Abstracts
This work involved the characterization of clays collected in the municipalities of São Luis, Rosário, Pinheiro and Mirinzal (state of Maranhão, Brazil), based on specific mass, specific surface area, cation exchange capacity (CEC), particle size distribution, X-ray diffraction (XRD), differential thermal analysis (DTA), thermogravimetric analysis (TG-DTA) and Atterberg limits. Technological tests for ceramic applications were also carried out on compacts pressed under 20 MPa and heat-treated at 850, 950, 1050, 1150 and 1250ºC. Our results indicated that two of the clays composed of kaolinite, quartz, and anatase with high plasticity limits, have excellent properties and can be used in the whiteware industry. The other ones are red-firing clays and have a mineralogical composition of quartz, kaolin, feldspar, montmorillonite, hematite and goethite. The latter showed low and moderate values of plasticity, which makes them suitable for the production of heavy clay products.
Raw materials; Maranhão; Characterization
Nesse trabalho, foram caracterizadas algumas argilas coletadas nos municípios de São Luís, Rosário, Pinheiro e Mirinzal. A caracterização foi realizada através dos ensaios de difração de raios X, massa específica real, capacidade de troca de cátions (CTC), área superficial, distribuição granulométrica, análise química, análise térmica (TG-DTA) e limites de Atterberg. Ensaios tecnológicos de retração linear, antes e após a queima, absorção de água e tensão de ruptura a flexão, em três pontos, foram realizados em corpos de prova prensados uniaxialmente a 20 MPa e tratados termicamente em 850, 950, 1050, 1150 e 1250ºC. Os resultados obtidos permitiram identificar duas argilas de queima branca, constituídas de quartzo, caolim, feldspato e anatásio, com excelentes propriedades para uso em cerâmica branca. As restantes são queima vermelha e possuem composição mineralógica de quartzo, caolim, feldspato, montmorilonita, hematita e goetita. Estas últimas apresentaram valores moderados de plasticidade e são adequadas para aplicações em cerâmica vermelha.
Matérias-primas cerâmicas; Maranhão; caracterização
METALURGIA E MATERIAIS METALLURGY AND MATERIALS
Ceramic raw materials from the State of Maranhão, Brazil. Part 1: chemical and mineralogical characterization and technological properties of clays from São Luis, Rosário, Pinheiro and Mirinzal
Matérias-primas cerâmicas do Estado do Maranhão. Parte 1: caracterização químico-mineralógica e propriedades tecnológicas de argilas dos municípios de São Luís, Rosário, Pinheiro e Mirinzal
José Manuel Rivas MercuryI; Gricirene Sousa CorreiaII; Nazaré Socorro Lemos Silva VasconcelosIII; Aluísio Alves Cabral Jr.IV; Rômulo Simões AngélicaV
IEngenheiro Químico, Doutor em Físico-Química Inorgânica, Professor do Programa de Pós-Graduação em Engenharia dos Materiais (PPGEM), Departamento de Química (DAQ) - Instituto Federal do Maranhão (IFMA). rivascefetma@gmail.com
IIEngenheiro Mecânico, M.Sc. em Engenharia dos Materiais (PPGEM/IFMA), Departamento de Mecânica e Materiais (DMM) IFMA. gricirene@gmail.com
IIIQuímica Industrial, Doutora em Química pela Universidade Estadual Paulista Júlio de Mesquita Filho, Professora do Programa de Pós-Graduação em Engenharia dos Materiais (PPGEM), Departamento de Química (DAQ) Instituto Federal do Maranhão (IFMA). ndsocorro@ifma.edu.br
IVLicenciado em Física, Doutor em Engenharia de Materiais, Professor do Programa de Pós-graduação em Engenharia dos Materiais (PPGEM), Departamento de Física (DCE) - Instituto Federal do Maranhão (IFMA). acabraljr@ifma.edu.br
VGeólogo, Doutor em Mineralogia e Geoquímica, Professor do Programa de Pós-Graduação em Geologia e Geoquímica Instituto de Geociências da UFPA. angelica@ufpa.br
ABSTRACT
This work involved the characterization of clays collected in the municipalities of São Luis, Rosário, Pinheiro and Mirinzal (state of Maranhão, Brazil), based on specific mass, specific surface area, cation exchange capacity (CEC), particle size distribution, X-ray diffraction (XRD), differential thermal analysis (DTA), thermogravimetric analysis (TG-DTA) and Atterberg limits. Technological tests for ceramic applications were also carried out on compacts pressed under 20 MPa and heat-treated at 850, 950, 1050, 1150 and 1250ºC. Our results indicated that two of the clays composed of kaolinite, quartz, and anatase with high plasticity limits, have excellent properties and can be used in the whiteware industry. The other ones are red-firing clays and have a mineralogical composition of quartz, kaolin, feldspar, montmorillonite, hematite and goethite. The latter showed low and moderate values of plasticity, which makes them suitable for the production of heavy clay products.
Keywords: Raw materials, Maranhão, Characterization.
RESUMO
Nesse trabalho, foram caracterizadas algumas argilas coletadas nos municípios de São Luís, Rosário, Pinheiro e Mirinzal. A caracterização foi realizada através dos ensaios de difração de raios X, massa específica real, capacidade de troca de cátions (CTC), área superficial, distribuição granulométrica, análise química, análise térmica (TG-DTA) e limites de Atterberg. Ensaios tecnológicos de retração linear, antes e após a queima, absorção de água e tensão de ruptura a flexão, em três pontos, foram realizados em corpos de prova prensados uniaxialmente a 20 MPa e tratados termicamente em 850, 950, 1050, 1150 e 1250ºC. Os resultados obtidos permitiram identificar duas argilas de queima branca, constituídas de quartzo, caolim, feldspato e anatásio, com excelentes propriedades para uso em cerâmica branca. As restantes são queima vermelha e possuem composição mineralógica de quartzo, caolim, feldspato, montmorilonita, hematita e goetita. Estas últimas apresentaram valores moderados de plasticidade e são adequadas para aplicações em cerâmica vermelha.
Palavras-chave: Matérias-primas cerâmicas, Maranhão, caracterização.
1. Introduction
The state of Maranhão today has more than 120 companies operating in the red ceramics sector. Most of these companies are situated in 15 microregions, which manufacture approximately 22 million bricks and 18 million roof tiles per month, generating over 5,000 direct jobs (IBGE, 2007; ABC, 2002; Santana et al., 2008). However, the raw materials used in many of these regions do not undergo a beneficiation process or proper characterization, which contributes to lower the quality levels of the manufactured products.
Despite the importance of these companies to the state's economy, the literature contains no data on the occurrence of ceramic raw materials, or scientific and technological studies to guide future investments of the private sector in this region (Mello, 2011).
Therefore, the purpose of this pioneering work was to characterize four clays collected in the municipalities of São Luis, Rosário, Pinheiro and Mirinzal, based on their chemical, mineralogical and technological properties. These materials are widely used in the manufacture of traditional ceramic materials. From our point of view, this paper contributes to the knowledge about the ceramic raw materials of Maranhão.
2. Materials and methods
Materials
The raw clays collected in the municipalities of São Luis, Rosário, Pinheiro and Mirinzal, situated 67, 86 and 154 km from São Luis, respectively, will hereinafter be identified as ASL (São Luis Clay), ARO (Rosário Clay), AP (Pinheiro Clay) and AM (Mirinzal Clay). The materials were sun-dried to obtain constant moisture content. Then they were cleaned (Souza Santos, 1987) and disagglomerated in an electric mortar mixer. Next, some aliquots were removed for granulometric testing. After quartering the rest of the materials (3 kg), they were ground and sifted through a sieve (150 mm mesh) to obtain a powder suitable for the remaining tests.
Methods
The samples were analyzed chemically by different methods: gravimetry, to determine the total SiO2 and the loss on ignition (LOI); complexometry (EDTA) to measure the total Al2O3; and colorimetry to estimate the total Fe2O3 and TiO2. The CaO, MgO, Na2O and K2O contents were analyzed by atomic absorption spectrometry (AAS) after alkali fusion.
The thermal behavior of the clays was examined by thermogravimetric analysis (TGA) and differential thermal analysis (DTA), using Al2O3 crucibles and an oxidizing atmosphere up to 1100ºC at a heating rate of 10ºC/min, in a PL Thermal Sciences thermal analyzer equipped with an STA 1000/150 simultaneous thermal analyzer (Stanton Redcroft Ltd.).
To determine the total fraction of the mineral phases (powder), the samples were analyzed in a back-loading sample holder to avoid orientation of the aggregates. The samples' clay fraction (< 2µm fraction) was separated by sedimentation after dispersion with NH4OH 0.1 mol L-1, using the slide orientation technique. In addition, they were air-dried, solvated with ethylene glycol for 24h and heat-treated at 500ºC/2h.
The XRD spectra of the total fraction were recorded with an X'Pert PRO diffractometer (PANalytical PW3040/60), equipped with a PW3050/60 goniometer (Theta/Theta) and a ceramic X-ray tube with Cu anode (CuKa1 = 1.5406 Å), operating with 40 mA current, 40 kV voltage, with a step size of 0.02º (2Q), a count time of 5 s, at an angular range of 5-70º (2Q). The diffractograms of fine fraction (< 2 mm) were recorded with the Co anode (CoKa1 = 1.78901 Å) to prevent efflorescence of iron in the angular region of 3-34º (2Q) with a step size of 0.02º (2Q) and a counting time of 10 s. The mineral phases were indexed using the PDF-ICDD database (Powder Diffraction File International Center for Diffraction Data).
The semiquantitative analysis of the crystalline phases was carried out based on the XRD results of the total fraction and the fine fraction with non-oriented aggregates, and on a chemical analysis (Coelho et al., 2002; Johnson et al., 1985; Loubser and Verryn, 2008) by X-ray fluorescence (XRF).
The physical characterization of the samples involved the following experiments: Granulometric distribution according to the Brazilian Association of Technical Standards ABNT standard NBR 7181 (ABNT, 1984); cation exchange capacity and surface area by the methylene blue saturation method (Ferreira, 1972); Atterberg limits: Plasticity Index (PI), Liquid Limit (LL) and Plasticity Limit (PL), according to the Brazilian NBR 6454 (ABNT, 1984), and NBR 7180 standards (ABNT, 1984); and Real Specific Mass and Water Absorption (WA) according to the NBR 6220 standard (ABNT, 1997).
Rectangular test specimens with dimensions of 8 x 2.0 x 0.5 cm, with moisture content of 8% in weight, were compacted uniaxially under a pressure of 20 MPa and dried at 110ºC for 24h. The compacts were heat-treated at 850, 950, 1050, 1150 and 1250ºC in an electric furnace (Nabertherm GmbH, Germany) in an oxidizing atmosphere, applying a heating rate of 5ºC/min with a dwell time of 3 hours. After the heat treatment, the following ceramic properties were determined: linear shrinkage (SL), water absorption (WA) and flexural stress rupture (FSR) under three-point bending (TIRA Test 2705), as recommended by the Brazilian ABNT standards NBR-6113/97 (ABNT, 1984b) and NBR 6220/97 (ABNT, 1984b).
3. Results and discussion
Mineralogical characterization
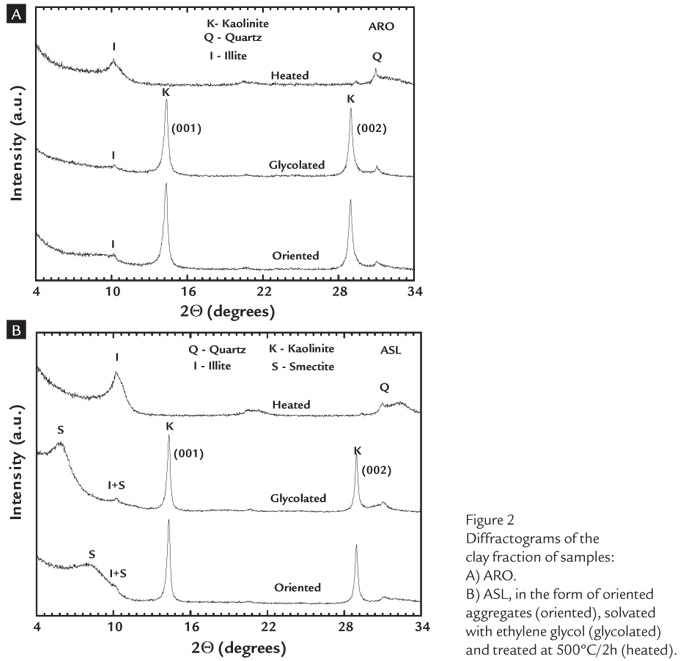
Figure 1 (A-B) illustrates the results of the XRD analysis of the total fraction of the AM, AP, ARO and ASL clays.
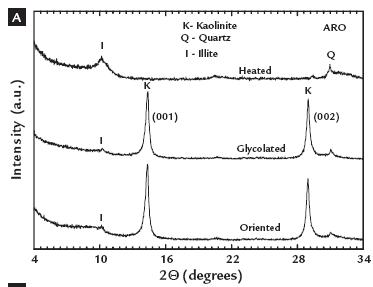
In Figure 1A, note that the AM and AP samples present a similar mineralogical composition, both showing the presence of the following minerals: quartz (26.64º (2Q); d = 3.34 Å (101)); kaolinite (12.30º (2Q); d =7.10 Å (001); 19.83º (2Q); d = 4.41Å (110); and 24.98º (2Q); d = 3.56 Å (002)); and anatase (25.28º (2Q); d = 3.52 Å; (001)). Neither of these samples (AM and AP) showed reflections corresponding to the mineral iron. In contrast, the ARO and ASL samples (Figure 1B) show the minerals quartz, kaolinite, anatase, illite (8.27º (2Q); d = 10.00 Å (001)), feldspar (27.48º (2Q); d = 3.25 Å) and minor contents of goethite (21.5 º (2Q); d = 4.18Å (101)) and hematite.
A more in-depth study of the mineralogy of the clays based on an analysis of the clay fraction (< 2 mm) by the oriented aggregation (OA) method indicated that the AM and AP clays are constituted primarily of the clay mineral kaolinite (Figure 2). In Figure 2A, note that the glycosylated OA of the ARO sample, after heat treatment at 500ºC/2h, shows reflections typical of kaolinite and illite (10.22º(2Q); d = 10.01 Å; 20.66º(2Q); d = 4.98 Å) of low intensity.
On the other hand, the diffractogram of the ASL sample (Figure 2B) presents reflections of low intensity of illite/smectite (10.22º(2Q); d = 10.01 Å; 20.66º(2Q); d = 4.98 Å) and a band centered at 7.94º 2Q(d=12.85 Å), which is shifted towards the low angle region (5.85º (2Q); d = 17.54 Å). This change indicates that the interplanar distance increases in the direction of the (001) plane due to the presence of a mineral of the smectite family (montmorillonite) (Garcia Verduch; 1985).
The presence of type 2:1 phyllosilicates deserves special attention due to their applications in other industrial areas (Souza Santos, 1992; Garcia Verduch, 1985; Vieira and Sousa Santos, 2007) and the impact of this mineral phase on the rheology of ceramic suspensions, the plasticity of pastes, and the mechanical properties of green ceramic materials after drying (Thorez, 1975; Díaz and Torrecillas, 2002).
Chemical analyses
Table 1 lists the results of the chemical analysis of the AM, AP, ARO and ASL clays. All the samples showed a high SiO2 content (51-72%).
The concentration of Al2O3 in the AM and AP samples is higher than 20%, with a SiO2/Al2O3 ratio of 1.76 and 2.45, respectively. The losses on ignition are close to the theoretical value of pure kaolinite (13.97%), suggesting the presence of kaolinite minerals and free quartz. This was confirmed in the XRD experiments, where it was observed that these clays are composed primarily of quartz and kaolinite with small amounts of anatase. It was also found that the Fe2O3 contents in all the samples are lower than 3%, which suggests that these clays may present pale colors (white, cream or rose) after firing in air. Nevertheless, no mineral phase containing iron was detected by XRD, indicating that it may be present in amorphous form. Alkaline oxides (Na2O + K2O < 2%) and alkaline earth oxides (CaO + MgO < 1%) are also present in small amounts. No feldspars or carbonates were detected by XRD, which indicates that these raw materials were subjected to very strong weathering (leaching).
The ARO and ASL clays present Al2O3 contents of less than 20%. The SiO2/Al2O3 ratio (3.33 and 5.23) is higher than 1.17, which indicates the presence of kaolinite minerals and free quartz in larger quantities than in the AM and AP samples. This finding is confirmed by the low values of loss on ignition (7.25 and 4.37%), the high SiO2 contents found in the chemical analysis, and by the identification of quartz by XRD.
The Fe2O3 content in the ARO sample is higher than in the other samples (5%) and lower than 4% in ASL clay, which is confirmed by the presence of goethite and hematite in both samples. Values of Fe2O3 above 3% indicate that both the clays should take on a reddish color after firing in air. The total alkalinity concentration (K2O + Na2O < 2%) is low in the ARO sample, but requires special attention in ASL clay, where it reaches values exceeding 4%. This high content of alkaline oxides promotes the formation of eutectic systems with a low melting point at 990ºC and 1050ºC in the K2O-SiO2-Al2O3 and Na2O-SiO2-Al2O3 ternary systems, respectively (Levin et al. , 1964; Garcia Verduch, 1985). TiO2 does not exceed 2% in either of the samples, and the corresponding mineral detected by XRD was anatase.
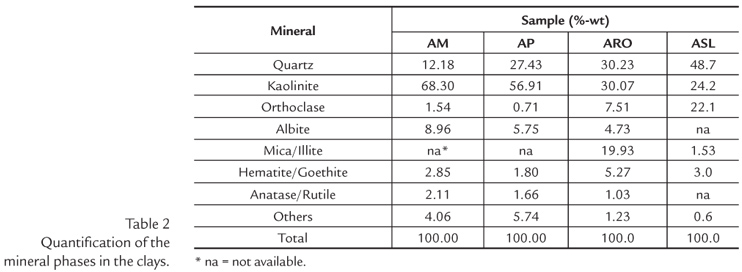
Quantification of the mineral phases
Taking into account the XRD results (AO and total fraction) and the chemical analysis of the total fraction, the mineral composition of the clays was calculated by means of normative calculation (Coelho et al., 2002; Johnson et al., 1985) (Table 2). The ideal formulas of the phases were considered, i.e., kaolinite (Al2O3∙2SiO2∙2H2O), quartz (SiO2), hematite (Fe2O3), anatase (TiO2), illite (K2O∙3Al2O3∙6SiO2∙2H2O), orthoclase (K2O∙Al2O3∙6SiO2), and albite (Na2O∙Al2O3∙6SiO2).
The quantification results confirm the XRD analysis, enabling us to state that the AM and AP clays are kaolinitic clays mixed with quartz and feldspar, while the ARO and ASL clays are mixtures of kaolinite, quartz, feldspar and illite.
Table 3 lists the real density, cation exchange capacity (CEC) and specific surface area of the samples under study. Note that the CEC of the AM and AP clays varies from 11.77 to 12.51 meq/100g, which is compatible with that of Brazilian reference plastic clays used in ceramic whiteware (Cardoso et al., 1998; Barba et al., 1997). On the other hand, the ASL and ARO clays presented CEC values of 8.10 and 8.32 meq/100 g. The densities of all samples changed from 2.51 to 2.63 g/cm3 and specific surface area ranging from 63.18 to 97.58 m2/g.
Thermal behavior
The thermal behavior of the clays determined by TGA and DTA is shown in Figure 3(A-B). The AM and AP clays presented a similar mass loss of 15.23 and 14.33%, which is higher than the theoretical mass loss of kaolinite (13.95%) and was attributed to hygroscopic water and organic matter in the samples. The ARO and ASL clays, in turn, presented a continuous mass loss of 8.77 and 4.30%, respectively. The four clays showed: (i) an endothermic effect with a maximum at 72ºC (AM and AP) and 65ºC (ARO and ASL), which corresponds to free water loss; (ii) another endothermic event whose maximum occurred at 525ºC, corresponding to the dehydroxylation of kaolinite with the formation of metakaolinite; (iii) an exothermic reaction with maximum at about 970ºC, which is attributed to the nucleation of primary mullite and/or Al:Si spinel-type phase (Souza Santos, 1987). No thermal events were detected associated with the decomposition of illite or other phases.
Particle size distributions and Atterberg limits
Table 4 describes the granulometric analysis obtained by sieving and sedimentation.
Table 5 lists the Atterberg limits of the samples. Note that the liquid limit (LL) varied from 44.70 to 57.60 %, the plastic limit (PL) from 27.41 to 30.26%, and the plasticity index (PI) ranged from 17.29 to 28.10%. These results allow us to state that these clays have plasticity indices above 15%, and are considered highly plastic, according to the criteria of soil mechanics (Cardoso et al., 1998).
Technological Properties
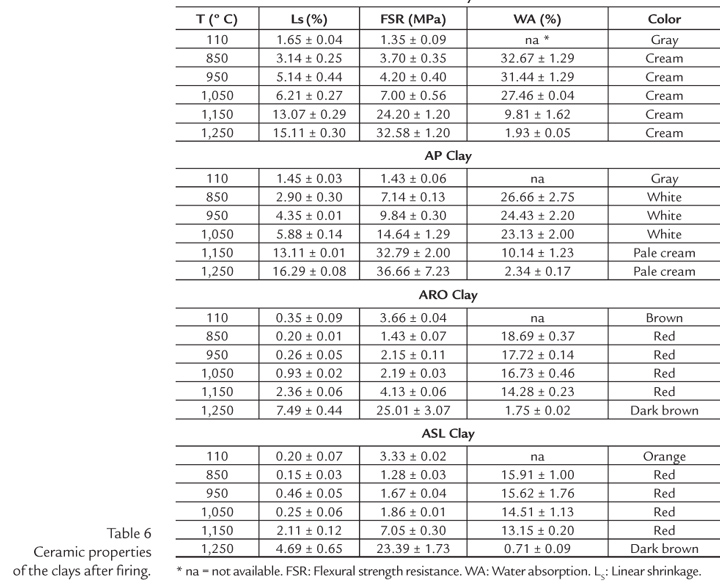
Table 6 lists the technological properties of the clays in the temperature interval of 110-1250ºC.
As can be seen, the flexural strength resistance (FSR) values of the AM and AP clays after drying at 110ºC were 1.35 and 1.43 MPa, respectively. These values are lower than those reported in the literature (Bouger, 1995; Powell, 1996; Wilson, 1998) for Brazilian and foreign ball clays, whose values vary from 4-10 MPa. The AM and AP clays show similar behavior upon firing, since their chemical and mineralogical compositions are identical. The WA of both samples decreases between 850-1250ºC, indicating that their linear shrinkage increase (sintering). The FSR reached values of 30 MPa at 1250ºC. These values are compatible with those of national reference clays used in ceramic whiteware. This finding is especially relevant because in Brazil high quality clays for ceramic whiteware applications are not common, and most of them are located in the state of São Paulo (Motta, 1993; Cardoso et al., 1998).
The ASL and ARO clays present higher FSR values at 110ºC than the AM and AP clays (3.33 MPa - ASL and 3.66 MPa - ARO), which is explained by their improved green packing and their corresponding granulometry (Table 4). The ASL and ARO clays heat-treated at 850ºC present lower LS values than the AM and AP samples. This is also due to the difference in granulometry (Table 2) and the high contents of non-plastic materials in their composition, which favor particle packing and reduce the thermal reactivity of ceramic bodies up to 1050ºC. Between 1050ºC and 1150ºC, both clays undergo a similar increase in their LS (≈ 2%), but at temperatures above 1150ºC the ASL sample shows a higher sintering rate than ARO clay due to the presence of a larger amount of K2O (Table 1), which acts as a fluxing agent, favoring the onset of the formation of liquid phase at temperatures above 985ºC, according to the K2O-Al2O3-SiO2 system (Levin et al., 1964).
Between 850 and 1050ºC, the FSR of ASL and ARO clays remains almost constant, reaching the highest value of 2 MPa. However, in the interval between 1150 and 1250ºC, the FSR increases rapidly until it reach values exceeding 20 MPa.
4. Conclusions
Based on the results of this work, the samples under study can be divided into two groups: (i) Kaolinic clays that present high plasticity, containing small amounts of quartz and accessory minerals in their composition. These raw materials fire in shades of cream to white and, based on their ceramic properties (WA, FSR, SL) in the firing interval applied here (850-1.250 ºC), they can be classified as suitable for us in ceramic whiteware. (ii) Kaolinic clays containing hematite, quartz, feldspar (4% of K2O), illite (ARO), small quantities of smectite (ASL) and of other accessory minerals in their composition, which can be used in red ceramics alone or together with others in the formulation of ceramic bodies. However, special attention should be given to their use in the manufacture of ceramic materials due to the abundance of free silica in their compositions, which may cause an increase in the coefficient of thermal expansion of products, with the appearance of cracks during firing due to the polymorphic transition a«b of cristobalite.
5. Acknowledgments
The authors gratefully acknowledge the Brazilian research funding agencies CAPES (PROCAD-AMAZÔNIA through the project "Map of the Clays of the State of Maranhão: Registration of Occurrences and Mineralogical and Technological Characterization of Ceramic Raw Materials") and FAPEMA for their financial support of this work.
6. References
Artigo recebido em 06 de março de 2012
Aprovado 27 em de junho de 2012
- ANUÁRIO BRASILEIRO DE CERÂMICA. ASSOCIAÇÃO BRASILEIRA DE CERÂMICA (ABC), p. 76 (2002).
- ABNT- ASSOCIAÇÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 6454:1984 - Determinação do limite de liquidez, método de ensaio Rio de Janeiro, 1984.
- _____ NBR 7180:1984 - Determinação do limite de plasticidade, método de ensaio Rio de Janeiro, 1984.
- _____ NBR 6457:1986 - Solo - análise granulométrica Rio de Janeiro, 1984.
- _____ NBR 6457:1986 - Amostras de solo - preparação para ensaios de compactação e ensaios de caracterização Rio de Janeiro, 1986.
- _____ NBR 6113:1997 - Materiais refratários densos conformados - determinação da resistência à flexão à temperatura ambiente. Rio de Janeiro, 1997.
- _____ NBR 6220:1997 - Materiais refratários densos conformados - determinação da densidade de massa aparente, porosidade aparente, absorção e densidade aparente da parte sólida Rio de Janeiro, 1997.
- BARBA, A. et al. Materias Primas para la Fabricación de Soportes de Baldosas Cerámicas Castellón, España: AICE, 1997.
- BOUGER, A. K. Ball Clay. Am. Ceram. Bull., v. 74, n. 6, p. 101-102, 1995.
- CARDOSO, S.R.F., SANTOS, H.S., COELHO A.V.C., SOUZA SANTOS P. Caracterização e propriedades cerâmicas de alguns caulins e argilas usados em cerâmica branca no Estado de São Paulo. Cerâmica Industrial, v. 4, n. 4-6, p. 39-47, 1998.
- _____ Caracterização e propriedades cerâmicas de alguns caulins e argilas usadas em cerâmica branca no Estado de São Paulo. Cerâmica Industrial v. 3, n. 3, p. 47-57, 1998.
- COELHO, C., ROQUEIRO N., HOTZA D.. Rational mineralogical analysis of ceramics. Material Letters, v. 52, n. 6, p. 394-398, 2002.
- DIAZ RODRIGUEZ, A., TORRECILLAS A. Arcillas cerámicas: una revisión de sus distintos tipos, significados y aplicaciones. Bol. Soc. Esp. Cerám. y Vidr, v. 41, n. 5, p. 459-470, 2002.
- EMILIANI G.P., CORBARA, F. Tecnología Cerámica - Le Materia Prime Faenza, Itália: Gruppo Editoriale Faenza Editrice, 1999. p. 115
- FERREIRA, H.C., CHEN, T.P., ZANDONADI, A.R., SOUZA SANTOS, P. Correlações Lineares entre áreas específicas de caulins do Nordeste Brasileiro (Estados da Paraíba e Rio Grande do Norte). Cerâmica, v. 18, n. 71, p. 333-344, 1972.
- GARCIA VERDUCH, A. Origen y composición de las arcillas cerámicas. Bol. Soc. Esp. Cerám. y Vidr., v. 24, n. 6, p. 396-404, 1985.
- CAPUTO, H. P. Mecânica dos solos e suas aplicações fundamentais Rio de Janeiro: Livros Técnicos, 1994. v. 1.
- INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA (IBGE). Em: Contagem da população 2007. http://www.ibge.gov.br/estadosat/perfil.php?sigla=ma Acessado em 10/2011.
- JOHNSON, L.J., CHU, C.H., HUSSEY G.A. Quantitative clay mineral analysis using simultaneous linear equations. Clays and Clay Minerals, v. 33, n. 2, p. 107-117, 1985.
- LEVIN, E.M., ROBBINS C. R., AND MCMURDIE H. F. Fig. 407 (p. 156, K,O-AI,O,-SO,) and Fig. 501 (p. 181, Na,O-Al,O,-SiO,) in Phase Diagrams for Ceramists Edited by M. K. Reser. American Ceramic Society, Columbus, OH, 1964.
- MELLO, I.S.C., MOTTA, J.F.M., BEZERRA, M.S., NESI, J.R., LORETI JR, R. Matérias-primas minerais cerâmicas do Nordeste Brasileiro São Paulo: CPRM, 184 p., 2011.
- MOTTA, J.F.M., TANNO L.C. e CABRAL Jr. M. argilas plásticas para cerâmica branca no Estado de São Paulo - potencialidade geológica. Revista Brasileira de Geociências, v. 23, n. 2, p 158-173, 1993.
- POWELL, P. S. Ball Clay Basics. Am. Ceram. Bull., v. 75, n. 6, p. 74-76, 1996.
- SANTANA A. et al. Cerâmica vermelha para construção: telhas, tijolos e tubos Série Mercado. In: Estudos de Mercado SEBRAE/ESPM, Setembro 2008. 95 p.
- SOUZA SANTOS, P. Ciência e tecnologia das argilas 2. ed. São Paulo: Edgard Blucher, 1992. v. 1.
- THOREZ, J. Phyllosilicates and clay minerals: a laboratory handbook for their x-ray diffraction analysis / Dison Belgium: Editions G. Lelotte, 1975. 579 p.
- VIEIRA COELHO A. C., SOUZA SANTOS P. Argilas especiais: o que são, caracterização e propriedades. Química Nova, v. 30, n. 1, p. 146-152, 2007.
- WILSON I. R. Constituição, avaliação e propriedades cerâmicas de "ball clays". Cerâmica, v. 44, n. 287-288, p. 88-117, 1998.
Publication Dates
-
Publication in this collection
13 Dec 2012 -
Date of issue
Dec 2012
History
-
Received
06 Mar 2012 -
Accepted
27 June 2012