Abstract:
Background:
Urticarias are frequent diseases, with 15% to 20% of the population presenting at least one acute episode in their lifetime. Urticaria are classified in acute ( ≤ 6 weeks) or chronic (> 6 weeks). They may be induced or spontaneous.
Objectives:
To verify the diagnostic and therapeutic recommendations in chronic spontaneous urticaria (CSU), according to the experience of Brazilian experts, regarding the available guidelines (international and US).
Methods:
A questionnaire was sent to Brazilian experts, with questions concerning diagnostic and therapeutic recommendations for CSU in adults.
Results:
Sixteen Brazilian experts answered the questionnaire related to diagnosis and therapy of CSU in adults and data were analyzed. Final text was written, considering the available guidelines (International and US), adapted to the medical practices in Brazil. Diagnostic work up in CSU is rarely necessary. Biopsy of skin lesion and histopathology may be indicated to rule out other diseases, such as, urticarial vasculitis. Other laboratory tests, such as complete blood count, CRP, ESR and thyroid screening. Treatment of CSU includes second-generation anti-histamines (sgAH) at licensed doses, sgAH two, three to fourfold doses (non-licensed) and omalizumab. Other drugs, such as, cyclosporine, immunomodulatory drugs and immunosuppressants may be indicated (non-licensed and with limited scientific evidence).
Conclusions:
Most of the Brazilian experts in this study partially agreed with the diagnostic and therapeutic recommendations of the International and US guidelines. They agreed with the use of sgAH at licensed doses. Increase in the dose to fourfold of sgAH may be suggested with restrictions, due to its non-licensed dose. Sedating anti-histamines, as suggested by the US guideline, are indicated by some of the Brazilian experts, due to its availability. Adaptations are mandatory in the treatment of CSU, due to scarce or lack of other therapeutic resources in the public health system in Brazil, such as omalizumab or cyclosporine.
Keywords:
Cyclosporine; Dapsone; Histamine antagonists; Methotrexate; Omalizumab; Urticaria; Urticaria/etiology; Urticaria/therapy
INTRODUCTION
Urticaria is characterized by the rapid onset of hives (edema in superficial dermis), which may be accompanied by angioedema (edema of deep dermis, fat tissue and gastrointestinal tract).11 Zuberbier T, Greaves MW, Juhlin L, Kobza-Black A, Maurer D, Stingl G, et al. Definition, classification, and routine diagnosis of urticaria: a consensus report. J Investig Dermatol Symp Proc. 2001;6:123-7.,22 Cooper KD. Urticaria and angioedema: diagnosis and evaluation. J Am Acad Dermatol. 1991;25:166-74. Hive, the dermatological lesion, consists of three typical features: (i) central edema of varying size, surrounded by reflex erythema; (ii) associated pruritus; and (iii) transient nature, with the skin returning to its normal appearance usually in a period ranging from 1 to 24 hours.11 Zuberbier T, Greaves MW, Juhlin L, Kobza-Black A, Maurer D, Stingl G, et al. Definition, classification, and routine diagnosis of urticaria: a consensus report. J Investig Dermatol Symp Proc. 2001;6:123-7.,22 Cooper KD. Urticaria and angioedema: diagnosis and evaluation. J Am Acad Dermatol. 1991;25:166-74. Angioedema is defined by: (i) sudden and marked edema of the deep dermis and and fat tissue; (ii) greater frequency of pain other than pruritus; (iii) frequent involvement of mucous membranes; and (iv) resolution of the condition at approximately 72 hours, slower than with hives.11 Zuberbier T, Greaves MW, Juhlin L, Kobza-Black A, Maurer D, Stingl G, et al. Definition, classification, and routine diagnosis of urticaria: a consensus report. J Investig Dermatol Symp Proc. 2001;6:123-7.
2 Cooper KD. Urticaria and angioedema: diagnosis and evaluation. J Am Acad Dermatol. 1991;25:166-74.-33 Criado PR, Criado RF, Maruta CW, Reis VM. Chronic urticaria in adults: state-of-the-art in the new millennium. An Bras Dermatol. 2015;90:74-89.
Urticaria is classified by progression as acute (up to 6 weeks) or chronic (beyond 6 weeks of clinical course).44 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868-87.,55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
1. Guidelines of the International Urticaria Consensus
(EAACI/GA2LEN/EDF/WAO (European Academy of Allergology and Clinical Immunology/The Global Allergy and Asthma European Network/The European Dermatology Forum/World Allergy Organization), with participation of the Brazilian Society of Dermatology.
The Guideline of the International Urticaria Consensus of the EAACI/GA2LEN/EDF/WAO (European Academy of Allergology and Clinical Immunology/The Global Allergy and Asthma European Network/The European Dermatology Forum/World Allergy Organization), published in 2018, was the result of a systematic with participation of experts, from several medical societies.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. The quality of the scientific evidence was assessed per the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) method using the GRADEpro Guideline Development Tool (GDT).55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
CLASSIFICATION55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
Chronic urticaria (CU) is subdivided into two types: “chronic spontaneous urticaria” (CSU, which is represented by urticaria with hives and/or angioedema of spontaneous onset, with an evolution of over 6 weeks, due to a known cause, such as autoreactivity, resulting from mast cells that are activated by autoantibodies, or unknown causes) and “induced urticarias” (symptomatic dermographism, cold urticaria, delayed pressure urticaria, solar urticaria, heat urticaria, vibratory angioedema, cholinergic urticaria, and aquagenic urticaria).
In this classification, conditions or diseases that may manifest with urticaria or angioedema, such as urticarial vasculitis, urticaria pigmentosa, autoinflammatory syndromes (in general, periodic syndromes cryopyrin-associated or Schnitzler syndrome), exercise-induced anaphylaxis, Gleich syndrome (episodic angioedema with eosinophilia), Wells syndrome (eosinophilic cellulitis), bullous pemphigoid prior to bullous lesions, angioedema mediated by non-mast cell mediators (in general, bradykinin-mediated angioedema), and other similar diseases, are not considered urticaria subtypes due to their different pathophysiological mechanisms.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
DIAGNOSTIC APPROACH TO CHRONIC URTICARIA55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
The diagnostic approach was recommended to meet three main objectives: (i) to exclude differential diagnoses, (ii) to assess disease activity and its impact and control, and (iii) to identify triggering or exacerbating agents or, where indicated, any underlying cause. The initial evaluation of patients with CSU should assess the disease activity with tools to which the patient responds (UAS, AAS) and questionnaires on quality of life (CU-Q2oL, AE-QoL) and disease control (UCT), which are indispensable to evaluate impact of the disease, to guide therapy, to help standardization of patient data in the follow-up. It should be emphasized that CSU has an impact in quality of life and a financial impact due to its prolonged treatment.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
6 O'Donnell BF, Lawlor F, Simpson J, Morgan M, Greaves MW. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136:197-201.
7 Parisi CA, Ritchie C, Petriz N, Morelo Torres C. Direct Medical Costs of Chronic Urticaria in a Private Health Organization of Buenos Aires, Argentina. Value Health Reg Issues. 2016;11:57-9.
8 Delong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic urticaria: a cost analysis of 50 nonimmunosuppressed patients. Arch Dermatol. 2008;144:35-9.
9 Williams PV, Kavati A, Pilon D, Xiao Y, Zhdanava M, Balp MM,et al. Treatment Patterns, Healthcare Resource Utilization, and Spending Among Medicaid-Enrolled Children with Chronic Idiopathic/Spontaneous Urticaria in the United States. Dermatol Ther (Heidelb). 2018;8:69-83.
10 Broder MS, Raimundo K, Antonova E, Chang E. Resource use and costs in an insured population of patients with chronic idiopathic/spontaneous urticaria. Am J Clin Dermatol. 2015;16:313-21.
11 O'Donnell BF. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014;34:89-104.
12 Al-Ahmad M, Alowayesh MS, Carroll NV. Economic burden of refractory chronic spontaneous urticaria on Kuwait's health system. Clinicoecon Outcomes Res. 2016;8:163-9.-1313 Graham J, McBride D, Stull D, Halliday A, Alexopoulos ST, Balp MM, et al. Cost Utility of Omalizumab Compared with Standard of Care for the Treatment of Chronic Spontaneous Urticaria. Pharmacoeconomics. 2016;34:815-27.
A medical history is essential in patients with urticaria, because of variable triggering and exacerbating factors.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Not all factors that are described as causative agents in CU should be investigated in all patients. The first step in the diagnosis is a detailed clinical history that takes into account the following questions:55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
-
Time of disease onset
-
Shape, size, frequency, duration, and distribution of hives/angioedema
-
Association with angioedema
-
Associated symptoms, such as bone or joint pain, fever, and abdominal pain
-
Personal and family history of hives and angioedema
-
Induction by physical agents or exercise
-
Occurrence in relation to time of day, weekend, menstrual cycle, holidays, and trips to countries abroad
-
Occurrence in relation to foods or medications (non-hormonal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors)
-
Occurrence in relation to infections or emotional stress
-
Prior or concurrent allergies, infections, internal or autoimune diseases, gastrointestinal problems, or other disorders
-
Social and occupational history, leisure activities
-
Previous treatments and response to treatments, including doses and duration of use
-
Previous diagnostic procedures and their results.
The second step in the diagnosis is to perform a detailed physical examination of the patient.5 Considering data from the history and physical examination, additional laboratory work up may be requested.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Full blood count, ESR (erythrocyte sedimentation rate), and C-reactive protein (CRP) levels are routinely measured.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. An extended research panel, based on the anamnesis for identifying the underlying causes or inducing factors and for excluding differential diagnoses, may be indicated if there are relevant data from the medical history or physical examination and should include the following measures: 1. suspected triggers (e.g., medications); 2. screening for infectious agents (e.g., Helicobacter pylori); 3. thyroid diseases (thyroid hormones and autoantibodies); 4. allergy (intradermal tests and tests to exclude allergens, in general, restriction diet); 5. presence of associated induced-urticaria; 6. associated systemic disease (e.g., serum tryptase levels); and 7. others (e.g., histopathology of skin lesion).55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
The frequency and relevance of infections vary considerably between patient groups and different areas.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Exclusion of malignancies with examinations is indicated only if the patient’s history implicates this possibility (in general, sudden and relevant weight loss).55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
Plasma D-dimer levels are significantly higher in patients with active CSU and decrease according to the clinical response to treatment with omalizumab. 55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. The recommendation on measuring D-dimer levels in all patients with CSU is still debated.
EVALUATION OF IMPACT OF CSU ACTIVITY AND ITS CONTROL55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
CSU activity may be evaluated using a simple unified validated system, the UAS7 score44 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868-87.,55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. UAS7 is based on the evaluation of key properties of urticaria, its signs (hives), and its symptoms (pruritus), which are documented by the patient.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. UAS7 consists of self-assessment over a 24-hour period once a day for several days, with summing the daily scores over 7 days. Maximum score each for daily hives and symptom intensity is 3, yielding a daily total score of between 0 and 6 and a weekly score of between 0 and 42 (Chart 1 and Figure 1). UAS7 should be performed in the week prior to medical consultation. It is a valuable tool for clinical evaluation of CSU.
The urticaria control test (UCT), in addition to UAS7, has become important in assessing the impact of the disease on quality of life and disease control, both in clinical practice and in research protocols.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. UCT was developed and validated to determine the level of disease control for all forms of urticaria (CSU and induced urticaria), because UAS does not evaluate angioedema or induced urticarias.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. UCT is composed of only four items, defining the limit between “well-controlled disease” and “poorly controlled disease;” thus, it is usable in clinical practice, with a cutoff point for “well-controlled disease” ranging from 12 and 16.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. The score varies from 0 to 16 and higher values reflect better disease control. It is an instrument that helps guide therapeutic decisions. 55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
UCT is under validation in Brazil by the Department of Allergy and Immunology of UFRJ, and the Portuguese version is described in chart 2.
MANAGEMENT OF PATIENTS WITH URTICARIA 55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
Three basic considerations are proposed in the treatment of these patients:
-
The goal is to treat urticaria until it enters remission
-
The therapeutic approach involves several aspects, such as:
-
Identification and elimination of underlying causes when possible
-
Eliminate triggering factors
-
Induction of tolerance in induced urticaria when possible
-
Use of pharmacological agents in the prevention of degranulation and the release of mast cell mediators and their effects
-
The treatment should follow the basic principles of treating as much as necessary and as little as possible; e.g., advancing in steps or retroactively in stages in the therapeutic escalation according to the course of disease.
-
In order to eliminate an underlying cause, an accurate diagnosis is necessary.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. The identification of a cause for CU, however, is difficult in most cases; for example, with infections, which may be a cause or an aggravating factor or have no relation to CU.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. The only definitive proof of the causal nature of a suspected or triggering agent is the remission of symptoms following its removal and their recurrence following re-exposure in a double-blind challenge.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. In practice, this approach is often not feasible. Spontaneous remission of urticaria may occur over time. Urticaria may go into remission with the elimination of a suspected cause or triggering factor coincidentally, without any cause-effect relationship.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
Pharmacological treatment of CSU has, as main goal, to relieve symptoms by reducing the effects of mast cell mediators, such as histamine and platelet-activating factor (PAF) and others, on target organs and tissues.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Many symptoms of urticaria are mediated primarily by the actions of histamine on H1 receptors on endothelial cells (resulting in hives) and sensory nerves (neurogenic erythema and pruritus).55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Thus, continuous treatment with antihistamines is fundamental in the treatment of urticaria; safety data are available for continuous use over several years.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
Other mast cell mediators (PAF, leukotrienes, and cytokines) may be involved, and a pronounced cellular infiltrate, including basophils, lymphocytes, and eosinophils, can be seen in the lesions.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. These patients may respond completely to a brief course of corticosteroids and be relatively refractory to antihistamines.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
First-generation antihistamines have prominent anticholinergic effects and sedative actions on the central nervous system (CNS) and have many interactions with alcohol and drugs that affect the CNS, such as analgesics, hypnotics, sedatives, and mood-altering substances. 55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. They can interfere with rapid eye movement sleep (REM sleep) and impact learning and cognitive performance.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Interference with the CNS is observed especially in multiple concurrent tasks and the performance of complex sensorimotor tasks, such as driving vehicles,55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. and should be indicated with caution. First-generation antihistamines with more pronounced adverse effects are promethazine, diphenhydramine, ketotifen, and chlorpheniramine.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
The recommended treatment algorithm for CU, per the 2018 International Guideline, is summarized in figure 2.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
INTERNATIONAL GUIDELINE 2018 - TREATMENT
First-line pharmacological treatment:
Oral antihistamines are key drugs in the treatment of chronic urticaria, especially nonsedating and low-sedating agents: H1 receptor antagonists, such as cetirizine, fexofenadine, loratadine, and ebastine and, more recently, levocetirizine, desloratadine, rupatadine, epinastine and bilastine.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Their efficacies are similar; however, due to the absence of hepatic metabolism, fexofenadine, desloratadine, and bilastine are indicated in liver diseases.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
Second-line pharmacological treatment:
A retrospective study with 549 CSU patients showed that more than 75% of subjects were refractory to first-line treatment with second-generation anti-H1 drugs, with only 31.8% of patients attaining complete control of disease with the use of the licensed doses. UAS7 was the only predictor of refractoriness to treatment with anti-H1.1414 Curto-Barredo L, Archilla LR, Vives GR, Pujol RM, Giménez-Arnau AM. Clinical Features of Chronic Spontaneous Urticaria that Predict Disease Prognosis and Refractoriness to Standard Treatment. Acta Derm Venereol. 2018;98:641-7
According to the Urticaria International Guideline, as second-line of treatment, use of up to fourfold doses of second-generation antihistamines is indicated, whenever licensed dose failed to control the disease. 44 Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868-87.,55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. The use of these drugs at maximum doses, such as desloratadine 20 mg/day, levocetirizine 20 mg/day, loratadine 40 mg/day, and cetirizine 40 mg/day, is not yet approved in Brazil, despite published international scientific literature.1515 Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H1-antihistamine up-dosing in chronic spontaneous urticaria: patients' perspective of effectiveness and side effects--a retrospective survey study. PLoS One. 2011;6:e23931. Due to their safety profile, in CSU patients without arrhythmia, without nephropathy or hepatopathy, these drugs may be indicated, with minimal side effects and increased efficacy, when doses are increased.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.,1616 Zuberbier T. Pharmacological rationale for the treatment of chronic urticaria with second-generation non-sedating antihistamines at higher-than-standard doses. J Eur Acad Dermatol Venereol. 2012 ;26:9-18.,1717 Ortonne JP. Urticaria and its subtypes: the role of second-generation antihistamines. Eur J Intern Med. 2012;23:26-30. Liver enzymes monitoring is indicated during this type of therapeutic approach, with careful patient orientation.33 Criado PR, Criado RF, Maruta CW, Reis VM. Chronic urticaria in adults: state-of-the-art in the new millennium. An Bras Dermatol. 2015;90:74-89.
Although antihistamines achieve CSU control when used at up to 4-fold the licensed doses, in many patients with CSU, alternative treatments may be required. Before changing the treatment to alternative therapies (adjuvants), it is recommended to wait 1-4 weeks to achieve complete effectiveness of the drugs that are in use.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
Because the severity of urticaria may vary and because spontaneous remission may occur over time, therapeutic re-evaluation of the need for continued treatment or a separate or adjunctive treatment is also recommended every 3 to 6 months.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
Third-line pharmacological treatment:
In recent years, the use of biological agents for urticaria, particularly omalizumab, has become more prominent as a third-line agent in chronic urticaria that is refractory to initial approaches (first-and second-lines of treatment).1818 Asero R, Tedeschi A, Cugno M. Treatment of refractory chronic urticaria: current and future therapeutic options. Am J Clin Dermatol. 2013;14:481-8 Omalizumab is a humanized monoclonal antibody against the cε3 domain of IgE, which lies near the binding site for FcεRI receptors on mast cells and basophils and FcεRII.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. The doses of omalizumab that have been used in several studies for chronic refractory urticaria have ranged from 150-300mg subcutaneously, once a month; ideal dose for urticaria is 300mg every 4 weeks.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.On average, half of all patients (52%) controlled their urticaria (UAS7 ≤ 6) after the 12th week versus 62% after the 24th week in phase III studies of the molecule; 11% did not respond to treatment.1919 Zhao ZT, Ji CM, Yu WJ, Meng L, Hawro T, Wei JF, et al. Omalizumab for the treatment of chronic spontaneous urticaria: A meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2016;137:1742-50.e4 Initial treatment should be continued for 24 weeks.1919 Zhao ZT, Ji CM, Yu WJ, Meng L, Hawro T, Wei JF, et al. Omalizumab for the treatment of chronic spontaneous urticaria: A meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2016;137:1742-50.e4 The total treatment duration for chronic urticaria has not been established; thus, the decision to discontinue treatment should be individualized.
The proposed mechanism of action for this drug is based on the finding that when there are high circulating IgE levels in the blood, mast cells and basophils express higher amounts of FcεRI receptors on their membranes, becoming vulnerable to binding with anti-FcεRIα IgG autoantibodies. Doses of omalizumab in CSU independent of serum IgE levels. The approved doses, and the treatment duration may vary by country.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. In Brazil, omalizumab was approved for CSU in children aged over 12 years, at 300 mg subcutaneously every 4 weeks for 6 consecutive months.
Treatment of Exacerbations
Oral corticosteroids, particularly prednisone at doses of 20-50mg per day, may be necessary for short periods of use (7 days, maximum 10 days) for significant exacerbations of chronic urticaria that does not respond completely to antihistamines or for sporadic episodes of exacerbation. 55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.Prolonged use should be avoided due to the side effects and development of comorbidities.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Their use should be avoided for more than 7 days each month. There is a strong recommendation for only using systemic corticosteroids under specialist supervision in the treatment of CSU.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
Fourth-line pharmacological treatments
Despite the absence of strong published scientific evidence, all fourth-line drugs may be valuable for patients in certain cases of refractoriness in the earlier stages, in the appropriate clinical settings.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
In patients with severe disease and persistent progression, with treatment failure to previous measures, cyclosporine therapy is an option to refractory CSU. Cyclosporine acts by inhibiting IL-2 production in lymphocytes. In urticaria, it is believed that an additional mechanism of action is its reduction of immunoglobulin production and reduction of the high-affinity IgE receptor. It has been studied in cohorts and placebo-controlled studies at doses of 1 to 5mg/kg/day. The effective dose in chronic urticaria appears to be 3 mg/kg/day for periods of 8 to 16 weeks, yielding success rates of 64% to 95%.2020 Koski R, Kennedy KK. Treatment with omalizumab or cyclosporine for resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2017;119:397-401.,2121 Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for Chronic Spontaneous Urticaria: A Meta-Analysis and Systematic Review. J Allergy Clin Immunol Pract. 2018;6:586-99. It is important to emphasize that before use, patients should perform blood pressure measures and evaluate renal function, magnesium, uric acid, and potassium; these tests should be repeated periodically.2020 Koski R, Kennedy KK. Treatment with omalizumab or cyclosporine for resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2017;119:397-401. Side effects appear to be dose-dependent and occur in more than half of all patients who are treated with moderate doses (4 to 5mg/kg/day).2121 Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for Chronic Spontaneous Urticaria: A Meta-Analysis and Systematic Review. J Allergy Clin Immunol Pract. 2018;6:586-99.
Other Treatments
Other non-licensed medications should be used in patients in whom previous steps of treatment failed. They have a low level of recommendation and have only been presented in case reports and studies of small series.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
-
Anti-inflammatory drugs: dapsone, colchicine, and montelukast are medications that present clinical studies with low scientific evidence.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.,2222 Criado RF, Criado PR, Martins JE, Valente NY, Michalany NS, Vasconcellos C. Urticaria unresponsive to antihistaminic treatment: an open study of therapeutic options based on histopathologic features. J Dermatolog Treat. 2008;19:92-6.
23 Morgan M, Cooke A, Rogers L, Adams-Huet B, Khan DA. Double-blind placebo-controlled trial of dapsone in antihistamine refractory chronic idiopathic urticaria. J Allergy Clin Immunol Pract. 2014;2:601-6.-2424 Pho LN, Eliason MJ, Regruto M, Hull CM, Powell DL. Treatment of chronic urticaria with colchicine. J Drugs Dermatol. 2011;10:1423-8. Montelukast showed good response in 20% to 50% of patients who did not responded to therapy with antihistamines alone.2525 Tedeschi A, Airaghi L, Lorini M, Asero R. Chronic urticaria: a role for newer immunomodulatory drugs? Am J Clin Dermatol. 2003;4:297-305 Reeves et al.2626 Reeves GE, Boyle MJ, Bonfield J, Dobson P, Loewenthal M. Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J. 2004;34:182-6. studied 18 CU patients who had been treated with hydroxychloroquine for 12 weeks, noting disease control and improved quality of life. This drug is relatively safe, but the possibility of retinopathy should be monitored. -
Other Immunosuppressants
-
- Methotrexate has been used at a mean weekly dose of 15 mg.2727 Sagi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91:303-6. This drug has anti-inflammatory and immunomodulatory properties, and its mechanism of action comprises an increase in adenosines, apoptosis of CD4 lymphocytes, and inhibition of neutrophil chemotaxis.2727 Sagi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91:303-6.,2828 Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-4.
-
- Other oral drugs, with immunomodulatory and immunossupressive effects, such as sulfasalazine, mycophenolate mofetil, azathioprine, cyclophosphamide, and tacrolimus. are available for use in CSU.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. There are no controlled studies, with relevant number of patients and efficacy; they mau be used as alternatives on failure with conventional therapy.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
-
-
Other Immunobiologicals:
-
- Anti-tumor necrosis factor (TNF) medications are indicated in CU due to increased production of TNF, implicated in the pathogenesis of the disease. Etanercept, infliximab, and adalimumab have been used in case reports and small series for various types of urticaria.2929 Ferrer M, Madamba R. Biologics in chronic urticaria. Allergol Immunopathol (Madr). 2017;45 (Suppl 1):S41-4.
-
- Interleukin 1 antagonists (anti-IL-1: canakinumab, anakinra), although formally indicated for autoimmune diseases, have been used in urticaria due to inflammatory cytokine production in the disease.2929 Ferrer M, Madamba R. Biologics in chronic urticaria. Allergol Immunopathol (Madr). 2017;45 (Suppl 1):S41-4. Canakinumab is under investigation in a placebo-controlled study, but the results have not been made available.2929 Ferrer M, Madamba R. Biologics in chronic urticaria. Allergol Immunopathol (Madr). 2017;45 (Suppl 1):S41-4.
-
- Rituximab (anti-CD20) is a chimeric monoclonal antibody against CD20, expressed on B cells, that decreases autoantibody production. The recommended dose is 375mg/m2 There are few case reports using this drug.2929 Ferrer M, Madamba R. Biologics in chronic urticaria. Allergol Immunopathol (Madr). 2017;45 (Suppl 1):S41-4.
-
- Intravenous immunoglobulin (IVIg) is an IgG purified polyclonal preparation derived from the plasma of several donors. In urticaria, IVIg has an immunomodulatory effect decreasing IgG anti-FcεRII and IgG anti-IgE.2929 Ferrer M, Madamba R. Biologics in chronic urticaria. Allergol Immunopathol (Madr). 2017;45 (Suppl 1):S41-4. Studies with series of patients showed improved response at a dose of 0.4mg/kg/day for 5 days. It may lead to rare side effects, such as kidney failure and anaphylactic reactions.2929 Ferrer M, Madamba R. Biologics in chronic urticaria. Allergol Immunopathol (Madr). 2017;45 (Suppl 1):S41-4.
-
US GUIDELINE 2014- TREATMENT3030 Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-7.
First-line treatment
The US Guideline for CSU, published in 2014, indicates use of second-generation anti-histamines (sgAH) at licensed doses as first-line of treatment.
Second-line of treatment
As second-line of treatment up-dosing (up to 4-fold) of second-generation antihistamines is indicated; furthermore, add other sgAH, H2-antagonists, anti-leukotriene (montelukast) or first-generation antihistamines at bedtime.
Third-line treatment
If there is no control with the previous steps, hydroxyzine or doxepin are indicated, as third-line treatment.
Fourth-line treatment
As fourth-line of treatment, omalizumab or cyclosporine are indicated, as well as, other anti-inflammatory drugs, immunossupressants ou immunobiological drugs (dapsone, sulfasalazine, hydroxychloroquine, coclchicine, tacrolimus, mycophenolate mofetil, sirolimus, cyclophosphamide, methotrexate, IVIg, anti-TNF, anti-IL-1 receptor and anti-CD20.3131 Antia C, Baquerizo K, Korman A, Alikhan A, Bernstein JA. Urticaria: A comprehensive review: Treatment of chronic urticaria, special populations, and disease outcomes. J Am Acad Dermatol. 2018;79:617-33.
Figure 3 shows the differences between the Internacional and US Guidelines in CSU.3232 Zuberbier T, Bernstein JA. A Comparison of the United States and International Perspective on Chronic Urticaria Guidelines. J Allergy Clin Immunol Pract. 2018;6:1144-51.
Pregnancy and lactation
Regarding treatment during gestation, to date, there are no reports of congenital defects in women who have used second-generation anti-H1 antihistamines during pregnancy.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. However, few studies are available regarding the use of cetirizine,3333 Weber-Schoendorfer C, Schaefer C. The safety of cetirizine during pregnancy. A prospective observational cohort study. Reprod Toxicol. 2008;26:19-23. and a large meta-analysis has examined the use of loratadine.3434 Schwarz EB, Moretti ME, Nayak S, Koren G. Risk of hypospadias in offspring of women using loratadine during pregnancy: a systematic review and meta-analysis. Drug Saf. 2008;31:775-88. Loratadine is metabolized in the liver, whereas desloratadine is not.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Due to safety profile, the preferred second-generation anti-histamines in pregnacy are loratadine, (with possible extrapolation to desloratadine) and cetirizine (with possible extrapolation to levocetirizine).55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. All anti-H1 antihistamines are excreted in human milk at low concentrations.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. First-generation anti-H1s should be avoided during breastfeeding.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. Omalizumab use in pregnancy has shown no evidence of maternal or fetal harm.55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.,3535 Namazy J, Cabana MD, Scheuerle AE, Thorp JM Jr, Chen H, Carrigan G, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135:407-12.
36 Ghazanfar MN, Thomsen SF. Successful and Safe Treatment of Chronic Spontaneous Urticaria with Omalizumab in a Woman during Two Consecutive Pregnancies. Case Rep Med. 2015;2015:368053.-3737 Wood RA, Khan DA, Lang DM, Fasano MB, Peden DB, Busse PJ, et al. AAAAI Response to the EAACI/GA(2)LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria 2017 Revision. Allergy. 2018 [Epub ahead of print].
Data obtained from the questionnaire sent to Brazilian experts
Sixteen specialists answered the questions that were sent about the number of patients who were seen in clinical practice, diagnostic procedures, and the treatment of CSU (Chart 3). Data concerning the questionnaire are summarized in table 1. The number of CSU patients who were seen by the participants varied: 7 participants (44%) attended up to 10 patients/month, 1 (6%) between 11-20 patients/month, 7 (44%) between 21-50 patients/month, and 1 (6%) between 51-100 patients/month, most of whom were part of public services. Participants reported that they conducted their CSU patients based on published treatment protocols in the international literature-63% in the International Guideline,55 Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414. and 13% in the US Guideline-but 50% of participants made use of recommendations from various treatment protocols or a combination of them.
Questionnaire on chronic spontaneous urticaria (CSU): Diagnosis and treatment - Brazilian Society of Dermatology
Laboratory tests were requested as required in the examination of CSU by 81% of participants; 19% did not request any tests. The most frequently requested tests (>50%) were full blood count, ESR, CRP, free T4, and TSH. The least commonly requested tests (50%) were autoantibodies to thyroid, stool parasitology, plasma D-dimer levels, hepatitis serology (particularly hepatitis B and C), ANA, total complement and fractions, total serum IgE, liver enzymes (AST, ALT, ALP, and gamma GT), renal analysis (urea and creatinine), and chest X-ray. Skin biopsy of the urticarial lesion and histopathology were indicated by 94% of participants in treatment-refractory patients or on suspicion of urticarial vasculitis, of whom 38% also performed direct immunofluorescence.
In the public service, within the treatment options for CSU, 81% of participants used non-sedating antihistamines (second-generation) as the initial option, versus 19% who administered sedating antihistamines (first-generation). As the second therapeutic option, 81% offered non-sedating antihistamines at doses of 1 to 4-folds the licensed dose; 13% used sedating antihistamines, and 6% indicated methotrexate, in addition to antihistamine treatment. As subsequent options, the use of cyclosporine, methotrexate, dapsone, montelukast, cyclosporine, systemic corticosteroids (short-term), and omalizumab were reported.
In private care, of the therapeutic options that are offered to patients with CSU, 94% used non-sedating antihistamines as the first option versus 6% for sedating antihistamines. As the second option, 81% administered non-sedating antihistamines at doses of 1 to 4-folds the licensed dose, compared with 13% for sedating antihistamines and 6% for omalizumab. Subsequent options included cyclosporine, dapsone, systemic corticosteroid (short-term), methotrexate and montelukast.
Regarding the side effects of medications, 56% of participants questioned the safety of continuous prescription of sedating antihistamines (first-generation). Further, 81% of participants opined that the prescription of non-sedating antihistamines at doses higher than those licensed was safe.
Regarding the indications for omalizumab use, when the antihistamines that were used did not show significant efficacy, 69% of participants favored its use, despite its high cost.
For other treatment options, after non-sedating antihistamines and omalizumab, participants indicated dapsone, colchicine, methotrexate, H2 antihistamines, cyclosporine, and montelukast.
Regarding lifestyle habits for CSU patients, 81% of participants suggested that patients should avoid use of nonhormonal anti-inflammatory drugs; 50% advised that foods with dyes and preservatives should be avoided; 75% questioned patients about perimenstrual aggravation (autoimmune progesterone dermatitis in the differential diagnosis); 13% inquired about the possibility of infestation by Toxocara canis (toxocariasis) due to the presence of domestic animals; and 94% considered the association with other general symptoms, such as fever and arthralgia.
For the evaluation of CSU activity, 62% of participants indicated the use of the UAS-7 form but noted inadequate comprehension and completion by many patients with CSU.
CONCLUSIONS
CSU treatment is constant evolving and remains a permanent challenge in most patients. This position paper, from sixteen Brazilian experts, based on data from literature and the International and US Guidelines, made the following recommendations to tailor it to actual clinical practice in Brazil in the public and private service:
-
There is no need for extensive work up in CSU if medical history and physical examination do not address the need for further laboratory testing other than general laboratory evaluation.
-
Antihistamine treatment should be continuous and always aim for complete disease control (UAS7=0) or UCT>12.
-
The use of medications at non-licensed doses, especially second-generation antihistamines, is supported by the literature; however, patients should understand and accept to use them. Monitoring cardiological and hepatic parameters (in antihistamines with liver metabolism) are always indicated.
-
Omalizumab is a safe and approved drug for use in CSU that is refractory, as adjuvant therapy to antihistamines anti-H1; its indications should be under the guidance of a team of experts in urticaria.
-
Other adjuvant drugs are available for off-label use in Brazil; thus, they may be useful and necessary for refractory cases of CSU, with strict clinical and laboratory monitoring and information of the possible benefits and risks when using them.
-
*
Work conducted at the Sociedade Brasileira de Dermatologia, Rio de Janeiro (RJ), Brazil.
-
Financial Support: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Code 001.
REFERENCES
-
1Zuberbier T, Greaves MW, Juhlin L, Kobza-Black A, Maurer D, Stingl G, et al. Definition, classification, and routine diagnosis of urticaria: a consensus report. J Investig Dermatol Symp Proc. 2001;6:123-7.
-
2Cooper KD. Urticaria and angioedema: diagnosis and evaluation. J Am Acad Dermatol. 1991;25:166-74.
-
3Criado PR, Criado RF, Maruta CW, Reis VM. Chronic urticaria in adults: state-of-the-art in the new millennium. An Bras Dermatol. 2015;90:74-89.
-
4Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868-87.
-
5Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393-414.
-
6O'Donnell BF, Lawlor F, Simpson J, Morgan M, Greaves MW. The impact of chronic urticaria on the quality of life. Br J Dermatol. 1997;136:197-201.
-
7Parisi CA, Ritchie C, Petriz N, Morelo Torres C. Direct Medical Costs of Chronic Urticaria in a Private Health Organization of Buenos Aires, Argentina. Value Health Reg Issues. 2016;11:57-9.
-
8Delong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic urticaria: a cost analysis of 50 nonimmunosuppressed patients. Arch Dermatol. 2008;144:35-9.
-
9Williams PV, Kavati A, Pilon D, Xiao Y, Zhdanava M, Balp MM,et al. Treatment Patterns, Healthcare Resource Utilization, and Spending Among Medicaid-Enrolled Children with Chronic Idiopathic/Spontaneous Urticaria in the United States. Dermatol Ther (Heidelb). 2018;8:69-83.
-
10Broder MS, Raimundo K, Antonova E, Chang E. Resource use and costs in an insured population of patients with chronic idiopathic/spontaneous urticaria. Am J Clin Dermatol. 2015;16:313-21.
-
11O'Donnell BF. Urticaria: impact on quality of life and economic cost. Immunol Allergy Clin North Am. 2014;34:89-104.
-
12Al-Ahmad M, Alowayesh MS, Carroll NV. Economic burden of refractory chronic spontaneous urticaria on Kuwait's health system. Clinicoecon Outcomes Res. 2016;8:163-9.
-
13Graham J, McBride D, Stull D, Halliday A, Alexopoulos ST, Balp MM, et al. Cost Utility of Omalizumab Compared with Standard of Care for the Treatment of Chronic Spontaneous Urticaria. Pharmacoeconomics. 2016;34:815-27.
-
14Curto-Barredo L, Archilla LR, Vives GR, Pujol RM, Giménez-Arnau AM. Clinical Features of Chronic Spontaneous Urticaria that Predict Disease Prognosis and Refractoriness to Standard Treatment. Acta Derm Venereol. 2018;98:641-7
-
15Weller K, Ziege C, Staubach P, Brockow K, Siebenhaar F, Krause K, et al. H1-antihistamine up-dosing in chronic spontaneous urticaria: patients' perspective of effectiveness and side effects--a retrospective survey study. PLoS One. 2011;6:e23931.
-
16Zuberbier T. Pharmacological rationale for the treatment of chronic urticaria with second-generation non-sedating antihistamines at higher-than-standard doses. J Eur Acad Dermatol Venereol. 2012 ;26:9-18.
-
17Ortonne JP. Urticaria and its subtypes: the role of second-generation antihistamines. Eur J Intern Med. 2012;23:26-30.
-
18Asero R, Tedeschi A, Cugno M. Treatment of refractory chronic urticaria: current and future therapeutic options. Am J Clin Dermatol. 2013;14:481-8
-
19Zhao ZT, Ji CM, Yu WJ, Meng L, Hawro T, Wei JF, et al. Omalizumab for the treatment of chronic spontaneous urticaria: A meta-analysis of randomized clinical trials. J Allergy Clin Immunol. 2016;137:1742-50.e4
-
20Koski R, Kennedy KK. Treatment with omalizumab or cyclosporine for resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2017;119:397-401.
-
21Kulthanan K, Chaweekulrat P, Komoltri C, et al. Cyclosporine for Chronic Spontaneous Urticaria: A Meta-Analysis and Systematic Review. J Allergy Clin Immunol Pract. 2018;6:586-99.
-
22Criado RF, Criado PR, Martins JE, Valente NY, Michalany NS, Vasconcellos C. Urticaria unresponsive to antihistaminic treatment: an open study of therapeutic options based on histopathologic features. J Dermatolog Treat. 2008;19:92-6.
-
23Morgan M, Cooke A, Rogers L, Adams-Huet B, Khan DA. Double-blind placebo-controlled trial of dapsone in antihistamine refractory chronic idiopathic urticaria. J Allergy Clin Immunol Pract. 2014;2:601-6.
-
24Pho LN, Eliason MJ, Regruto M, Hull CM, Powell DL. Treatment of chronic urticaria with colchicine. J Drugs Dermatol. 2011;10:1423-8.
-
25Tedeschi A, Airaghi L, Lorini M, Asero R. Chronic urticaria: a role for newer immunomodulatory drugs? Am J Clin Dermatol. 2003;4:297-305
-
26Reeves GE, Boyle MJ, Bonfield J, Dobson P, Loewenthal M. Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J. 2004;34:182-6.
-
27Sagi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91:303-6.
-
28Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162:191-4.
-
29Ferrer M, Madamba R. Biologics in chronic urticaria. Allergol Immunopathol (Madr). 2017;45 (Suppl 1):S41-4.
-
30Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-7.
-
31Antia C, Baquerizo K, Korman A, Alikhan A, Bernstein JA. Urticaria: A comprehensive review: Treatment of chronic urticaria, special populations, and disease outcomes. J Am Acad Dermatol. 2018;79:617-33.
-
32Zuberbier T, Bernstein JA. A Comparison of the United States and International Perspective on Chronic Urticaria Guidelines. J Allergy Clin Immunol Pract. 2018;6:1144-51.
-
33Weber-Schoendorfer C, Schaefer C. The safety of cetirizine during pregnancy. A prospective observational cohort study. Reprod Toxicol. 2008;26:19-23.
-
34Schwarz EB, Moretti ME, Nayak S, Koren G. Risk of hypospadias in offspring of women using loratadine during pregnancy: a systematic review and meta-analysis. Drug Saf. 2008;31:775-88.
-
35Namazy J, Cabana MD, Scheuerle AE, Thorp JM Jr, Chen H, Carrigan G, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135:407-12.
-
36Ghazanfar MN, Thomsen SF. Successful and Safe Treatment of Chronic Spontaneous Urticaria with Omalizumab in a Woman during Two Consecutive Pregnancies. Case Rep Med. 2015;2015:368053.
-
37Wood RA, Khan DA, Lang DM, Fasano MB, Peden DB, Busse PJ, et al. AAAAI Response to the EAACI/GA(2)LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria 2017 Revision. Allergy. 2018 [Epub ahead of print].
Publication Dates
-
Publication in this collection
03 June 2019 -
Date of issue
Mar-Apr 2019
History
-
Received
06 Sept 2018 -
Accepted
07 Dec 2018


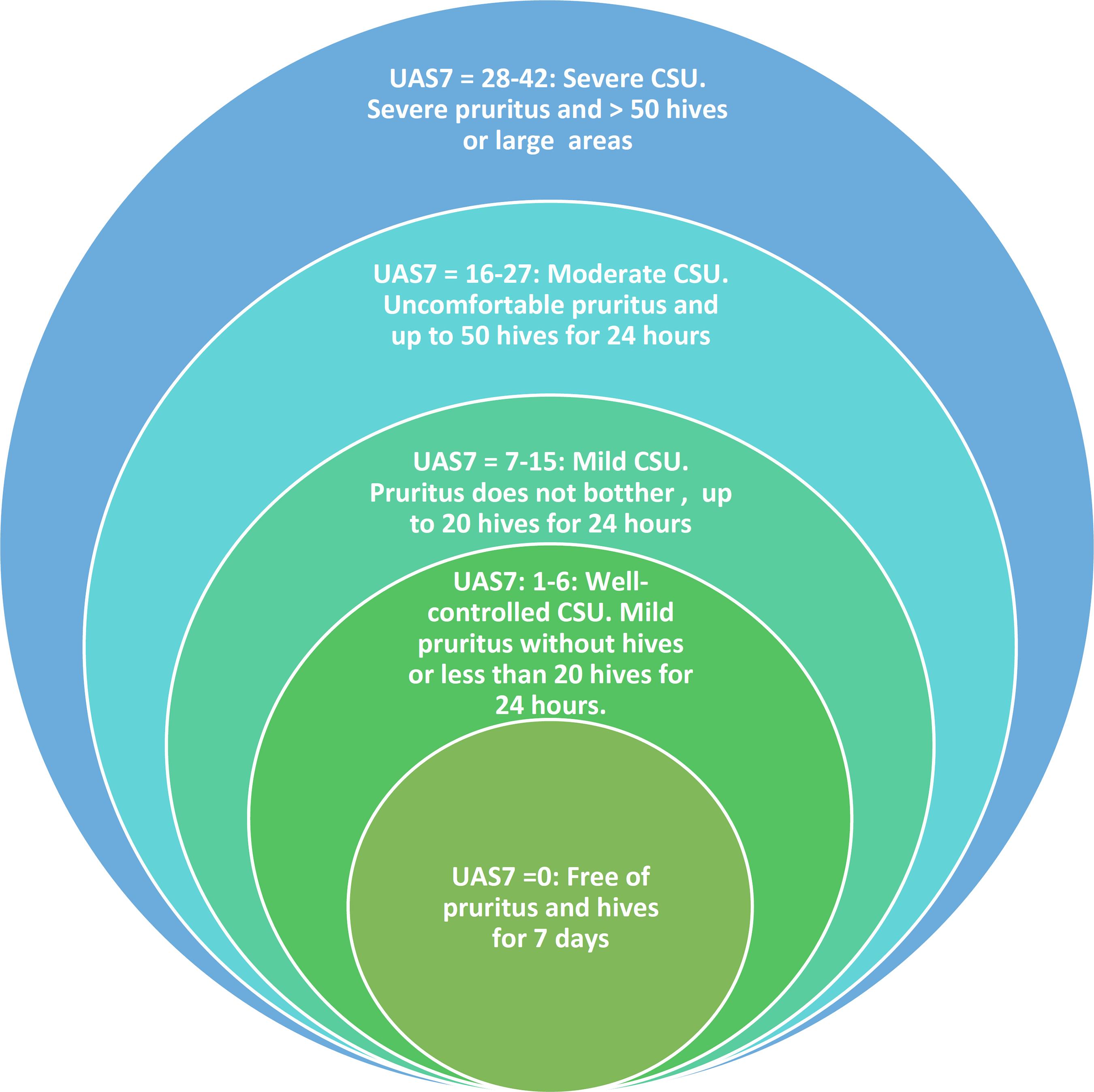
 Adaptaded from: Zuberbier, et al, 2018.
Adaptaded from: Zuberbier, et al, 2018. Adaptaded from: Zuberbier, et al, 2018.
Adaptaded from: Zuberbier, et al, 2018.