Abstracts
Two series of silicate glasses were processed to micron-size, sub-micron size, and nanoparticles using three different milling systems: ball milling, attrition, and high-energy milling. The effect of milling time and media size on particle size and contamination were investigated in aqueous and isopropanol suspensions. The particle size was determined using a laser-diffraction particle size analyzer and scanning electron microscopy. The smallest glass particles with a median particle size of 0.3 µm were achieved by a two-step comminution process in a high energy mill.
comminution; dental composites; glass fillers; nano-particles; particle processing
Foram moídas duas séries de vidros silicatos para a obtenção de partículas com dimensões nas faixas micrométrica, submicrométrica e nanométrica, utilizando três tipos distintos de processos de moagem: moagem em moinho de esferas, em atritor, e de alta energia. Foram investigados os efeitos do tempo e do tipo do meio de moagem no tamanho de partícula, em suspensões aquosas e em isopropanol. Foram utilizados um granulômetro com o princípio de difração de laser e um microscópio eletrônico de varredura para a determinação do tamanho de partícula. A distribuição de tamanho de partículas com o menor tamanho médio de partícula igual a 0,3 µm foi obtida para vidros submetidos ao processo de cominuição em duas etapas, utilizando um moinho de alta energia.
cominuição; compósitos odontológicos; glass fillers; nano-partículas; processamento de partículas
Processing of yttrium aluminosilicate (YAS) glasses for dental composites
Processamento de vidros de aluminosilicatos de ítrio (YAS) para compósitos odontológicos
M. VelezI; Y. HeI; D. E. DayII; T. P. SchumanIII; K. V. KilwayIV; J. R. MelanderV; R. A. WeilerIV; B. D. MillerIV; E. L. NalvarteV; J. D. EickV
IMo-Sci Corporation, Rolla, MO
IIMaterials Science and Engineering Department, Missouri University of Science and Technology, Rolla, MO
IIIDepartment of Chemistry, Missouri University of Science and Technology, Rolla, MO
IVDepartment of Chemistry, College of Arts and Sciences, University of Missouri, Kansas City, MO
VDepartment of Oral Biology, School of Dentistry, University of Missouri, Kansas City, MO
ABSTRACT
Two series of silicate glasses were processed to micron-size, sub-micron size, and nanoparticles using three different milling systems: ball milling, attrition, and high-energy milling. The effect of milling time and media size on particle size and contamination were investigated in aqueous and isopropanol suspensions. The particle size was determined using a laser-diffraction particle size analyzer and scanning electron microscopy. The smallest glass particles with a median particle size of 0.3 µm were achieved by a two-step comminution process in a high energy mill.
Keywords: comminution, dental composites, glass fillers, nano-particles, particle processing.
RESUMO
Foram moídas duas séries de vidros silicatos para a obtenção de partículas com dimensões nas faixas micrométrica, submicrométrica e nanométrica, utilizando três tipos distintos de processos de moagem: moagem em moinho de esferas, em atritor, e de alta energia. Foram investigados os efeitos do tempo e do tipo do meio de moagem no tamanho de partícula, em suspensões aquosas e em isopropanol. Foram utilizados um granulômetro com o princípio de difração de laser e um microscópio eletrônico de varredura para a determinação do tamanho de partícula. A distribuição de tamanho de partículas com o menor tamanho médio de partícula igual a 0,3 µm foi obtida para vidros submetidos ao processo de cominuição em duas etapas, utilizando um moinho de alta energia.
Palavras-chave: cominuição, compósitos odontológicos, glass fillers, nano-partículas, processamento de partículas.
INTRODUCTION
Composite restorative dental materials have been investigated extensively during the past 50 years. The state of the art of dental composites has been reported [i.e., 1, 2], and several reviews describing dental adhesives and composites are available [3-6]. Nanofiller technology has been introduced to improve the properties of dental composites [7]. Glass nanofillers can be used in a variety of prosthetic, dental, bone, and orthodontic applications as reinforcements in silorane-based resin-systems used for restorative composites and cements [8-11].
Nanoparticles are commonly obtained by bottom-up processes (precipitation from solutions). However, it is difficult to produce nanoparticles with complex compositions, such as glasses with more than five oxides [12]. A more efficient approach is the high-energy milling (top-down process) of larger glass particles (< 100 microns).
In this work, two series of glasses were milled to micron-size, sub-micron size, and nanoparticles using three different milling systems: ball milling, attrition, and high-energy milling. The first glass series composition (coded M-series) included alkali-free and alkali-containing borosilicate glasses. Alkali-free glasses are preferred for silorane-based dental composites as the alkalies interfere with the polymerization of cationically curing polymer resins. The M-series glasses were made by conventional melting of the raw-material batches at 1200 ºC to 1400 ºC. The quenched glasses were crushed, ground, and milled to obtain glass fillers for dental composites. The second glass series composition (coded DY-series) included alkali-free and alkali-containing yttrium aluminosilicate (YAS) glasses.
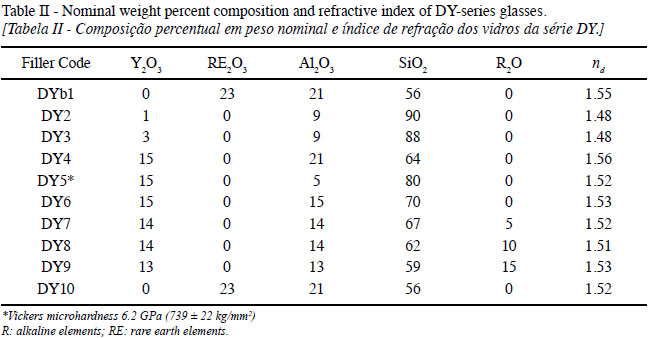
YAS glasses possess a fully cross-linked, 'strong' silica-like structure and have many properties similar to those found in fused silica glass. Such properties include: optical transparency throughout the visible spectrum, high electrical resistivity, excellent chemical durability, high strength, and UV absorption. Requirements for glass fillers for dental composites include the following: X-ray opacity (radiopacity) and hardness, no alkalies in the surface of the filler particles, match the refractive index (nd) of the dental polymer resin during curing, match the cured resin color and translucency (or visual opacity), filler content between 50 to 70 wt.%, particle size ranges from 0.05 µm to 5 µm, and PSD and particle shape to allow flowability of uncured composite.
YAS glasses possess higher nd values (1.54-1.75) and higher Vickers hardness (6.4-8.3 GPa) comparable to those of fused silica glass (1.46 and 5.9 GPa, respectively) [13]. The nd of YAS glasses can be tailored to match the nd of resins for dental applications, and should provide similar radiopacity as observed, for instance, with Ba-Sr silicate glasses [14].
Glass making
The glass fillers were made by either melting batches (M-series and alkali-containing DY-series) or by sintering batches and spheroidization of the sinter (alkali-free DY-series). The sinter/spheroidization process was used for batches that melted above 1550 ºC. The nominal chemical composition and ndof the M-series glasses are given in Table I.
The nominal chemical composition and ndof the DY-series glasses are listed in Table II. The ndof the glasses varied from 1.48 to 1.56 depending on composition, a range of values which is needed for blue-light (440 nm)-curable dental composites. The glass batch was wet-mixed, sintered, crushed, and sieved to separate particles < 210 µm, which were spheroidized by injecting the particles into a propane-oxygen-flame. The glass microspheres were then milled to produce glass filler powders and mixing with nanoparticles via ball milling [15].
Glass milling
Selected glass compositions from the M-series and DY-series were chosen for milling experiments based on polymerization (curing) tests of silorane-resins [16]. Three milling processes were tested to control the particle size distribution (PSD) of glass fillers (M-series and DY-series): (1) a ball mill (high-alumina porcelain jar), (2) an attrition mill, and (3) a high energy mill. Wet milling was performed in every case, using de-ionized water for non-alkali-containing glasses and isopropanol for alkali-containing glasses. The milling media was Tosoh zirconia: 10 mm diameter for coarse or primary grinding, and either 1 mm or 0.5 mm diameter media for fine grinding (below 1 micron size particles).
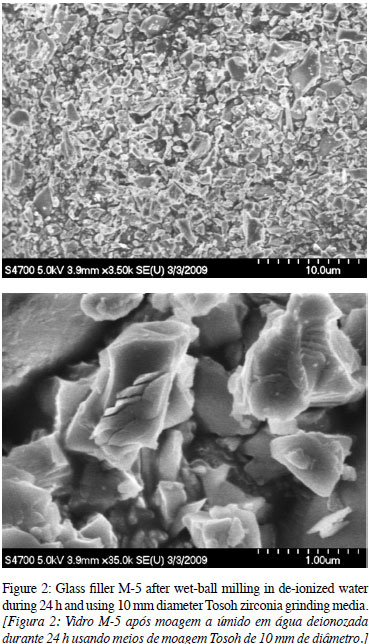
Wet Ball Milling. One hundred grams of glass fillers M-5 through M-10 were wet-ball milled for two days with ZrO2 grinding media. Samples from each processed glass filler were taken for chemical analysis and for PSD measurement. A Beckman Coulter LS 13 320 particle size analyzer was used for PSD and specific surface area (SSA) measurements. The PSD of the processed glass fillers, M-5 through M-10, are shown in Fig. 1. All glass fillers have a bi-modal PSD, with the larger fraction centered on 2.6 to 3.5 microns and the smaller fraction centered on 0.25 to 0.35 microns. Glass fillers M-5 and M-6 have low alkali content (0.9 wt.%); M-10 is a soda-lime glass.
The general particle shape after wet-ball milling glass filler M-5 frit (starting median 150 µm) for 24 h is shown in Fig. 2. All milled glass filler powders obtained from ball-milling have a ZrO2 contamination estimated between 4 and 8 weight % from XRF and SEM/EDAX analysis.
One kilogram of DY-5 glass filler was made by sintering raw materials, spheroidizing, grinding, and sieving to micron-size particles. Zirconia media was used to grind the glass via wet ball-milling. The PSD parameters and density of the processed glass filler are summarized in Table III. The median (d50) is below 3 µm. The differential volume per cent and cumulative PSD for glasses DY-5 (Fig. 3) is basically a bi-modal distribution. This glass filler was mixed with ZrO2 nanoparticles for incorporation into a silorane resin.
Grinding of Glass Fillers in Attrition Mill. One-hundred-fifty grams of essentially alkali-free glass filler M-5 frit (starting size 150 µm) were wet-milled with de-ionized water in an attrition mill at a speed of 525 rpm. Solids loading was 40 wt.% with 2 mm diameter Tosoh zirconia grinding media. The general particle shape after wet-milling the glass filler M-5 for 8 h is shown in Fig. 4. The attrition milling proved to be more efficient than wet-ball milling as similar median fractions were obtained in less time (∼ 1/4 of the required in wet ball milling) and the ZrO2 contamination was estimated below 0.1 wt.%.
The cumulative size fractions of glass filler M-5 as a function of milling time, for milling times below 5 h, is shown in Fig. 5. All particle size distributions obtained either by wet- attrition milling were bi-modal or multiple-modal distributions. The span of the PSD can be measured by [17]:
The d10, d50, and d90 values are size values corresponding to the cumulative distribution at 10%, 50%, and 90%. The d50 value corresponds to the median diameter. A span below 1.3 is recommended for fillers for dental resins [17]. The measured specific surface area (SSA) of the powders is included in Table IV.
The attrition milling test was expanded in an attempt to obtain sub-micron size glass particles. The test included two glass fillers (M-5, essentially an alkali-free glass, and
M-8, an alkali-containing glass) with two suspending media (de-ionized water and 2-propanol), and two different ZrO2 grinding media size (2 mm diameter and 0.5 mm diameter). The objective was to investigate the effect of three variables (alkali-presence, suspending media, and grinding media size) on particle size and PSD, particle morphology, and ZrO2 contamination.
The dependence of particle size vs. milling time for tests made with 2 mm zirconia milling media is shown in Fig. 6. The non-alkali and alkali glass had a smaller particle median (d50) after 8 h when milled in de-ionized water than when milled in isopropanol (Table V). This is likely due to some amount of leaching of water-soluble components during milling. The effect is more pronounced with the alkali glass due to leaching of the alkali constituents. Additionally, the alkali-containing glass had a smaller d50 than the non-alkali glass when milled in both de-ionized water and isopropanol (Table V).
No bimodal distributions were observed after three hours in any of the attrition milling tests as observed with ball-milling. This result means that there was consistent milling-all particles were being milled at roughly the same rate. The pH of the isopropanol remained at 6 throughout the milling experiments for both glasses. Additionally, the pH of the deionized water remained at 7 for the non-alkali glass; while for the alkali-glass, the pH was between 11 and 12 (initial pH of de-ionized water was 6). The particle median diameter (d50) versus milling time for the alkali-glass milled in deionized water with the 0.5 mm zirconia media is shown in Fig. 7. A particle median (d50) of ∼ 2 µm was reached after 6 h when milled in de-ionized water. Milling in isopropanol with the 0.5 mm zirconia media was not as effective as milling with de-ionized water. This result is most likely due to some amount of leaching of water-soluble components during milling in de-ionized water.
Particles milled in either de-ionized water or isopropanol were irregular and jagged, with no strong indication of a plate-like morphology (Fig. 8). The micrographs are consistent with the particle size analysis, with a larger number of smaller glass particles (∼ 1 µm) present after milling in de-ionized water.
The zirconia contamination, measured by X-ray fluorescence (XRF), was below 0.1 wt.% after 8 h of milling. Wet attrition milling with de-ionized water is more effective than with isopropanol in achieving a smaller particle size in the same milling time. The 0.5 mm diameter zirconia media was not as effective as the 2 mm diameter media, and its use would require a 2-step milling operation: (1) milling with the 2 mm media, and then milling with the 0.5 mm media.
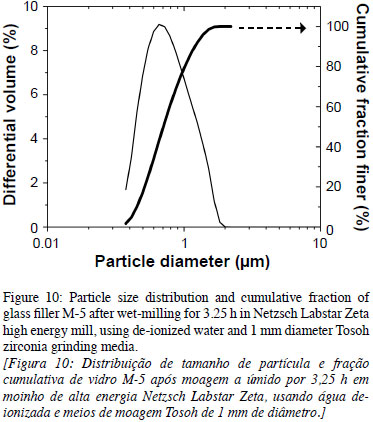
Grinding of Glass Filler M-5 in High-energy Mill. Two kilograms of alkali-free M-5 glass frit (150 µm median size) were wet-milled in de-ionized water in a Netzsch Labstar Zeta high-energy mill using 1 mm diameter Tosoh zirconia grinding media. The general shape and size of glass filler M-5 after wet-milling 3.25 h and using 1 mm diameter Tosoh zirconia grinding media is shown in Fig. 9.
The high-energy milling proved to be more efficient than wet-attrition milling as the particle-size distributions are single-modal distributions of narrower widths (smaller spans according to Eqn. 1), as compared to those fractions obtained from wet-ball milling or from attrition milling. The PSD and cumulative fraction of glass filler M5 after wet-milling 3.25 h and using 1 mm diameter Tosoh zirconia grinding media is shown in Fig. 10. The ZrO2 contamination from the milling media was estimated below 0.1 wt.%. The SSA increases with milling time for both milling processes, the attrition and the high energy mill. A maximum SSA of 3.23 m2/g was obtained for the high energy mill after 3.25 h of milling.
The size of different powder fractions after milling and their span is listed in Table VI. The high energy mill seems to be the best option for obtaining single mode powder fractions, in less milling time and with minimum media contamination. The zirconia contamination of the M-5 powder was ∼ 0.1 wt.%, similar to the contamination of the M-5 powder in the attrition mill.
Sub-micron size particles. Four silicate glass fillers (DY-5, DY-9, M-2, and M-8) were ground in a Netzsch Labstar Zeta mill to obtain sub-micron size glass particles. All glasses were crushed, dry-milled, and sieved below 60 µm, and the powders were dispersed in ∼30% solid suspensions for the high energy mill.
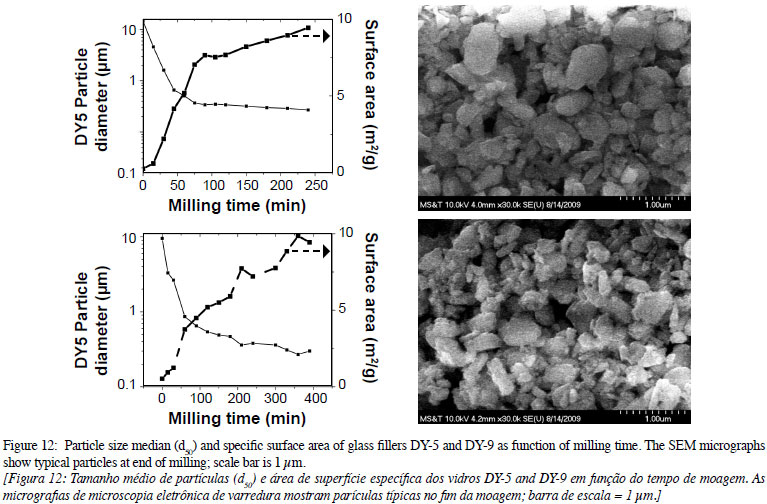
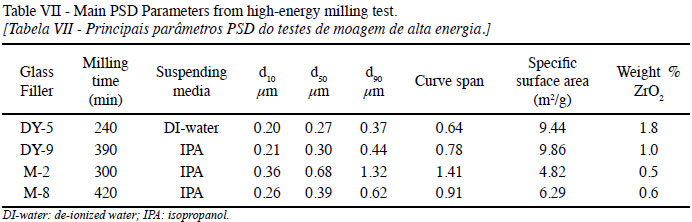
The PSD of the glass fillers at the end of the milling test (4 to 6 h) is shown in Fig. 11. All curves are single-mode distributions with medians (d50) below 0.5 µm, except for M-2 (which was milled for shorter times than DY-9 and M-8, also dispersed in isopropanol) due to the alkali content of these glasses. The milling time for each glass, the suspending media, main parameters of the distribution curves, and the ZrO2 contamination is listed in Table VII. The glass particles were reduced to a median particle size between 0.2 and 0.7 µm with milling times up to 7 h.
The particle size median (d50) for each glass filler and the specific surface area as a function of milling time is shown in Fig. 12. The medians approach values between 0.2 and 0.7 µm for milling times up to 7 h, which is the maximum milling time recommended as the energy input is too high for further particle size reduction. At the end of the milling test, the micrographs show that the particles are irregular but with rounded edges, which is required for high flowability of mixes of resin and glass fillers.
CONCLUSIONS
Two series of glasses were processed to produce micron-size, sub-micron size, and nanoparticles using three different milling systems: high-energy, attrition, and ball milling. Wet-milling of glass fillers using the attrition mill was more efficient than wet-ball milling since a shorter time is required for reaching a similar particle size. Contamination from the milling media was negligible (∼0.1 wt.%) compared to contamination from wet-ball-milling (4 to 8 wt.% ZrO2).
Wet-milling of glass fillers using the Netzsch Labstar mill was more efficient than either wet-ball milling or wet-milling using an attrition mill. Single mode fractions of sub-micron-size particles can be obtained in less time and with a narrower distribution range. Contamination from the milling media is negligible compared to contamination obtained during wet-ball-milling.
Alkali-free/Y2O3-Yb2O3-Al2O3-SiO2 glass-fillers were prepared with properties suitable for use in dental composites. High melting-temperature glass-fillers can be produced by sintering, flame-spheroidizing, and wet-ball-milling. The glass-fillers can be ground to a bi-modal PSD that includes a micron-size fraction and a sub-micron-size fraction. Glass fillers for dental resins can be made by mixing micron-size particles (from wet-ball milling), sub-micron size particles (from a high-energy mill), and commercial nanoparticles (size below 0.1 µm).
ACKNOWLEDGEMENTS
This work was supported in part by NIH/NIDCR Grant R21 DEO18336. The attrition milling data was obtained by A. Doering, S. Heise, J. Murray, and C. Urrutia in 2009 for their Senior Design course in Materials Science at Missouri University of Science & Technology, supervised by Prof. R. K. Brow.
(Rec. 28/04/2010, Ac. 08/06/2010)
- [1] K. J. Anusavice, Phillips' Science of Dental Materials, Saunders, 11th Ed. (2003).
- [2] J. De Munck, K. Van Landuyt, M. Peumans, A. Poitevin, P. Lambrechts, M. Braem, B. Van Meerbeek, "A Critical Review of the Durability of Adhesion to Tooth Tissue: Methods and Results", J. Dent. Res. 84 (2005) 118-32.
- [3] R. R. Braga, R.Y. Ballester, J. L. Ferracane, "Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review", Dent. Mater. 21 (2005) 962-70.
- [4] J. L. Ferracane, "Developing a More Complete Understanding of Stresses Produced in Dental Composites During Polymerization", Dent. Mater. 21 (2005) 36-42.
- [5] H. Lu, J. W. Stansbury, S. H. Dickens, F. C. Eichmiller, C. N.Bowman, "Probing the Origins and Control of Shrinkage Stress in Dental Resin-Composites: I. Shrinkage Stress Characterization Technique", J. Mater. Sci. Mater. Med. 15 (2004) 1097-1103.
- [6] M. J. Tyas, M. F. Burrow, "Adhesive Restorative Materials: a Review", Austr. Dent. J. 49 (2004) 112-21.
- [7] K. Pflug, "Dental Material Having a Nanoscale Filler", U.S. Patent 6,693,143 B2, assigned to Dentsply DeTrey GmbH, DE (2004).
- [8] C. C. Chappelow, C. S. Pinzino, J. D. Eick, A. J. Holder, S.S. Chen, L. Jeang, "Dioxiranyl Tetraoxaspiro[5.5] undecanes", U.S. Patent No. 6,825,364, assigned to the Curators of the University of Missouri (2004).
- [9] C. C. Chappelow, C. S. Pinzino, J. D. Eick, "Spiroorthocarbonates Containing Epoxy Groups", U.S. Patent No. 6,653,486, assigned to the Curators of the University of Missouri (2003).
- [10] R. E. Smith, C. S. Pinzino, C. C. Chappelow, A. J. Holder, E. L. Kostoryz, J. R. Guthrie, M. D. Miller, D. M. Yourtee, J. D. Eick, "Photopolymerization of an Expanding Monomer with an Aromatic Dioxirane", J. Appl. Polym. Sci. 92 (2004) 62-71.
- [11] T. J. Byerley, C. C. Chappelow, J. D. Eick, "Polymeric Compositions and Composites Prepared from Spiroorthocarbonates and Epoxy Monomers", U. S. Patent No. 6,022,940, assigned to the Curators of the University of Missouri (2000).
- [12] A. Vital, S. Zurcher, R. Dittmann, M. Trottmann, P. Lienemann, B. Bommer, T. Graule, E. Apel, W. Holand, "Ultrafine Comminution of Dental Glass in a Stirred Media Mill", Chem. Eng. Sci. 63 (2008) 484-494.
- [13] J. E. White, D. E. Day, "Rare Earth Aluminosilicate Glasses for In Vivo Radiation Delivery", Key Eng. Mater. 94-95 (1994) 181-208.
- [14] M. Zhou, J. L. Drummond, L. Hanly, "Barium and Strontium Leaching from Aged Glass Particle/Resin Matrix Dental Composites", Dent. Mater. 21 (2005) 145-155.
- [15] B. Chelluri, E. A. Knoth, E. J. Schumacher, R. D. Evans, J.L. Maloney, "Method of Producing Uniform Blends of Nano and Micron Powders", WO/2008/034043, assigned to the Timken Company.
- [16] C. Chappelow, C. Pinzino, S. Chen, S. Kotha, A. Glaros, J.D. Eick, "Tetraoxaspiroalkanes for Polymerization Stress Reduction of Silorane Resins", J. Appl. Polym. Sci. 108, 6 (2008) 3738-3747.
- [17] G. B. Blackwell, K. Utz, "Dental Composite Restorative Material and Method of Restoring a Tooth" U.S. Patent 7,001,932 B2, assigned to Dentsply DeTrey GmbH, DE (2006).
Publication Dates
-
Publication in this collection
10 May 2011 -
Date of issue
Mar 2011
History
-
Received
28 Apr 2010 -
Accepted
08 June 2010