-36.
-
41Augey F, Gunera-Saad N, Bensaid B, Nosbaum A, Berard F, Nicolas JF. Chronic spontaneous urticaria is not an allergic disease. Eur J Dermatol. 2011;21:349-53.
-
42Caliskaner Z, Ozturk S, Turan M, Karaayvaz M.Skin Test positivity to aeroallergens in the patients with chronic urticaria without respiratory disease. J Investig Allergol Clin Immunol. 2004;14:50-4.
-
43Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387-91.
-
44Młynek A, Zalewska-Janowska A, Martus P, Staubach P, Zuberbier T, Maurer M. How to assess disease activity in patients with chronic urticaria? Allergy. 2008;63:777-80.
-
45Grattan CEH. The urticaria spectrum: recognition of clinical patterns can help management. Clin Exp Dermatol. 2004;29:217-21.
-
46Malmros H. Autoserumtest. Nordisk Med 1946;29:150-1.
-
47Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006;154:813-9.
-
48Yıldız H, Karabudak O, Doğan B, Harmanyeri Y. Evaluation of autologous plasma skin test in patients with chronic idiopathic urticaria. Br J Dermatol. 2011;165:1205-9.
-
49Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes whealand- flare reaction much more frequently than frequently than autologous serum. J Allergy Clin Immunol. 2006;117:1113-7.
-
50Metz M, Gimenez-Arnau A, Borzova E, Grattan CE, Magerl M, Maurer M. Frequency and clinical implications of skin autoreactivity to serum versus plasma in patients with chronic urticaria. J Allergy Clin Immunol. 2009;123:705-6.
-
51Yıldız H, Karabudak O, Doğan B, HarmanyeriY.Evaluation of autologous plasma skin test in patients with chronic idiopathic urticaria.Br J Dermatol. 2011;165:1205-9.
-
52Criado PR, Criado RFJ, Maruta CW, Martins JEC, Rivitti EA. Urticaria. An Bras Dermatol 2005;80;613-30.
-
53Criado PR, Criado RFJ, Maruta CW, Machado CDA. Histamine, histamine receptors and antihistamines: new concepts. An Bras Dermatol. 2010;85:195-210.
-
54Pires JS, de Ue APF, Furlani EJ, de Souza PK, Rotta O. Dapsone as an alternative to the treatment of chronic urticaria non-responsive to antihistamines. An Bras Dermatol. 2008;83:413-8.
-
55Criado RF, Criado PR, Martins JE, Valente NY, Michalany NS, Vasconcellos C. Urticaria unresponsive to antihistaminic treatment: an open study of therapeutic options based on histopathologic features. J Dermatolog Treat. 2008;19:92-6.
-
56Jauregui I, Ferrer M, Montoro J, Davila I, Bartra J, del Cuvillo A, et al. Antihistamines in the treatment of chronic urticaria. J Investig Allergol Clin Immunol. 2007;17:41-52.
-
57Kalivas J, Breneman D, Tharp M, Bruce S, Bigby M. Urticaria: clinical efficacy of cetirizine in comparison with hydroxyzine and placebo. J Allergy ClinImmunol. 1990;86:1014-8.
-
58Zuberbier T, Bindslev-Jensen C, Canonica W, Grattan CE, Greaves MW, Henz BM, et al. EAACI/GA2LEN/EDF. EAACI/GA2LEN/EDF guideline: management of urticaria. Allergy. 2006;61:321-31.
-
59Asero R. Chronic unremitting urticaria: is the use of antihistamines above the licensed dose effective? A preliminary study of cetirizine at licensed and above-licensed doses. Clin Exp Dermatol. 2007;32:34-8.
-
60Black AK, Greaves MW. Antihistamines in urticaria and angioedema. Clin Allergy Immunol. 2002;17:249-86.
-
61Kozel MM, Sabroe RA. Chronic urticaria: aetiology, management and current and future treatment options. Drugs. 2004;64:2515-36.
-
62Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H1- Antihistamine Up-Dosing in Chronic Spontaneous Urticaria: Patients' Perspective of Effectiveness and Side Effects - A Retrospective Survey Study. PLoS One. 2011;6:e23931.
-
63Sanchez-Borges M, Caballero-Fonseca F, Capriles-Hulett A. Treatment of recalcitrant chronic urticaria with nonsedating antihistamines: is there evidence for updosing? J Investig Allergol Clin Immunol. 2013;23:141-4.
-
64Fedorowicz Z, van Zuuren EJ, Hu N. Histamine H2-receptor antagonists for urticaria. Cochrane Database Syst Rev. 2012;3:CD008596.
-
65Grattan CE, O'Donnell BF, Francis DM, Niimi N, Barlow RJ, Seed PT, et al. Randomized double-blind study of cyclosporine in chronic 'idiopathic' urticaria. Br J Dermatol. 2000;143:365-72.
-
66Hollander SM, Joo SS, Wedner HJ. Factors that predict the success of cyclosporine treatment for chronic urticaria. Ann Allergy Asthma Immunol. 2011;107:523-8.
-
67Di Leo E, Nettis E, Aloia AM, Moschetta M, Carbonara M, Dammacco F, et al. Cyclosporin-A efficacy in chronic idiopathic urticaria. Int J Immunopathol Pharmacol. 2011;24:195-200.
-
68Vena GA, Cassano N, Colombo D, Peruzzi E, Pigatto P; Neo-I-30 Study Group.Neo-I-30 Study Group. Cyclosporine in chronic idiopathic urticaria: a double-blind, randomized, placebo-controlled trial. J Am Acad Dermatol. 2006;55:705-9.
-
69Hollander SM, Joo SS, Wedner HJ. Factors that predict the success of cyclosporine treatment for chronic urticaria. Ann Allergy Asthma Immunol. 2011;107:523-8.
-
70Neverman L, Weinberger M. Treatment of chronic urticaria in children with antihistamines and cyclosporine. J Allergy Clin Immunol Pract. 2014;2:434-8.
-
71Kessel A, Toubi E. Cyclosporine-A in severe chronic urticaria: the option for longterm therapy. Allergy. 2010;65:1478-82.
-
72Sagi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91:303-6.
-
73Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-4.
-
74Asero R. Oral cyclophosphamide in a case of cyclosporine and steroid-resistant chronic urticaria showing autoreactivity on autologous serum skin testing. Clin Exp Dermatol. 2005;30:582-3.
-
75O'Donnell BF, Barr RM, Black AK, Francis DM, Kermani F, Niimi N, et al. Intravenous immunoglobulin in autoimmune chronic urticaria. Br J Dermatol. 1998;138:101-6.
-
76Pereira C, Tavares B, Carrapatoso I, Loureiro G, Faria E, Machado D, et al. Lowdose intravenous gammaglobulin in the treatment of severe autoimmune urticaria. Eur Ann Allergy Clin Immunol. 2007;39:237-42.
-
77Mitzel-Kaoukhov H, Staubach P, Muller-BrenneT. Effect of high-dose intravenous immunoglobulin treatment in therapy-resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2010;104:253-8.
-
78Maurer M, Altrichter S, Bieber T, Biedermann T, Brautigam M, Seyfried S, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202-209.e5.
-
79Khan DA. Alternative Agents in Refractory Chronic Urticaria: Evidence and Considerations on Their Selection and Use. J Allergy Clin Immunol Pract. 2013;1:433-440.e1.
-
80Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy ClinImmunol. 2011;128:567-73.e1.
-
81Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924-35.
-
82Asero R, Tedeschi A, Cugno M. Treatment of chronic urticaria. Immunol Allergy Clin North Am. 2014;34:105-16.
-
83Chakravarty SD, Yee AF, Paget SA. Rituximab successfully treats refractory chronic autoimmune urticaria caused by IgE receptor autoantibodies. J Allergy ClinImmunol. 2011;128:1354-5.
-
84Arkwright PD.Anti-CD20 or anti-IgE therapy for severe chronic autoimmune urticaria. J Allergy Clin Immunol. 2009;123:510-1.
-
85Mallipeddi R, Grattan CE. Lack of response of severe steroid-dependent chronic urticaria torituximab. Clin Exp Dermatol. 2007;32:333-4.
-
86Wilson LH, Eliason MJ, Leiferman KM, Hull CM, Powell DL. Treatment of refractory chronic urticaria with tumor necrosis factor-alfainhibitors. J Am Acad Dermatol. 2011;64:1221-2.
-
87Piconi S, Trabattoni D, Iemoli E, Fusi ML, Villa ML, Milazzo F, et al. Immune profiles of patients with chronic idiopathic urticaria. Int Arch Allergy Immunol. 2002;128:59-66.
-
88Hermes B, Prochazka AK, Haas N, Jurgovsky K, Sticherling M, Henz BM. Upregulation of TNF-alpha and IL-3 expression in lesional and uninvolved skin in different types of urticaria. J Allergy Clin Immunol. 1999;103:307-14.
-
89Reeves GE, Boyle MJ, Bonfield J, Dobson P, Loewenthal M. Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J. 2004;34:182-6.
-
90Pho LN, Eliason MJ, Regruto M, Hull CM, Powell DL. Treatment of chronic urticaria with colchicine. J Drugs Dermatol. 2011;10:1423-8.
-
91Noda S, Asano Y, Sato S. Long-term complete resolution of severe chronic idiopathic urticaria after dapsone treatment. J Dermatol. 2012;39:496-7.
-
92Engin B, Ozdemir M. Prospective randomized non-blinded clinical trial on the use of dapsone plus antihistamine vs. antihistamine in patients with chronic idiopathic urticaria. J Eur Acad Dermatol Venereol. 2008;22:481-6.
-
93Khan S, Lynch N. Efficacy of montelukast as added therapy in patients with chronic idiopathic urticaria.Inflamm Allergy Drug Targets. 2012;11:235-43.
-
94Criado PR, Antinori LC, Maruta CW, Reis VM. Evaluation of D-dimer serum levels among patients with chronic urticaria, psoriasis and urticarial vasculitis. An Bras Dermatol. 2013;88:355-60.
-
95Asero R, Tedeschi A, Cugno M. Heparin and tranexamic Acid therapy may be effective in treatment-resistant chronic urticaria with elevated d-dimer: a pilot study. Int Arch Allergy Immunol. 2010;152:384-9.
-
96Samarasinghe V, Marsland AM. Class action of oral coumarins in the treatment of a patient with chronic spontaneous urticaria and delayed-pressure urticaria. Clin Exp Dermatol. 2012;37:741-3.
-
97Chua SL, Gibbs S. Chronic urticaria responding to subcutaneous heparin sodium. Br J Dermatol. 2005;153:216-7.
-
98Parslew R, Pryce D, Ashworth J, Friedmann PS. Warfarin treatment of chronic idiopathic urticaria and angio-oedema. Clin Exp Allergy. 2000;30:1161-5.
-
99Karaayvaz M, Calişkaner Z, Turan M, Akar A, Ozturk S, Ozanguc N. Levothyroxine versus ketotifen in the treatment of patients with chronic urticaria and thyroid autoimmunity. J Dermatolog Treat. 2002;13:165-72.
-
100Temboury Molina C, AlinsSahun Y, Cerecedo Carballo I. [Recurrent urticaria and autoimmune thyroiditis: the influence of thyroxin treatment on the outcome of the urticaria]. An Pediatr (Barc). 2012;77:66-7.
-
101Gupta MA, Gupta AK. Chronic idiopathic urticaria and post-traumatic stress disorder (PTSD): an under-recognized comorbidity. Clin Dermatol. 2012;30:351-4.
-
102Hunkin V, Chung MC. Chronic idiopathic urticaria, psychological co-morbidity and posttraumatic stress: the impact of alexithymia and repression. Psychiatr Q. 2012;83:431-47.
-
103Staubach P, Dechene M, Metz M, Magerl M, Siebenhaar F, Weller K, et al. High prevalence of mental disorders and emotional distress in patients with chronic spontaneous urticaria. Acta Derm Venereol. 2011;91:557-61.
-
104O'Donnell BF. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014;34:89-104.
-
105Rabelo-Filardi R, Daltro-Oliveira R, Campos RA.Parameters associated with chronic spontaneous urticaria duration and severity: a systematic review. Int Arch Allergy Immunol. 2013;161:197-204.
-
106Sanchez-Borges M, Asero R, Ansotegui IJ, Baiardini I, Bernstein JA, Canonica GW, et al. Diagnosis and treatment of urticaria and angioedema: a worldwide perspective. World Allergy Organ J. 2012;5:125-47.
-
107Ye YM, Jin HJ, Hwang EK, Nam YH, Kim JH, Shin YS, Co-existence of chronic urticaria and metabolic syndrome: clinical implications. Acta Derm Venereol. 2013;93:156-60.
Publication Dates
-
Publication in this collection
Jan-Feb 2015
History
-
Received
05 Mar 2014 -
Accepted
04 June 2014