Abstract
Type I collagen is the main dermal component, and its evaluation is relevant to quantitative studies in dermatopathology. However, visual gradation (0 to 4+) has low precision and high subjectivity levels. This study aimed to develop and validate a digital morphometric analysis technique to estimate type I collagen levels in the papillary dermis. Four evaluators visually quantified (0 to 4+) the density of type I collagen in 63 images of forearm skin biopsies marked by immunohistochemistry and two evaluators analyzed the same images using digital morphometric techniques (RGB split colors (I) and color deconvolution (II)). Automated type I collagen density estimation in the papillary dermis (two techniques) were correlated with visual evaluations (Spearman's rho coefficients of 0.48 and 0.62 (p<0.01)). With regard to the inter-observer repeatability, the four evaluators who used visual classification had an intraclass correlation coefficient (for absolute agreement) of 0.53, while the other two evaluators who used digital analysis (algorithm II) had an intraclass correlation coefficient of 0.97.
Keywords:
Collagen; Collagen Type I; Image Processing, Computer-Assisted; Immunohistochemistry
Type I collagen, the most common type in the post-fetal human body, is the main component of the dermis and it is also found in tendons, bones and cartilage. The aggregation of its molecules makes up fibrils, named collagen fibers.11 Murphy GF. Histology of the skin. In: Elder D, Elenitsas R, Jaworsky C, Johnson Jr B, editors. Lever's Histopathology of the skin. Philadelphia: Lippincott-Raven; 1997. p. 42-3.
Many diseases are characterized by direct injury to or insufficient or abnormal formation of collagen fibers. Therefore the study of the density of type I collagen in the papillary dermis is important for better understanding photoaging, rheumatic diseases, skin repair and healing.
In research, collagen fiber density calculations are usually performed visually by trained evaluators (ranking from 0 to 4+). However, visual gradations suffer systematic interference of subjectivity and show low precision, which encourages the use of automated techniques of image analysis to estimate these results.22 Oberholzer M, Ostreicher M, Christen H, Bruhlmann M. Methods in quantitative image analysis. Histochem Cell Biol. 1996;105:333-55.
3 Brianezi G, Miot HA. Desenvolvimento e validação de técnica quantitativa de análise de imagem para avaliação do teste do cometa corado pela prata. J Bras Patol Med Lab. 2009;45:325-34.
4 Lee ES, Kim JH, Im S, Lee KB, Sohn S, Kang WH. Application of computerized image analysis in pigmentary skin diseases. Int J Dermatol. 2001;40:45-9.-55 Miot HA, Brianezi G. Morphometric analysis of dermal collagen by color clusters segmentation. An Bras Dermatol. 2010;85:361-4.
Digital morphometric techniques are widely employed in the objective measurement of microscopic structures. Nevertheless, they must be properly validated before use in quantitative research. Moreover, the availability of freeware for image analysis supports wider use of this technology for quantifying these structures.66 Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671-5.
7 Hamilton NA. Open source tools for fluorescent imaging. Methods Enzymol. 2012;504:393-417.
8 Stuurman N, Swedlow JR. Software tools, data structures, and interfaces for microscope imaging. Cold Spring Harb Protoc. 2012;2012:50-61.-99 Papadopulos F, Spinelli M, Valente S, Foroni L, Orrico C, Alviano F, et al. Common tasks in microscopic and ultrastructural image analysis using ImageJ. Ultrastruct Pathol. 2007;31:401-7.
This study intended to validate a technique of digital morphometric analysis for type I collagen estimation in the papillary dermis.
Sixty-three pictures from adult (50 to 75 years-old) skin biopsies (healthy ventral forearm skin) were marked by immunohistochemistry with anticollagen I antibody (purified rabbit anti-human type I collagen, Dako, d-13, 974), incubated with diaminobenzidine (DAB) and counterstained with Hematoxylin. The study was approved by the institutional review board.
Images of interfollicular spaces were acquired in a standardized way, with a light microscope (Olympus BX 40) under 400x magnification, with 1280x960 pixels, 24-bit color pixels, ISO 80, speed 1/2000, and stored as JPG files (high quality).
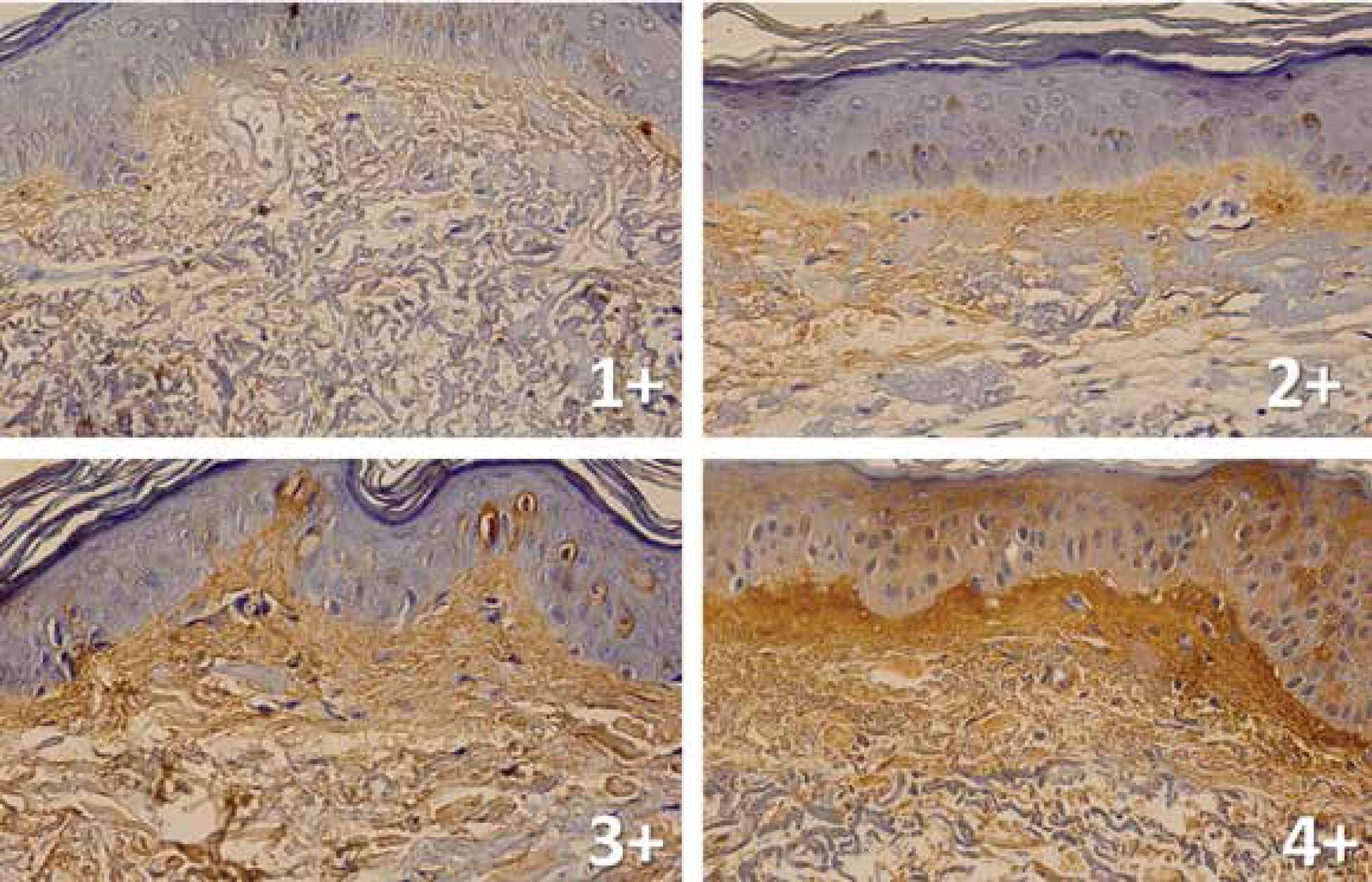
Qualitative assessments (ranking from zero to 4+) of collagen type I density in the papillary dermis seen in the images were performed by two senior dermatopathologists (Ev3 and Ev4) and two boarded dermatologists (Ev1 and Ev2) (Figure 1).
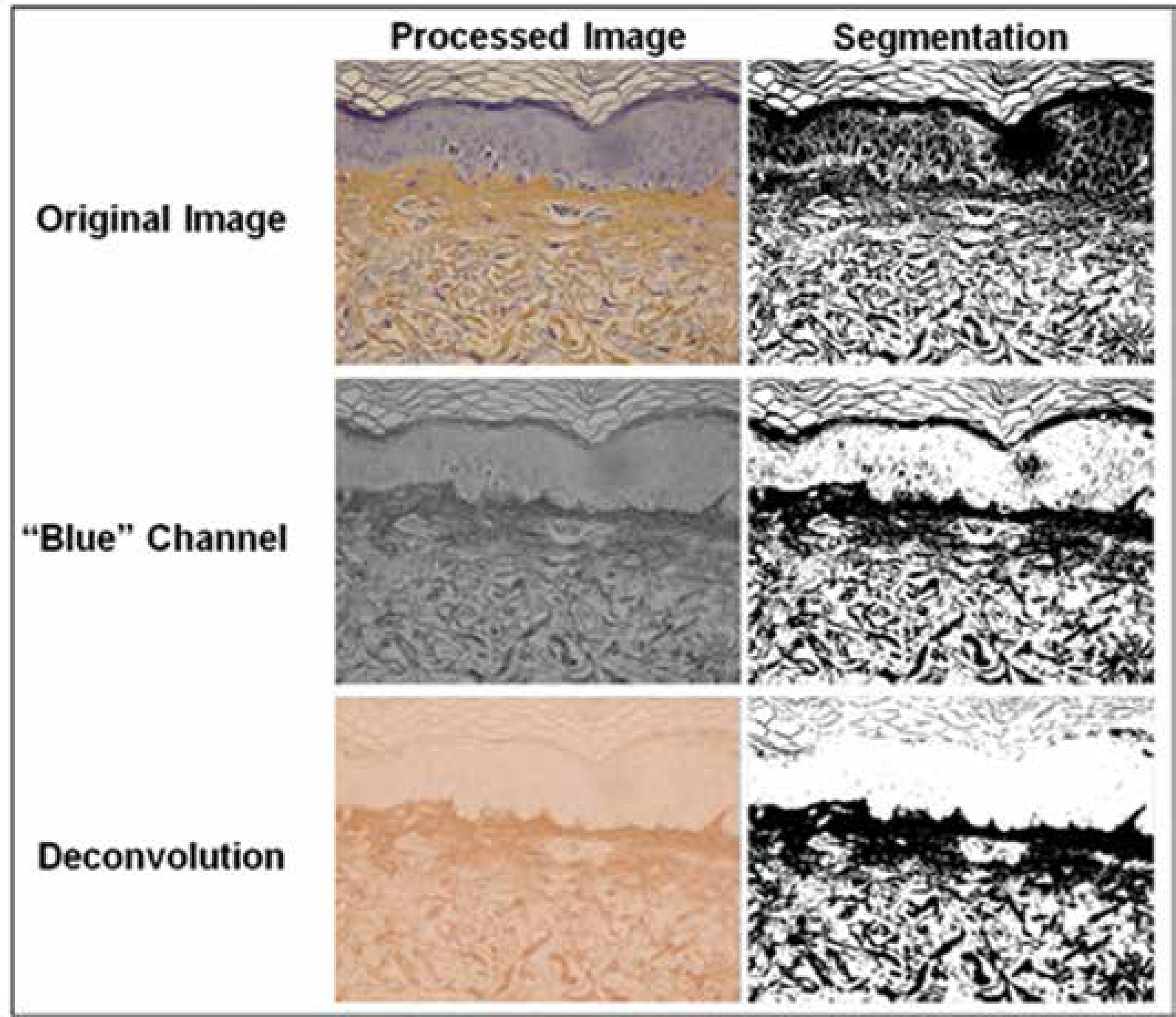
ImageJ 1.46 software was used for morphometric analysis. Labeled areas associated with the papillary dermis were evaluated following automatic segmentation (ISODATA) after using two algorithms.66 Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671-5.,1010 Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25-30.The first algorithm uses the RGB color decomposition tool for channel "blue", and the second algorithm uses the color deconvolution technique (H&E-DAB) (Figure 2).1111 Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291-9.
Algorithms used in the image processing. Algorithm 1 – split channels "blue", algorithm 2 – colors deconvolution "H&E DAB"
The statistical analysis was carried out using the SPSS 20 software.1212 Statistical Package for Social Science (SPSS). IBM SPSS 20.0 for Windows. 20 ed. Chicago (IL): SPSS Incorporation; 2011.Agreement between measurements was assessed by the intraclass coefficient of correlation (ICC -for complete agreement). The correlation between the morphometric estimate and the median of all four estimates calculated by the evaluators was determined by Spearman's rank correlation coefficient. All tests considered a two tailed p value of 0.05 as significant.1313 Vargha P. A critical discussion of intraclass correlation coefficients. Stat Med. 1997;16:821-3.,1414 Norman GR, Streiner DL. Biostatistics. The bare essentials. 3rd ed. Shelton, Connecticut: People's Medical Publishing House; 2008.
The gradations of all four evaluators are shown intable 1. The intraclass coefficient of correlation (ICC) between the visual scores of the four evaluators was 0.52, (p <0.01). The 2x2 concordance between evaluators is shown intable 2.
The estimate densities of collagen type I by color decomposition technique (channel blue -algorithm I) and color deconvolution (algorithm II) are shown infigure 3. Correlation coefficients (Spearman's rho) between the image analysis techniques and the median of all four estimates calculated by the evaluators are shown intable 3.
Original Image, channel "blue" and color deconvolution products and the respective results of the automatic segmentation (ISOdata)
Correlation coefficients (Spearman's rho) between the image analysis techniques and the median of the evaluators´ scores
The agreement (ICC) between the digital estimate of papillary dermal collagen calculated by two independent evaluators using the color deconvolution (algorithm II) method was 0.97 (p <0.01).
Usually, histological estimation of collagen is performed qualitatively by an experienced dermatopathologist using visual gradation for each microscopic field, stained by picrosirius "red" or labeled by immunohistochemistry. However, in scientific research, the low interobserver reproducibility and the low accuracy of visual grading encourage the use of computerized morphometric estimators for such evaluation, because they allow greater objectivity and agility of results, as well as greater reproducibility among evaluators.55 Miot HA, Brianezi G. Morphometric analysis of dermal collagen by color clusters segmentation. An Bras Dermatol. 2010;85:361-4.,1515 Brianezi G, Minicucci EM, Marques ME, Miot HA. Evaluation epidermal p53 immunostaining by digital image analysis. Skin Res Technol. 2013;19:e108-12.
The reproducibility of the results provided by algorithm II showed greater concordance between estimates than the one found between different evaluators using visual classification, which suggests a superior reliability of the digital method. Moreover, the visual classification, even if performed by the same observer, can be influenced by psychological factors such as stress and fatigue. In addition, assessments performed on different days may present varying grading standards.
The automated morphometric analysis of the density of pixels representing type I collagen in digital images is more accurate and objective than visual qualitative gradation. It also allows the comparison of smaller samples, the statistical detection of less exuberant differences and earlier detection of phenomena; favoring blinding, reproducibility between laboratories and operationalization of the research process.1616 Miot HA, Brianezi G, Tamega Ade A, Miot LD. Techniques of digital image analysis for histological quantification of melanin. An Bras Dermatol. 2012;87:608-11.
The segmentation of the image directly from the pixel histogram has lower discriminating performance, underestimating the components of the study, which adds noise to the analysis. Therefore, it is important to use filters that extract the information and which are strictly enforced in a standardized way to ensure complete reliability of the results, besides the validation of the technique used, as presented in this study.
The first algorithm uses a RGB split color channel as a tool and the color channel "blue", which retains information of brown pigments. It is the most commonly used algorithm for the segmentation of immunohistochemical staining (DAB). However it has not shown the best correlation with visual classification.1616 Miot HA, Brianezi G, Tamega Ade A, Miot LD. Techniques of digital image analysis for histological quantification of melanin. An Bras Dermatol. 2012;87:608-11.,1717 Brey EM, Lalani Z, Johnston C, Wong M, McIntire LV, Duke PJ, et al. Automated selection of DAB-labeled tissue for immunohistochemical quantification. J Histochem Cytochem. 2003;51:575-84.
Algorithm II showed a better performance in the discrimination of pixels related to collagen type I, and also with regard to reproducibility and correlation to the scores given by the visual evaluators. Indeed, color deconvolution tools have been used in the analysis of DAB staining immunohistochemistry for research in other areas of knowledge.1818 Helps SC, Thornton E, Kleinig TJ, Manavis J, Vink R. Automatic nonsubjective estimation of antigen content visualized by immunohistochemistry using color deconvolution. Appl Immunohistochem Mol Morphol. 2012;20:82-90.
19 Safadi RA, Musleh AS, Al-Khateeb TH, Hamasha AA. Analysis of immunohistochemical expression of k19 in oral epithelial dysplasia and oral squamous cell carcinoma using color deconvolution-image analysis method. Head Neck Pathol. 2010;4:282-9.-2020 Halushka MK, Cornish TC, Lu J, Selvin S, Selvin E. Creation, validation, and quantitative analysis of protein expression in vascular tissue microarrays. Cardiovasc Pathol. 2010;19:136-46.
Color deconvolution algorithms use all pixel color information of the image and presuppose the effect of superimposed colors. They create a matricial operation that subtracts planes directed by vectors of predefined colors, providing an effective separation of stainings in histological specimens.1111 Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291-9.
This study proposes a digital technique for the analysis of type I collagen in the superficial dermis, based on color deconvolution. It correlates satisfactorily with the visual perception of the evaluators and can be used in this assessment, since adequate standardization of acquisition and processing of the specimens were warranted. This process can be extrapolated to other collagen markers labeled by Hematoxylin-DAB immunohistochemistry.
In conclusion, we developed and validated a technique for the assessment of type I collagen in the papillary dermis, using a color deconvolution algorithm. This technique provides adequate correlation with the evaluator's visual estimate and shows high reproducibility.
-
Financial Support: None.
-
How to cite this article: Brianezi G, Grandi F, Bagatin E, Enokihara MMSS, Miot HA. Dermal type I collagen assessment by digital image analysis. An Bras Dermatol. 2015;90(5):723-7.
-
*
Study conducted at the Dermatology Department, Botucatu Medical School – Universidade Estadual Paulista "Júlio de Mesquita Filho" (FMB-Unesp) – Botucatu (SP), Brazil.
REFERENCES
-
1Murphy GF. Histology of the skin. In: Elder D, Elenitsas R, Jaworsky C, Johnson Jr B, editors. Lever's Histopathology of the skin. Philadelphia: Lippincott-Raven; 1997. p. 42-3.
-
2Oberholzer M, Ostreicher M, Christen H, Bruhlmann M. Methods in quantitative image analysis. Histochem Cell Biol. 1996;105:333-55.
-
3Brianezi G, Miot HA. Desenvolvimento e validação de técnica quantitativa de análise de imagem para avaliação do teste do cometa corado pela prata. J Bras Patol Med Lab. 2009;45:325-34.
-
4Lee ES, Kim JH, Im S, Lee KB, Sohn S, Kang WH. Application of computerized image analysis in pigmentary skin diseases. Int J Dermatol. 2001;40:45-9.
-
5Miot HA, Brianezi G. Morphometric analysis of dermal collagen by color clusters segmentation. An Bras Dermatol. 2010;85:361-4.
-
6Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671-5.
-
7Hamilton NA. Open source tools for fluorescent imaging. Methods Enzymol. 2012;504:393-417.
-
8Stuurman N, Swedlow JR. Software tools, data structures, and interfaces for microscope imaging. Cold Spring Harb Protoc. 2012;2012:50-61.
-
9Papadopulos F, Spinelli M, Valente S, Foroni L, Orrico C, Alviano F, et al. Common tasks in microscopic and ultrastructural image analysis using ImageJ. Ultrastruct Pathol. 2007;31:401-7.
-
10Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25-30.
-
11Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291-9.
-
12Statistical Package for Social Science (SPSS). IBM SPSS 20.0 for Windows. 20 ed. Chicago (IL): SPSS Incorporation; 2011.
-
13Vargha P. A critical discussion of intraclass correlation coefficients. Stat Med. 1997;16:821-3.
-
14Norman GR, Streiner DL. Biostatistics. The bare essentials. 3rd ed. Shelton, Connecticut: People's Medical Publishing House; 2008.
-
15Brianezi G, Minicucci EM, Marques ME, Miot HA. Evaluation epidermal p53 immunostaining by digital image analysis. Skin Res Technol. 2013;19:e108-12.
-
16Miot HA, Brianezi G, Tamega Ade A, Miot LD. Techniques of digital image analysis for histological quantification of melanin. An Bras Dermatol. 2012;87:608-11.
-
17Brey EM, Lalani Z, Johnston C, Wong M, McIntire LV, Duke PJ, et al. Automated selection of DAB-labeled tissue for immunohistochemical quantification. J Histochem Cytochem. 2003;51:575-84.
-
18Helps SC, Thornton E, Kleinig TJ, Manavis J, Vink R. Automatic nonsubjective estimation of antigen content visualized by immunohistochemistry using color deconvolution. Appl Immunohistochem Mol Morphol. 2012;20:82-90.
-
19Safadi RA, Musleh AS, Al-Khateeb TH, Hamasha AA. Analysis of immunohistochemical expression of k19 in oral epithelial dysplasia and oral squamous cell carcinoma using color deconvolution-image analysis method. Head Neck Pathol. 2010;4:282-9.
-
20Halushka MK, Cornish TC, Lu J, Selvin S, Selvin E. Creation, validation, and quantitative analysis of protein expression in vascular tissue microarrays. Cardiovasc Pathol. 2010;19:136-46.
Publication Dates
-
Publication in this collection
Sep-Oct 2015
History
-
Received
18 Dec 2013 -
Accepted
23 June 2014