Abstract:
Background:
Hereditary angioedema is a rare autosomal dominantly inherited immunodeficiency disorder characterized by potentially life-threatening angioedema attacks.
Objective:
We aimed to investigate the clinical and genetic features of a family with angioedema attacks.
Methods:
The medical history, clinical features and C1-INH gene mutation of a Turkish family were investigated and outcomes of long-term treatments were described.
Results:
Five members had experienced recurrent swellings on the face and extremities triggered by trauma. They were all misdiagnosed as familial Mediterranean fever (FMF) depending on frequent abdominal pain and were on colchicine therapy for a long time. They had low C4 and C1-INH protein concentrations and functions. A mutation (c.1247T>A) in C1-INH gene was detected. They were diagnosed as having hereditary angioedema with C1-INH deficiency (C1-INH hereditary angioedema) for the first time. Three of them benefited from danazol treatment without any significant adverse events and one received weekly C1 esterase replacement treatment instead of danazol since she had a medical history of thromboembolic stroke.
Study limitations:
Small sample size of participants.
Conclusion:
Patients with C1-INH hereditary angioedema may be misdiagnosed as having familial Mediterranean fever in regions where the disorder is endemic. Medical history, suspicion of hereditary angioedema and laboratory evaluations of patients and their family members lead the correct diagnoses of hereditary angioedema. Danazol and C1 replacement treatments provide significant reduction in hereditary angioedema attacks.
Keywords:
Complement C1 inhibitor protein; Danazol; Hereditary Angioedema Types I and II
INTRODUCTION
Hereditary angioedema (HAE) is a rare autosomal dominantly inherited disorder characterized by potentially life-threatening angioedema attacks. It affects about 1:10000 to 50000 individuals of different populations.11 Kesim B, Uyguner ZO, Gelincik A, Mete Gökmen N, Sin AZ, Karakaya G, et al. The Turkish Hereditary Angioedema Pilot Study (TURHAPS): the first Turkish series of hereditary angioedema. Int Arch Allergy Immunol. 2011;156:443-50. Hereditary angioedema is classified into two subtypes: HAE with C1 inhibitor deficiency (C1-INH-HAE) and HAE with normal C1-INH caused by an unknown origin or associated with factor XII mutations. 22 Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K,et al. HAWK under the patronage of EAACI (European Academy of Allergy and Clinical Immunology). Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602-16. C1-INH is a serine protease inhibitor that regulates inflammatory, complement and kinin forming pathways. 33 Zuraw BL. The Pathophysiology of Hereditary Angioedema. World Allergy Organ J. 2010;3:S25-8. C1-INH gene (or SERPING1) mutations cause alterations in the structure and function of C1-INH protein, which lead to two phenotypic variants of C1-INH-HAE. C1-INH gene mutations leading to low levels of C1-INH protein causes type 1 of the disease. Type 2 presents normal or elevated levels of plasma protein with low C1-INH function.22 Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K,et al. HAWK under the patronage of EAACI (European Academy of Allergy and Clinical Immunology). Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602-16.
C1-INH/SERPING1 gene is located on chromosome 11q12-q13.1. More than 345 mutations have been described causing C1-INH-HAE in unrelated patients (http://portal.biobase-international.com/hgmd). Missense mutations and frame shift alterations are mainly involved in the pathogenesis. Although systematic mutation analysis of C1- INH gene has been performed in many different ethnic populations, correlation between genotype and phenotype of HAE could not be defined. 22 Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K,et al. HAWK under the patronage of EAACI (European Academy of Allergy and Clinical Immunology). Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602-16.,44 Gompels MM, Lock RJ, Abinun M, Bethune CA, Davies G, Grattan C, et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005;139:379-94.,55 Kalmár L, Hegedüs T, Farkas H, Nagy M, Tordai A. HAEdb: a novel interactive,locus-specific mutation database for the C1 inhibitor gene. Hum Mutat. 2005;25:1-5. However, some recent studies indicated that carrying C1-INH gene missense mutations may represent a less severe form of C1-INH-HAE phenotype and patients with genetic alterations in the genes encoding proteins other than C1-INH gene may be involved in the pathogenesis of angioedema. 66 Speletas M, Szilagyi A, Psarros F, Moldovan D, Magerl M, Kompoti M, et al. Hereditary angioedema: molecular and clinical differences among European populations. J Allergy Clin Immunol. 2015;135:570-3.,77 Bors A, Csuka D, Varga L, Farkas H, Tordai A, Füst G, et al. Less severe clinical manifestations in patients with hereditary angioedema with missense C1INH gene mutations. J Allergy Clin Immunol. 2013;131:1708-11.
Patients with HAE should be treated both during angioedema attacks and for prophylaxis. On demand therapy (therapy during angioedema attacks) is made by fresh frozen plasma or C1- INH concentrate to replace the deficient C1-INH esterase inhibitor. Kallikrein inhibitors (such as ecallantide) and bradykinin B2 receptor antagonists (e. g. icatibant) are additional, possible therapeutic options for the treatment of edema attacks. Long-term prophylaxis is very important for patients who experience frequent and severe episodes of angioedema. Danazol is the main agent used for such kind of prophylaxis and follow-up and monitoring of drug adverse events and toxicity is mandatory. 22 Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K,et al. HAWK under the patronage of EAACI (European Academy of Allergy and Clinical Immunology). Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602-16.
Epidemiology of HAE, genotypic features of the disorder, efficacy and adverse events of treatments applied have been investigated in large case and family studies in different populations. However, knowledge on clinical and genetic features of Turkish population is limited. In the present study, we investigated the clinical and genetic features of seven related patients and described the outcomes of long-term treatment of C1-INH-HAE.
METHODS
Subjects
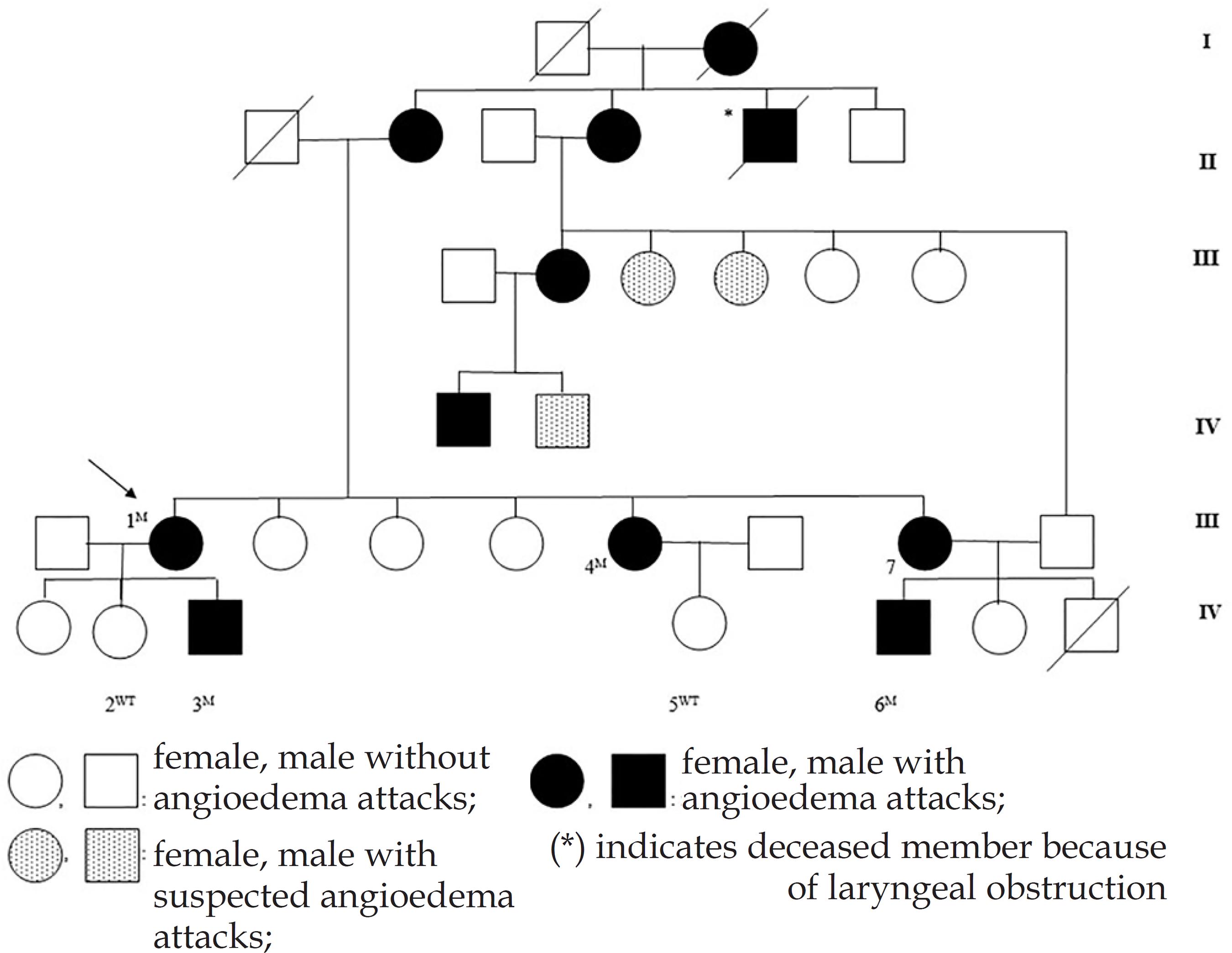
The proband (Patient 1) was a 58-year-old woman who was referred from emergency department for periorbital, lip and uvula edema. She had hit her head on a door before swellings. She has had angioedema attacks without urticarial lesions for about 30 years. Her family history revealed that her mother, son, sisters, aunt, some cousins and nephews had similar swelling attacks (pedigree is in figure 1). Her uncle had died because of laryngeal obstruction during an edema attack. Among the family members, proband's son (Patient 3), her two sisters (Patients 4 and 7) and one nephew (Patient 6) who had angioedema attacks and her daughter (Patient 2) and nephew (Patient 5), who were asymptomatic, were available for clinical and laboratory investigations for HAE.
Pedigree of the family with hereditary angioedema. Symbols with a slash indicate deceased family members. The arrow indicates the index patient. Numbers indicate the cases genetically investigated in this study. Superscripts show the results of genetic analysis (M: mutant for p.Leu416X (c.1247T>A) mutation in the 7th exon of C1-INH gene; WT: wild type).
Genetic analysis
All these patients, except for Patient 7, were accepted for genetic analysis for C1-INH gene mutation. Peripheral blood samples were collected into 5ml K3EDTA tubes and DNA was isolated by a solution-based kit (Mammalian Blood Isolation Kit-Roche, Istanbul). Deep intronic primers were designed to cover all eight exons of C1-INH gene (NM_000062.2), including the exon-intron boundaries and promoter region to secure to identify all the point mutations, small insertion and deletions described up to date (Table 1). Each region was amplified in 50µl reaction mixtures as directed in the instructions contained in the Taq Polymerase Enzyme Kit (Fermentas-Elips, Istanbul) under optimum Tm temperatures (+/-2°C) of the primers in PTC-200 (MJ Research-Biorad, Medgen, Istanbul). PCR products were purified (GeneMark-Genova, Istanbul) and sequenced in ABI 3130 (Duzen Laboratory, Istanbul). Sequencing chromatograms were analyzed by using the public free blast programs (http://genome.ucsc.edu/ and http://www.ncbi.nlm.nih.gov/).
The identified mutations were confirmed by independent reactions and by family members' DNA whose samples were specifically sequenced for the exon that was shown to carry a mutation in the index.
RESULTS
Clinical and laboratory evaluation and C1-INH gene mutation results
Demographic and clinical features of all cases are summarized in table 2. Swelling attacks emerged between one (Patient 7) and 28 (Patient 1) years of age. Trauma was the most frequent trigger for angioedema. The proband had swellings attacks every two weeks, lasting up to one week. The daughter (Patient 2) and one of the nephews (Patient 5) of Patient 1 did not have any swelling attacks until now. Abdominal attacks were common and all symptomatic five patients were misdiagnosed as having familial Mediterranean fever (FMF) in other health centers and put on colchicine treatment for a long time. Patient 7 had the most severe and frequent angioedema attacks as laryngeal obstruction and severe abdominal pain. Her medical history revealed stroke due to thrombosis. Patient 3 had experienced severe abdominal attacks initiated in pre-puberty until about his twenties. He had no angioedema attacks for about 13 years. Total blood counts with differentials, liver, kidney and thyroid function tests, anti-thyroid autoantibodies, and fibrinogen levels were within normal limits in all patients. Levels of C4, C1-INH protein and function are given in table 3. C1-INH gene mutation analyses demonstrated p.Leu416X (c.1247T>A) mutation in the 7th exon of C1-INH gene leading to a premature stop codon in Patients 1, 3, 4 and 6 (Graph 1).
Serum levels of C4, C1-INH protein and C1-INH activity, and results of C1-INH gene mutation of patients
Depending on medical and family history, clinical and laboratory findings, all these symptomatic patients, for the first time, were diagnosed as having C1-INH-HAE type I.
Managements
Patient 1 was treated with fresh frozen plasma and swellings completely regressed. She was put on 200mg/d danazol prophylaxis. After two months, the dosage was reduced to 100mg/d. The patient is now taking 50mg danazol every other day and she is still on her regular follow-ups without any other angioedema attacks for five years. Although she was symptom free, danazol therapy was not stopped due to her willing to continue the therapy because of being very anxious for having a serious attack otherwise. Patient 4 also initiated danazol at the same dosages; however, she suffered drug-induced dizziness, which required reducing the dosage to 50mg/d. She is still on 50mg/d danazol therapy for two years, having only infrequent mild swellings on the extremities. Patient six was also put on danazol therapy with a dosage of 100mg/d for 6 months, and then 50mg/g for two years. Weekly C1 esterase replacement therapy was initiated for angioedema prophylaxis for Patient 7 since danazol therapy was not suitable due to previous thrombotic stroke event. During the first year of follow-up period, treatment provided significant reduction of severity and frequency of life threatening attacks. Since Patient 3 was free of attacks for years, no therapy was administered and avoidance of trauma and short-term prophylaxis when needed were recommended (Table 4).
No laboratory abnormalities were detected during long-term prophylactic treatment. Annual ultrasonography examinations of liver were normal in Patient 1. Liver ultrasonography revealed two small lesions, suggesting hemangiomas in Patient 4. Dynamic contrast enhanced magnetic resonance imaging confirmed hemangiomas to be smaller than reported in ultrasonography. Gastroenterology consultation recommended imaging examinations of liver every six months during the minimal effective danazol maintenance therapy. Grade 3 hepatosteatosis was reported on ultrasonography in the last visit of Patient 6. Switching to fibrinolytic therapy was not successful. Since normal liver structure was detected in magnetic resonance imaging, minimal danazol therapy was continued with close follow-up for liver functions.
DISCUSSION
This study demonstrated a nucleotide substation T>A encoding leucine amino acid in exon 7 of C1- INH gene resulting in a premature stop codon causing C1-INH-HAE type I in a Turkish family. This kind of mutation was described by Pappalardo et al.88 Pappalardo E, Caccia S, Suffritti C, Tordai A, Zingale LC, Cicardi M. Mutation screening of C1 inhibitor gene in 108 unrelated families with hereditary angioedema: functional and structural correlates. Mol Immunol. 2008;45:3536-44. This is a nonsense mutation in the upstream from the reactive site that may affect mRNA or protein stability. The knowledge about genetic features of Turkish patients having HAE is limited to only one study in which mutation analyses of 10 patients were reported.11 Kesim B, Uyguner ZO, Gelincik A, Mete Gökmen N, Sin AZ, Karakaya G, et al. The Turkish Hereditary Angioedema Pilot Study (TURHAPS): the first Turkish series of hereditary angioedema. Int Arch Allergy Immunol. 2011;156:443-50. The results of that study showed five novel mutations consisted of regulatory mutation, initiation codon change, splice mutation, nonsense mutation and 9bp-deletion mutation. Although C1-INH-HAE type I is usually inherited in an autosomal dominant manner, two Turkish families with autosomal recessive inheritance were reported in the literature. 11 Kesim B, Uyguner ZO, Gelincik A, Mete Gökmen N, Sin AZ, Karakaya G, et al. The Turkish Hereditary Angioedema Pilot Study (TURHAPS): the first Turkish series of hereditary angioedema. Int Arch Allergy Immunol. 2011;156:443-50.,99 Büyüköztürk S, Eroğlu BK, Gelincik A, Uzümcü A, Ozşeker F, Colakoğlu B, et al. A Turkish family with a novel mutation in the promoter region of the C1 inhibitor gene. J Allergy Clin Immunol. 2009;123:962-4. In our family cases, the inheritance was autosomal dominant. One of the twin children of the index patient had the mutation and the other did not.
The results of our study demonstrated that C1-INH-HAE type I may present with diverse clinical features in patients carrying the same mutation for C1-INH gene. Onset of clinical symptoms and signs of HAE started in a time period ranging between very early years of life to adulthood. Besides, symptoms varied in degree from very mild to severe as defined by different members of the family. When the proband was correctly diagnosed as C1-INH-HAE type I, the other family members were invited for examination and had the chance of correct diagnoses. Symptoms of HAE include mainly swelling on extremities, abdominal cramps, facial and laryngeal edema. 11 Kesim B, Uyguner ZO, Gelincik A, Mete Gökmen N, Sin AZ, Karakaya G, et al. The Turkish Hereditary Angioedema Pilot Study (TURHAPS): the first Turkish series of hereditary angioedema. Int Arch Allergy Immunol. 2011;156:443-50.,1010 Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angioedema: comparison of treated and untreated patients. J Allergy Clin Immunol. 1997;99:194-6. Laryngeal edema without asphyxia was experienced by our patients whereas one older relative had died due to airway obstruction. As observed in our patients, abdominal attacks are very common in Turkish patients with HAE. 11 Kesim B, Uyguner ZO, Gelincik A, Mete Gökmen N, Sin AZ, Karakaya G, et al. The Turkish Hereditary Angioedema Pilot Study (TURHAPS): the first Turkish series of hereditary angioedema. Int Arch Allergy Immunol. 2011;156:443-50. In the present study, all patients were first misdiagnosed as having FMF and administered colchicine treatment for years. As expected, patients did not have any benefit from colchicine. Misdiagnosis as FMF was also reported in six of 70 patients in the study of Kesim et al. and a case report from Turkey. 11 Kesim B, Uyguner ZO, Gelincik A, Mete Gökmen N, Sin AZ, Karakaya G, et al. The Turkish Hereditary Angioedema Pilot Study (TURHAPS): the first Turkish series of hereditary angioedema. Int Arch Allergy Immunol. 2011;156:443-50.,1111 Sarici G, Koca R, Tekin NS, Altinyazar HC. A family with hereditary angioedema having been followed as familial Mediterranean fever. Turkderm 2009;43:29-31. Abdominal angioedema without skin swelling is a challenging problem in countries where FMF is an endemic disorder. Abdominal pain in HAE may also mimic acute abdominal disorders, which require urgent surgical procedures. High prothrombin fragment F1 and F2 and D-dimer levels are suggested to support HAE during acute angioedema attacks, which prevent patients from undergoing unnecessary abdominal surgery. 1212 Cugno M, Zanichelli A, Bellatorre AG, Griffini S, Cicardi M. Plasma biomarkers of acute attacks in patients with angioedema due to C1-inhibitor deficiency. Allergy. 2009;64:254-7. Besides, elevated D-dimers in HAE attacks were not found to be associated with increased thrombotic risk. 1313 Reshef A, Zanichelli A, Longhurst H, Relan A, Hack CE. Elevated D-dimers in attacks of hereditary angioedema are not associated with increased thrombotic risk. Allergy. 2015;70:506-13. Overlooking patients with HAE gave rise to a very long lag time for diagnosis - up to 52 years as observed in one of our patients. Taking a detailed personal and familial medical history and suspicion for HAE is the key approach to make a correct diagnosis.
Treatment of C1-INH-HAE type I includes on demand or attack therapy and prophylaxis. The proband in our study was first observed when she was having a severe facial angioedema attack and put on fresh frozen plasma therapy immediately since C1-INH concentrate was not available at the time. Then, she was followed-up by long-term prophylaxis with danazol. Danazol therapy was administered for three patients of the family and very well tolerated. We observed that a minimum dose of danazol could provide a very satisfying disease control. On the other hand, the patients were closely followed for potential adverse events on hormonal status and liver. Danazol may cause hormonal adverse events including virilization, decreased breast size, irregular vaginal bleeding or decreased libido, changes on lipid profiles leading to atherosclerosis, and hepatotoxicity. Cicardi et al. reported that menstrual irregularities were the main adverse events of danazol or danazol and stanozolol combination treatment. 1010 Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angioedema: comparison of treated and untreated patients. J Allergy Clin Immunol. 1997;99:194-6. Zotter et al. observed that hirsutism was the most common adverse event, however, long-term danazol treatment with the lowest effective dose had resulted only a mild virilizing effect. 1414 Zotter Z, Veszeli N, Csuka D, Varga L, Farkas H. Frequency of the virilising effects of attenuated androgens reported by women with hereditary angioedema. Orphanet J Rare Dis. 2014;9:205. Bork et al. reported that adverse events including hormonal disturbances, headache, weight gain, depression, and liver adenoma, resulted in discontinuation of therapy in 30 of 118 patients. They concluded that patients should be closely monitored due to the high risk of adverse events. 1515 Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100:153-61. Birjmohun et al. found no significant risk for atherosclerosis whereas Széplaki et al. recommended monitoring of lipid profiles to detect any development of early atherosclerosis. 1616 Birjmohun RS, Kees Hovingh G, Stroes ES, Hofstra JJ, Dallinga-Thie GM, Meijers JC, et al. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: two clinical trials in healthy volunteers and patients with hereditary angioedema. Clin Ther. 2008;30:2314-23.,1717 Széplaki G, Varga L, Valentin S, Kleiber M, Karádi I, Romics L, et al. Adverse effects of danazol prophylaxis on the lipid profiles of patients with hereditary angioedema J Allergy Clin Immunol. 2005;115:864-9. In a recent study, no hematological abnormalities, including erythrocytosis and/or polyglobulia, were detected during long-term danazol treatment.1818 Kőhalmi KV, Veszeli N, Zotter Z, Csuka D, Benedek S, Imreh É, et al. The effect of long-term danazol treatment on haematological parameters in hereditary angioedema. Orphanet J Rare Dis. 2016;11:18. Our observations on patients receiving a very long period of danazol therapy did not revealed hormonal adverse events, as well as hematological or lipid profile abnormalities.
Hepatotoxicity is a well-known adverse event of danazol that may cause development of adenomas, focal nodular hyperplasia, and rarely hepatocellular carcinoma.1919 Bork K, Schneiders V. Danazol-induced hepatocellular adenoma in patients with hereditary angio-oedema. J Hepatol. 2002;36:707-9.,2020 Helsing P, Nielsen EW. Hepatocellular focal nodular hyperplasia after danazol treatment for hereditary angio-oedema. Acta Derm Venereol. 2006;86:272-3. Risk for hemangioma development increases in patients receiving danazol more than 10 years. Besides, danazol induced adenomas were also observed even after discontinuing the therapy.1919 Bork K, Schneiders V. Danazol-induced hepatocellular adenoma in patients with hereditary angio-oedema. J Hepatol. 2002;36:707-9. Hepatocellular carcinoma is detected in very rare cases, especially in individuals treated for more than 10 years with high doses such as >200mg/d. 1919 Bork K, Schneiders V. Danazol-induced hepatocellular adenoma in patients with hereditary angio-oedema. J Hepatol. 2002;36:707-9. On the other hand, the lowest effective dose of danazol was not found to induce liver injury in HAE patients. 2121 Farkas H, Czaller I, Csuka D, Vas A, Valentin S, Varga L, et al. The effect of long-term danazol prophylaxis on liver function in hereditary angioedema-a longitudinal study. Eur J Clin Pharmacol. 2010;66:419-26. According to the World Allergy Organization (WAO) guideline for the management of hereditary angioedema, liver ultrasound is recommended to be performed before danazol therapy, yearly during therapy, and 6 months after ceasing therapy. 2222 Craig T, Aygören-Pürsün E, Bork K, Bowen T, Boysen H, Farkas H, et al. WAO Guideline for the Management of Hereditary Angioedema. World Allergy Organ J. 2012;5:182-99. We observed development of liver hemangioma and hepatosteatosis with normal liver structure in two patients. They were all very satisfied with control of attacks. Patients were recommended to come to their routine follow-up visits since they were on a minimum dose of danazol and switching to tranexamic acid did not work well in one of them.
Androgens have anticoagulant and fibrinolytic effects; however, a possible thrombogenic effect of exogenous androgens is an important concern. Androgens may affect platelet aggregation, coagulation, and facilitate thrombosis. 2323 Ferenchick GS. Anabolic/androgenic steroid abuse and thrombosis: is there a connection? Med Hypotheses. 1991;35:27-31. Although the link between thrombosis or premature cardiovascular disease and danazol is still unclear, danazol-induced thrombosis was reported in several cases.2424 Sahraian MA, Mottamedi M, Azimi AR, Moghimi B. Androgen-induced cerebral venous sinus thrombosis in a young body builder: case report. BMC Neurol. 2004;4:22.
25 Kumar GS, Roopesh Kumar VR, Gopalakrishnan MS, Shankar Ganesh CV, Venkatesh MS. Danazol-induced life-threatening cerebral venous thrombosis in a patient with aplastic anemia. Neurol India. 2011;59:762-4.-2626 Alvarado RG, Liu JY, Zwolak RM. Danazol and limb-threatening arterial thrombosis: two case reports. J Vasc Surg. 2001;34:1123-6. Danazol therapy was found to increase activation of coagulation in patients treated for more than two years. 1616 Birjmohun RS, Kees Hovingh G, Stroes ES, Hofstra JJ, Dallinga-Thie GM, Meijers JC, et al. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: two clinical trials in healthy volunteers and patients with hereditary angioedema. Clin Ther. 2008;30:2314-23. Depending on these reports, we did not initiate danazol prophylaxis in one patient having thrombotic cerebrovascular disease. Instead, she was put on weekly C1-INH concentrate infusions, which reduced the frequency of angioedema attacks. The efficacy of C1-INH concentrate is clearly demonstrated on both attack treatment and long-term prophylaxis in patients with HAE. 2727 Bork K, Craig TJ, Bernstein JA, Feuersenger H, Machnig T, Staubach P. Efficacy of C1 esterase inhibitor concentrate in treatment of cutaneous attacks of hereditary angioedema. Allergy Asthma Proc. 2015;36:218-24.,2828 Pedrosa M, Lobera T, Panizo C, Jurado J, Caballero T. Long-term prophylaxis with C1-inhibitor concentrate in patients with hereditary angioedema. J Investig Allergol Clin Immunol. 2014;24:271-3. Intravenous infusion of C1-INH concentrate, the only available and approved drug for HAE in Turkey, needs to be administered in health centers. Patient compliance is mandatory for the treatment. Self-administration of C1-INH concentrate at home needs training of patients and their relatives. 2222 Craig T, Aygören-Pürsün E, Bork K, Bowen T, Boysen H, Farkas H, et al. WAO Guideline for the Management of Hereditary Angioedema. World Allergy Organ J. 2012;5:182-99. Subcutaneous icatibant seems to be a relatively easier treatment for patients who live far away from health centers. On the other hand, for an easier administration, subcutaneous form of C1-INH concentrate is under investigation and phase II study results showed to be well tolerated and caused a dose-dependent increase in plasma levels. 2929 Zuraw BL, Cicardi M, Longhurst HJ, Bernstein JA, Li HH, Magerl M, et al. Phase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrate. Allergy. 2015;70:1319-28.
CONCLUSION
In the present study, we demonstrated a genetic mutation of C1-INH gene in a Turkish family with HAE and showed that long-term danazol prophylaxis might be effective for disease control. Patients with HAE may be overlooked in countries where FMF is endemic. Since phenotypic diversity may occur in the same family, clinicians should be aware of the signs and symptoms of HAE and consider it in the differential diagnosis to make an early diagnosis leading to prompt initiation of treatment.
-
*
Study conducted at the Ankara Ataturk Training and Education Hospital - Ankara, Turkey.
-
Financial support: None.
REFERENCES
-
1Kesim B, Uyguner ZO, Gelincik A, Mete Gökmen N, Sin AZ, Karakaya G, et al. The Turkish Hereditary Angioedema Pilot Study (TURHAPS): the first Turkish series of hereditary angioedema. Int Arch Allergy Immunol. 2011;156:443-50.
-
2Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K,et al. HAWK under the patronage of EAACI (European Academy of Allergy and Clinical Immunology). Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014;69:602-16.
-
3Zuraw BL. The Pathophysiology of Hereditary Angioedema. World Allergy Organ J. 2010;3:S25-8.
-
4Gompels MM, Lock RJ, Abinun M, Bethune CA, Davies G, Grattan C, et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005;139:379-94.
-
5Kalmár L, Hegedüs T, Farkas H, Nagy M, Tordai A. HAEdb: a novel interactive,locus-specific mutation database for the C1 inhibitor gene. Hum Mutat. 2005;25:1-5.
-
6Speletas M, Szilagyi A, Psarros F, Moldovan D, Magerl M, Kompoti M, et al. Hereditary angioedema: molecular and clinical differences among European populations. J Allergy Clin Immunol. 2015;135:570-3.
-
7Bors A, Csuka D, Varga L, Farkas H, Tordai A, Füst G, et al. Less severe clinical manifestations in patients with hereditary angioedema with missense C1INH gene mutations. J Allergy Clin Immunol. 2013;131:1708-11.
-
8Pappalardo E, Caccia S, Suffritti C, Tordai A, Zingale LC, Cicardi M. Mutation screening of C1 inhibitor gene in 108 unrelated families with hereditary angioedema: functional and structural correlates. Mol Immunol. 2008;45:3536-44.
-
9Büyüköztürk S, Eroğlu BK, Gelincik A, Uzümcü A, Ozşeker F, Colakoğlu B, et al. A Turkish family with a novel mutation in the promoter region of the C1 inhibitor gene. J Allergy Clin Immunol. 2009;123:962-4.
-
10Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angioedema: comparison of treated and untreated patients. J Allergy Clin Immunol. 1997;99:194-6.
-
11Sarici G, Koca R, Tekin NS, Altinyazar HC. A family with hereditary angioedema having been followed as familial Mediterranean fever. Turkderm 2009;43:29-31.
-
12Cugno M, Zanichelli A, Bellatorre AG, Griffini S, Cicardi M. Plasma biomarkers of acute attacks in patients with angioedema due to C1-inhibitor deficiency. Allergy. 2009;64:254-7.
-
13Reshef A, Zanichelli A, Longhurst H, Relan A, Hack CE. Elevated D-dimers in attacks of hereditary angioedema are not associated with increased thrombotic risk. Allergy. 2015;70:506-13.
-
14Zotter Z, Veszeli N, Csuka D, Varga L, Farkas H. Frequency of the virilising effects of attenuated androgens reported by women with hereditary angioedema. Orphanet J Rare Dis. 2014;9:205.
-
15Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100:153-61.
-
16Birjmohun RS, Kees Hovingh G, Stroes ES, Hofstra JJ, Dallinga-Thie GM, Meijers JC, et al. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: two clinical trials in healthy volunteers and patients with hereditary angioedema. Clin Ther. 2008;30:2314-23.
-
17Széplaki G, Varga L, Valentin S, Kleiber M, Karádi I, Romics L, et al. Adverse effects of danazol prophylaxis on the lipid profiles of patients with hereditary angioedema J Allergy Clin Immunol. 2005;115:864-9.
-
18Kőhalmi KV, Veszeli N, Zotter Z, Csuka D, Benedek S, Imreh É, et al. The effect of long-term danazol treatment on haematological parameters in hereditary angioedema. Orphanet J Rare Dis. 2016;11:18.
-
19Bork K, Schneiders V. Danazol-induced hepatocellular adenoma in patients with hereditary angio-oedema. J Hepatol. 2002;36:707-9.
-
20Helsing P, Nielsen EW. Hepatocellular focal nodular hyperplasia after danazol treatment for hereditary angio-oedema. Acta Derm Venereol. 2006;86:272-3.
-
21Farkas H, Czaller I, Csuka D, Vas A, Valentin S, Varga L, et al. The effect of long-term danazol prophylaxis on liver function in hereditary angioedema-a longitudinal study. Eur J Clin Pharmacol. 2010;66:419-26.
-
22Craig T, Aygören-Pürsün E, Bork K, Bowen T, Boysen H, Farkas H, et al. WAO Guideline for the Management of Hereditary Angioedema. World Allergy Organ J. 2012;5:182-99.
-
23Ferenchick GS. Anabolic/androgenic steroid abuse and thrombosis: is there a connection? Med Hypotheses. 1991;35:27-31.
-
24Sahraian MA, Mottamedi M, Azimi AR, Moghimi B. Androgen-induced cerebral venous sinus thrombosis in a young body builder: case report. BMC Neurol. 2004;4:22.
-
25Kumar GS, Roopesh Kumar VR, Gopalakrishnan MS, Shankar Ganesh CV, Venkatesh MS. Danazol-induced life-threatening cerebral venous thrombosis in a patient with aplastic anemia. Neurol India. 2011;59:762-4.
-
26Alvarado RG, Liu JY, Zwolak RM. Danazol and limb-threatening arterial thrombosis: two case reports. J Vasc Surg. 2001;34:1123-6.
-
27Bork K, Craig TJ, Bernstein JA, Feuersenger H, Machnig T, Staubach P. Efficacy of C1 esterase inhibitor concentrate in treatment of cutaneous attacks of hereditary angioedema. Allergy Asthma Proc. 2015;36:218-24.
-
28Pedrosa M, Lobera T, Panizo C, Jurado J, Caballero T. Long-term prophylaxis with C1-inhibitor concentrate in patients with hereditary angioedema. J Investig Allergol Clin Immunol. 2014;24:271-3.
-
29Zuraw BL, Cicardi M, Longhurst HJ, Bernstein JA, Li HH, Magerl M, et al. Phase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrate. Allergy. 2015;70:1319-28.
Publication Dates
-
Publication in this collection
Sep-Oct 2017
History
-
Received
08 Apr 2016 -
Accepted
13 July 2016