Abstracts
CD55 and CD59 are glycosylphosphatidylinositol-anchored proteins with regulatory properties on the activating cascades of the complement system. This regulation occurs through inhibition of the C3-convertase formation by CD55, and prevention of the terminal polymerization of the membrane attack complex by CD59. Deficiency in the expression of these proteins can be associated with increased susceptibility to complement-mediated cell death. Systemic lupus erythematosus patients with hemolytic anemia and lymphopenia seem to have an acquired deficiency of CD55 and CD59 proteins. However, the mechanisms involved in this deficiency and its impact on the clinical manifestation of SLE needs to be further investigated.
systemic lupus erythematosus (SLE); CD55; CD59; complement
CD55 e CD59 são proteínas de membrana ancoradas por glicosilfosfatidilinositol que apresentam propriedades reguladoras da ativação da cascata do complemento. Essa regulação ocorre através da inibição da C3 convertase pelo CD55 e prevenção da etapa final de polimerização do complexo de ataque à membrana pelo CD59. Deficiência na expressão dessas proteínas pode estar associada a uma maior ativação do sistema complemento, inclusive do complexo de ataque à membrana, levando à morte celular. Pacientes com lúpus eritematoso sistêmico, com anemia hemolítica e linfopenia, parecem apresentar uma deficiência adquirida de CD55 e CD59. Contudo, os mecanismos que modulam essa diminuída expressão continuam desconhecidos e o seu impacto nas manifestações do lúpus eritematoso sistêmico precisa ser mais bem estudado.
lúpus eritematoso sistêmico (LES); CD55; CD59; complemento
REVIEW ARTICLE
IBiochemical Pharmacist of the Clinical Pathology Service and Rheumatology Service at Hospital de Clínicas de Porto Alegre
IIRheumatologist of the Rheumatology Service at Hospital de Clínicas de Porto Alegre
IIIAssistant Professor of the Department of Internal Medicine at Faculdade de Medicina/UFGRGS
Correspondence to
ABSTRACT
CD55 and CD59 are glycosylphosphatidylinositol-anchored proteins with regulatory properties on the activating cascades of the complement system. This regulation occurs through inhibition of the C3-convertase formation by CD55, and prevention of the terminal polymerization of the membrane attack complex by CD59. Deficiency in the expression of these proteins can be associated with increased susceptibility to complement-mediated cell death. Systemic lupus erythematosus patients with hemolytic anemia and lymphopenia seem to have an acquired deficiency of CD55 and CD59 proteins. However, the mechanisms involved in this deficiency and its impact on the clinical manifestation of SLE needs to be further investigated.
Keywords: systemic lupus erythematosus (SLE), CD55, CD59, complement.
INTRODUCTION
Complement system
Complement system (CS) is traditionally defined as a sequentially activated soluble serum proteins cascade, leading to cell death through direct lysis and/or phagocyte activation. CS in mammals has more than 30 serum and cell membrane proteins produced mainly by the liver. However, many cell types, like monocytes, fibroblasts, epithelial and endothelial cells, may also synthesize most components of the complement system.1
Evidences in the literature suggest that CS can perform an important immunoregulatory function through its role in humoral immunity,2 T cells immunity modulation,3 and tolerance regulation for own nuclear antigens.4 Although CS is well known for its highly efficient role in defense against pathogens such as bacteria, virus and parasite-infected cells, it has been calling attention of researchers due to its potential to damage cells in the organism.5
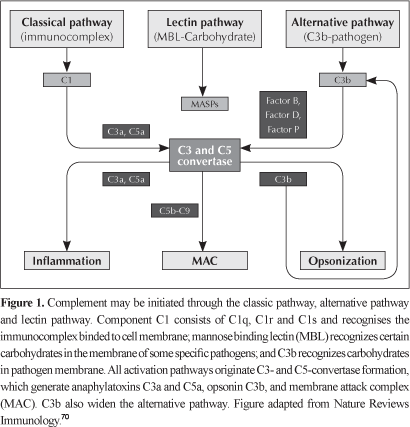
Activating cascades of the complement system may be initiated through the classic pathway (antibody dependent), alternative pathway (spontaneous) or lectin pathway (mediated by mannose-binding lectins pathway). After its activation, fragments generated from complement work modulating humoral and cell reactions, especially chemotaxis and anaphylaxis, through interaction of these activation fragments with cell receptors or through deposition of protein complexes in cell membrane.6
The classic pathway, a powerful mechanism of humoral immunity, is activated by C1 component interaction with the constant fraction (CF) of immunoglobulin (Ig) IgM or IgG complex to antigen (antibody-antigen immune complex). C1 consists of three proteins (C1q, C1r, C1s), and for C1 complex activation at least two of its six globular sites should bind to Ig molecules linked to the pathogen. After this binding, C1q suffers a conformation change generating C1r activation and C1s cleavage that can cleave C4 and C2. C4b fragment is bound to the pathogen cell membrane and allows the binding of C2a; the formed C4b2a complex is the C3-convertase of the classic pathway.7,8
The alternative pathway is directly activated when there are no antibodies by particles rich in carbohydrates found in the invasive microorganism surface, involving C3b binding (found soluble in plasma) and other components in the alternative pathway: factor B, factor D and properdin (factor P).9 Factor B is a serine protease, which is homologous to C2. Factor B, after factor D cleavage, binds to C3b forming C3bBb (C3-convertase in the alternative pathway). Properdin can stable C3bBb complex, which can cleave other C3 molecules.10
Lectin pathway is activated by MBL - mannose-binding lectin, a soluble component in our organism, with carbohydrates found in the target microorganism surface. MBL is a member of calcium-dependent lectins and has a C1q-similar structure. After its activation, occurs interaction with MBL-associated serine protease - MASPs, including MASP-1, MASP-2 and MASP-3, which can cleave structures in complement C4 and C2 and generate C3-convertase (C4b2a) and C5-convertase (C4b2a3b).11,12
Therefore, the three activation pathways converge to generate proteolytic enzymes, called C3-convertases, that cleave protein C3 in C3a and C3b. Fragment C3b generated combines with C3-convertase originating C5-convertase, which cleaves C5 into C5a and C5b. Fragments C3a and C5a are powerful anaphylatoxins. Fragment C5b aggregates with C6 and C7 to form the C5b-7 insertion complex; after this step, the recruitment of C8 and multiple units of C9 in the target cell membrane occurs, forming the MAC - membrane attack complex13,14 (Figure 1). MAC's functional unit is a pore inserted in the phospholipid bilayer that interferes in selective permeability of membrane, allowing water, ions and small molecules to enter to target cell cytosol, leading to its rupture.15
Additionally to an effector action against pathogens, the complement has other biological activities in the organism, such as: opsonization and phagocytosis, solubilization and removal of immune complexes and apoptotic cells, interface between innate and adaptative immunity, and proinflammatory action. These effects occur through the binding of activation products to specific membrane receptors found in different types of cells.1, 6, 13
When complement is activated by external antigen-directed antibodies, but also eventually by its own antigens, explosive and uncertain activation of the final common pathway and excessive formation of inflammation may damage tissues and autological cells. In order to protect against or restrain these damages, CS is strongly regulated by soluble substances or cell membrane bound substances.16
CD55/CD59 COMPLEMENT REGULATORY PROTEINS
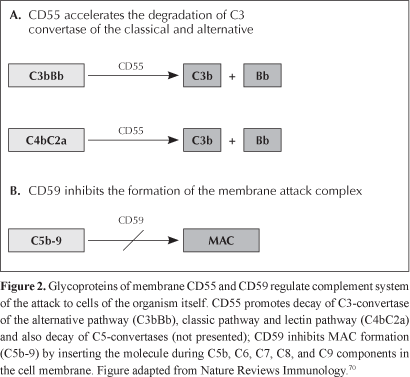
Normal cells, which are resistant to autologous lysis mediated by complement, have a regulatory complement system in cell membrane made of proteins, and the main proteins are CD55, DAF - decay accelerating factor, and CD59, or MIRL - membrane inhibitor of reactive lysis (Table 1). CD55 inhibits formation of new C3- and C5-convertases, preventing C3 and C5 cleavage, additionally to accelerating the preformed enzyme decay.17 CD59 protein is the only membrane regulator that interferes directly on the MAC structure through its physical incorporation to the forming complex, avoiding the binding of C9 units to the C5b-8 complex18 (Figura 2).
CD55, reviewed in Mikesch et al.,19 is a globular glycoprotein anchored to the cell membrane by glycosylphosphatidylinositol (GPI), with molecular weight ranging from 50 to 100 kDa in different cell types. It is found in soluble form in plasma, tear fluid, saliva, urine, synovial liquid and liquor.20 Additionally to being complement regulator, CD55 seems to protect cells against lysis mediated by NK- natural killers cells. CD55 may also work as an intercellular adhesion binder, interacting with CD97 in leucocytes, and as a receptor for certain virus and microorganisms.21
CD59, reviewed by Kimberley et al.,16 is a small globular glycoprotein, also anchored by GPI, of almost 20 kDa, belonging to the family of leukocytary antigen 6 (Ly-6). Due to its crucial role in preventing damages to the organism itself through inadequate deposition of MAC lithium complex, this protein is widely expressed in most tissues and in all circulating cells.
The pathologic consequence of complement regulatory deficiency found in the membrane was initially observed in paroxysmal nocturnal hemoglobinuria (PNH). This acquired hematologic disease was first described in 1866 by William Gull, and in 1882 by Paul Strubing, as a different form of rare hemolytic anemia, associated with hemoglobinuria during the night.22 PNH is characterized by increasing lysis in erythrocytes due to reduction of membrane proteins bound to GPI, especially CD55 and CD59, responsible for inhibiting autologous cell lysis of complement.23
PNH is a clonal disorder in which a PIG-A gene (phosphatidylinositol glycan A) mutation of chromosome X occurs, leading to an abnormal biosynthesis of GPI anchor for lipidic membrane.22 As it is a clonal disorder in hematopoietic stem cells, all cell blood lines are affected, and in NPH patients subpopulations of deficient and normal cells are normally found. Among GPI-anchored proteins, we find complement regulatory proteins, like CD55, CD59, and CD46 (membrane cofactor protein); and other proteins involved in immune function,24,25 like receptor FC (CD16) in granulocytes and NK cells, lipopolysaccharide (CD14) in monocytes, cell adhesion molecule (CD58) in all hematopoietic cells, and CD24 in lymphocytes, with still unknown activity.
There are few reports in the literature about the normal expression pattern of these proteins in blood cells. Araten et al.,26 in 1999, and Hu et al.,27 in 2005, showed that clones with PIG-A gene mutation are found in normal subjects. Oelschlaegel et al.,23 in 1999, analyzed blood samples of 52 healthy donors by flow cytometry (FC) and observed 3% of CD55/CD59 deficiency in normal granulocytes and erythrocytes. Isolated CD55 deficiency in human beings was not associated with intravascular hemolysis or with other evidence of failure in complement regulation. However, isolated CD59 deficiency was associated with signs and symptoms similar to HPN28 due to the fact that CD59 is a more effective inhibitor in complement, as it blocks the formation of membrane attack complex.
CD55 and CD59 deficiency has been studied in other diseases and correlated with its severity. 29-36 Yamaguchi et al.37 showed that 28.6% of the patients with aplastic anemia (AA) and 27.8% of the patients with myelodysplasia syndrome (MDS) presented a poor population of CD59 in erythrocytes. Wang et al.38 observed a significant CD55 and CD59 reduction in 52% of neutrophils in patients with AA not treated. This deficiency causes hemolytic processes mediated by complement similar to those found in HPN.
In 2007 Isoda et al.,39 evaluated 40 healthy subjects for flow cytometry as a control to evaluate if patients with lymphoproliferative disease of granular lymphocytes shared a HPN phenotype. Cutoff value obtained for the negative cells ratio in healthy subjects was lower than 0.04% in granulocytes and lower than 0.07% in erythrocytes, both for CD55 and CD59. DLLG patient cells did not demonstrate change in CD55 and CD59 expression, excepting granular lymphocytes with CD16+CD56- phenotype, which presented deficiency of these proteins.
Resistance of carcinogenic cells to lysis mediated by complement is one of the strategies acquired by these cells, characterizing an obstruction in developing immunotherapies based on antitumor antibodies that fix complement.40 Recently, studies evaluated the over expression of complement regulatory proteins in cells and tissues as a cellular defense mechanism against an exacerbated attack from complement system. 41,45 This mechanism may generate resistance to drugs used in immunotherapy with complement-mediated action, such as the case of rituximab, CD20 molecule-directed chimerical monoclonal antibody, which promotes lymphocyte B depletion. We believe that one of the action mechanisms is the signaling and induction of complement-mediated B cell apoptosis. This drug has been more and more used as an efficient and specific treatment, especially in lymphoproliferation B (particularly lymphomas) and autoimmune diseases.46-49
CD55/CD59 COMPLEMENT REGULATORY PROTEINS IN AUTOIMMUNE DISEASES
Recent studies in animal models with autoimmune diseases concurrent with removal of complement regulatory proteins, through addition of monoclonal antibodies or gene deletion,50-53 have evaluated the role of CD55 and CD59 in organism cells.54
Multiple sclerosis (MS) is among the pathologies studied in this context; this disease attack the central nervous system (CNS) and is more common in young adults. Its etiology is still unknown, but there is evidence of formation of autoantibodies against antigens in the myelin layer. In MS, loss of myelin (demyelination) interferes in pulse transmission, causing varied symptoms of the disease.55 Some trials with CD55 and CD59 gene deficiency50,51 in models of experimental autoimmune encephalomyelitis (animal model for MS studies) have shown that these animals presented a more severe level of disease when compared with controls. Mead et al. also reported that C6 deficient rats, incapable to form MAC, did not present axonium damage nor demyelination, and clinical manifestations were less intense.56
Antineutrophil cytoplasmic autoantibodies (ANCA) are cytoplasmatic antiproteins specific for neutrophils and monocytes, and myeloperoxidase and proteinase 3 are the main target antigens in patients with vasculitis and glomerulonephritis. Xiao et al.57 suggest that ANCA-stimulation of neutrophils releases factors that activate complement through the alternative pathway, causing inflammatory amplification of the disease. Matsuo et al.58 reported that neutralization with anti-CD59 monoclonal antibodies in kidney cells in rats causes an exacerbation of the disease in experimental models of glomerulonephritis.
In myasthenia gravis ( mg), the immune system produces antibodies against nicotinic acetylcholine receptors located in the neuromuscular junction, preventing muscular activation. It is suggested that the complement system participates in the mg pathology based on the identification of complement activation products in plasma and deposit in the motor plate of patients.59 Kaminki et al. showed in studies with mice that increasing expression of CD55 and CD59 protects against the loss of acetylcholine and reduces muscle weakness.31
THE ROLE OF CD55/CD59 PROTEINS AND COMPLEMENT IN CYTOPENIAS SECONDARY TO SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease that affects especially young women, characterized by attacking multiple organs and presenting changes in the immune response, with the presence of antibodies against proteins in the organism itself.60 Hematological abnormalities are commonly found in SLE patients, and anemia and lymphopenia are the most frequent changes.61-63 Anemia in chronic disease, due to iron deficiency, and autoimmune hemolytic anemia (AIHA) are the most common forms in patients with SLE, and drug-induced myelotoxicity and anemia due to chronic kidney failure may also occur.64 Lymphopenia is particularly present during active disease and is strongly related with IgM cryoglobulins, fixation of complement and antilymphocytes antibodies. Autoantibodies against blood cells may cause cell lysis for antibody-dependent cytotoxicity mechanisms, opsonization, apoptosis and receptors blocks, and others.42
Antibodies produced in autoimmune diseases may connect to cell surface antigens or form immune complexes after binding to circulating antigens. These immune complexes tend to be deposited in organs, like kidney glomerulus, with subsequent activation of complement system by the classic pathway, damaging tissues.6 Despite the known effector action of complement in damage of organs in autoimmune diseases, little is known about the mechanism of regulatory proteins of complement membrane in severity modulation of this damage.65
A work published by Miwa et al.66 showed that Daf-1 gene deletion, which codifies the CD55 molecule in MRL/lpr mice, an experimental model widely used to study LES, exacerbated the severity of the autoimmune disease. These animals presented stressed lymphadenopathy and esplenomegalia, higher levels of anti-chromatin antibodies and more severe dermatitis than controls.
Until today few studies have been carried out about CD55 and CD59 expression profile in lymphocytes and erythrocytes of patients with LES,67 and no study evaluated expression in granulocytes and monocytes. Richaud-Patin et al.68 for instance, evaluated the intensity of CD55 and CD59 expression in erythrocyte membrane in patients with AHAI, and a reduction of these proteins was found in erythrocytes of patients with lupus who presented secondary AHAI. In this study, authors evaluated the presence of IgG and IgM antiphospholipid antibodies and no correlation between these immunoglobulins and the CD55 and CD59 expression was found.
Later, the same authors42 researched the intensity of CD55 and CD59 expression in T and B lymphocytes in LES patients with and without lymphopenia and showed that cells in patients with lymphopenia presented reduction of CD55 and CD59 expression when compared with controls. Interestingly, they found that, in patients with SLE who did not present lymphopenia, lymphocytes presented a higher intensity of these proteins than controls. Another finding of this study was that titer of tested autoantibodies (anti-SSA, anti-dsDNA and ribosomal anti-P) was higher in lymphopenic patients. However, the prevalence of antibody positivity was equal, excepting anti-SSA, which was significantly higher in the group of patients with lymphopenia - findings that corroborate those previously reported in the literature.63,65,67,68
In order to evaluate apoptosis in vitro in autoimmune diseases, Tsunoda et al.69 observed a reduced expression of CD59 in T CD8+lymphocytes, but not in T CD4+ lymphocytes, both in patients with LES and in patients with Sjögren's syndrome and predominantly in active T CD8+cells expressing CD45RO+ and HLADR+. In this same study, it was shown that TCD8+CD59dim cells (low expression) were more susceptible to apoptosis in vitro. According to the data found in this study, authors suggest that reduction of CD59 expression in activated T CD8+ cells could be related with the disease activity and activation or induction of apoptosis in those patients.
Arosa et al.43 evaluated CR1 expression (complement receptor 1), CD55 and CD59 in erythrocytes and glomerulum cells of lupus patients with diffuse proliferative glomerulonephritis; CR1 expression was reduced in patients with LES and DPG, both in glomerulum cells and erythrocytes, and, interestingly, CD55 and CD59 were increasing in these cells. Authors suggest that this CD55 and CD59 increase happens to compensate reduced CR1 expression (complement regulator in C3- and C5-convertase level) and as an attempt of the cell to protect against complement action.
CONCLUSION
Few studies on CD55 and CD59 expression profile in patients with SLE are found in the literature. Acquired deficiency of these proteins in SLE does not seem to be dependent on genetic mutations, as in HPN, and was not correlated with autoantibody production either. On the other hand, it seems that there is an association with the disease activity. Additionally, the role of these proteins in the induction of SLE cytopenias is not very well defined. However, studies suggest that levels of CD55 and CD59 cell expression below normal provide low protection to exacerbated lysis mediated by complement. Interestingly, cells expressing high levels of these proteins appear to be involved with a mechanism to protect cytolic actions of complement.
REFERENCES
-
1Walport MJ. Complement. First of two parts. N Engl J Med 2001;344(14):1058-66.
-
2Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J et al MarkedLy impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA 1996;93(8):3357-61.
-
3Kaya Z, Afanasyeva M, Wang Y, Dohmen KM, Schlichting J, Tretter T et al Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat Immunol 2001;2(8):739-45.
-
4Carroll MC. The role of complement in B cell activation and tolerance. Adv Immunol 2000;74:61-88.
-
5Welch TR. Complement in glomerulonephritis. Nat Genet 2002;31(4):333-4.
-
6Walport MJ. Complement. Second of two parts. N Engl J Med 2001;344(15):1140-4.
-
7Schumaker VN, Zavodszky P, Poon PH. Activation of the first component of complement. Annu Rev Immunol 1987;5:21-42.
-
8Gewurz H, Ying SC, Jiang H, Lint TF. Nonimmune activation of the classical complement pathway. Behring Inst Mitt 1993;(93):138-47.
-
9Farries TC, Lachmann PJ, Harrison RA. Analysis of the interactions between properdin, the third component of complement (C3), and its physiological activation products. Biochem J 1988;252(1):47-54.
-
10Gupta R, Ahuja T, Agraharkar M. Disorders of the complement system: an overview. Saudi J Kidney Dis Transpl 2002;13(2):119-25.
-
11Monticielo OA, Mucenic T, Xavier RM, Brenol JC, Chies JA. The role of mannose-binding lectin in systemic lupus erythematosus. Clin Rheumatol 2008;27(4):413-9.
-
12Dahl MR, Thiel S, Matsushita M, Fujita T, Willis AC, Christensen T et al MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity 2001;15(1):127-35.
-
13Song WC, Sarrias MR, Lambris JD. Complement and innate immunity. Immunopharmacology 2000;49(1-2):187-98.
-
14Mollnes TE, Song WC, Lambris JD. Complement in inflammatory tissue damage and disease. Trends in immunology 2002;23(2):61-4.
-
15Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J 1989;264(1):1-14.
-
16Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Molecular immunology 2007;44(1-3):73-81.
-
17Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol 1989;7:35-58.
-
18Farkas I, Baranyi L, Ishikawa Y, Okada N, Bohata C, Budai D et al CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9. J Physiol 2002;539(Pt 2):537-45.
-
19Mikesch JH, Buerger H, Simon R, Brandt B. Decay-accelerating factor (CD55): a versatile acting molecule in human malignancies. Biochim Biophys Acta 2006;1766(1):42-52.
-
20Medof ME, Walter EI, Rutgers JL, Knowles DM, Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med 1987;165(3):848-64.
-
21Hamann J, Vogel B, van Schijndel GM, van Lier RA. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J Exp Med 1996;184(3):1185-9.
-
22Tomita M. Biochemical background of paroxysmal nocturnal hemoglobinuria. Biochim Biophys Acta 1999;1455(2-3):269-86.
-
23Oelschlaegel U, Besson I, Arnoulet C, Sainty D, Nowak R, Naumann R et al A standardized flow cytometric method for screening paroxysmal nocturnal haemoglobinuria (PNH) measuring CD55 and CD59 expression on erythrocytes and granulocytes. Clin Lab Haematol 2001;23(2):81-90.
-
24Holguin MH, Fredrick LR, Bernshaw NJ, Wilcox LA, Parker CJ. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest 1989;84(1):7-17.
-
25Blanchard D, Navenot JM, Petit-Le Roux Y, Willem C, Loirat MJ. Flow cytometry and immunoblotting analysis of monoclonal antibodies directed to complement regulatory proteins. Transfus Clin Biol 1997;4(1):131-4.
-
26Araten DJ, Nafa K, Pakdeesuwan K, Luzzatto L. Clonal populations of hematopoietic cells with paroxysmal nocturnal hemoglobinuria genotype and phenotype are present in normal individuals. Proc Natl Acad Sci USA 1999;96(9):5209-14.
-
27Hu R, Mukhina GL, Piantadosi S, Barber JP, Jones RJ, Brodsky RA. PIG-A mutations in normal hematopoiesis. Blood 2005;105(10):3848-54.
-
28Motoyama N, Okada N, Yamashina M, Okada H. Paroxysmal nocturnal hemoglobinuria due to hereditary nucleotide deletion in the HRF20 (CD59) gene. Eur J Immunol 1992;22(10):2669-73.
-
29Meletis J, Terpos E, Samarkos M, Meletis C, Konstantopoulos K, Komninaka V et al Detection of CD55 and/or CD59 deficient red cell populations in patients with aplastic anaemia, myelodysplastic syndromes and myeloproliferative disorders. Haematologia (Budap) 2001;31(1):7-16.
-
30Azenishi Y, Ueda E, Machii T, Nishimura J, Hirota T, Shibano M et al CD59-deficient blood cells and PIG-A gene abnormalities in Japanese patients with aplastic anaemia. Br J Haematol 1999;104(3):523-9.
-
31Kaminski HJ, Kusner LL, Richmonds C, Medof ME, Lin F. Deficiency of decay accelerating factor and CD59 leads to crisis in experimental myasthenia. Exp Neurol 2006;202(2):287-93.
-
32Meletis J, Terpos E, Samarkos M, Meletis C, Apostolidou E, Komninaka V et al Detection of CD55- and/or CD59-deficient red cell populations in patients with plasma cell dyscrasias. Int J Hematol 2002;75(1):40-4.
-
33Meletis J, Terpos E, Samarkos M, Meletis C, Apostolidou E, Komninaka V et al Detection of CD55- and/or CD59-deficient red cell populations in patients with lymphoproliferative syndromes. Hematol J 2001;2(1):33-7.
-
34Ruiz P, Weppler D, Tryphonopoulos P, Nishida S, Moon J, Kato T et al CD55 and CD59 deficiency in transplant patient populations: possible association with paroxysmal nocturnal hemoglobinuria-like symptoms in Campath-treated patients. Transplant Proc 2006;38(6):1750-2.
-
35Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M et al Minor population of CD55-CD59- blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood 2006;107(4):1308-14.
-
36Yang LB, Li R, Meri S, Rogers J, Shen Y. Deficiency of complement defense protein CD59 may contribute to neurodegeneration in Alzheimer's disease. J Neurosci 2000;20(20):7505-9.
-
37Yamaguchi M, Machii T, Azenishi Y, Nishimura J, Shibano M, Kanakura Y et al Detection of small populations of CD59-deficient erythrocytes in patients with aplastic anemia or myelodysplastic syndrome and normal individuals. Blood Cells Mol Dis 2000;26(3):247-54.
-
38Wang H, Chuhjo T, Yamazaki H, Shiobara S, Teramura M, Mizoguchi H et al Relative increase of granulocytes with a paroxysmal nocturnal haemoglobinuria phenotype in aplastic anaemia patients: the high prevalence at diagnosis. Eur J Haematol 2001;66(3):200-5.
-
39Isoda A, Tsukamoto N, Mitsui T, Yamane A, Hatsumi N, Matsushima T et al Expression of CD55 and CD59 on peripheral blood cells in patients with lymphoproliferative disease of granular lymphocytes. Int J Lab Hematol 2007;29(1):52-7.
-
40Green MC, Murray JL, Hortobagyi GN. Monoclonal antibody therapy for solid tumors. Cancer Treat Rev 2000;26(4):269-86.
-
41Li SH, Szmitko PE, Weisel RD, Wang CH, Fedak PW, Li RK et al C-reactive protein upregulates complement-inhibitory factors in endothelial cells. Circulation 2004;109(7):833-6.
-
42Garcia-Valladares I, Atisha-Fregoso Y, Richaud-Patin Y, Jakez-Ocampo J, Soto-Vega E, Elias-Lopez D et al Diminished expression of complement regulatory proteins (CD55 and CD59) in lymphocytes from systemic lupus erythematosus patients with lymphopenia. Lupus 2006;15(9):600-5.
-
43Arora M, Arora R, Tiwari SC, Das N, Srivastava LM. Expression of complement regulatory proteins in diffuse proliferative glomerulonephritis. Lupus 2000;9(2):127-31.
-
44Cuida M, Legler DW, Eidsheim M, Jonsson R. Complement regulatory proteins in the salivary glands and saliva of Sjogren's syndrome patients and healthy subjects. Clin Exp Rheumatol 1997;15(6):615-23.
-
45Qin C, Cai XY. [Research progression on complement regulatory proteins CD46, CD55, and CD59 in tumor immunotherapy]. Ai Zheng 2006;25(11):1450-3.
-
46Golay J, Lazzari M, Facchinetti V, Bernasconi S, Borleri G, Barbui T et al CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood 2001;98(12):3383-9.
-
47Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S et al Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood 2000;95(12):3900-8.
-
48Takei K, Yamazaki T, Sawada U, Ishizuka H, Aizawa S. Analysis of changes in CD20, CD55, and CD59 expression on established rituximab-resistant B-lymphoma cell lines. Leuk Res 2006;30(5):625-31.
-
49Thatayatikom A, White AJ. Rituximab: a promising therapy in systemic lupus erythematosus. Autoimmun Rev 2006;5(1):18-24.
-
50Mead RJ, Neal JW, Griffiths MR, Linington C, Botto M, Lassmann H et al Deficiency of the complement regulator CD59a enhances disease severity, demyelination and axonal injury in murine acute experimental allergic encephalomyelitis. Lab Invest 2004;84(1):21-8.
-
51Liu J, Miwa T, Hilliard B, Chen Y, Lambris JD, Wells AD et al The complement inhibitory protein DAF (CD55) suppresses T cell immunity in vivo. J Exp Med 2005;201(4):567-77.
-
52Tuzun E, Saini SS, Morgan BP, Christadoss P. Complement regulator CD59 deficiency fails to augment susceptibility to actively induced experimental autoimmune myasthenia gravis. J Neuroimmunol 2006;181(1-2):29-33.
-
53Lin F, Salant DJ, Meyerson H, Emancipator S, Morgan BP, Medof ME. Respective roles of decay-accelerating factor and CD59 in circumventing glomerular injury in acute nephrotoxic serum nephritis. J Immunol 2004;172(4):2636-42.
-
54Holt DS, Botto M, Bygrave AE, Hanna SM, Walport MJ, Morgan BP. Targeted deletion of the CD59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood 2001;98(2):442-9.
-
55Lutterotti A, Berger T, Reind LM. Biological markers for multiple sclerosis. Curr Med Chem 2007;14(18):1956-65.
-
56Mead RJ, Singhrao SK, Neal JW, Lassmann H, Morgan BP. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol 2002;168(1):458-65.
-
57Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol 2007;170(1):52-64.
-
58Matsuo S, Nishikage H, Yoshida F, Nomura A, PiddLesden SJ, Morgan BP. Role of CD59 in experimental glomerulonephritis in rats. Kidney Int 1994;46(1):191-200.
-
59Chamberlain-Banoub J, Neal JW, Mizuno M, Harris CL, Morgan BP. Complement membrane attack is required for endplate damage and clinical disease in passive experimental myasthenia gravis in Lewis rats. Clin Exp Immunol 2006;146(2):278-86.
-
60D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet 2007;369(9561):587-96.
-
61Keeling DM, Isenberg DA. Haematological manifestations of systemic lupus erythematosus. Blood Rev 1993;7(4):199-207.
-
62Nossent JC, Swaak AJ. Prevalence and significance of haematological abnormalities in patients with systemic lupus erythematosus. Q J Med 1991;80(291):605-12.
-
63Wenzel J, Gerdsen R, Uerlich M, Bauer R, Tueting T, Bieber T. Lymphocytopenia in lupus erythematosus: close in vivo association to autoantibodies targeting nuclear antigens. Br J Dermatol 2004;150(5):994-8.
-
64Voulgarelis M, Kokori SI, Ioannidis JP, Tzioufas AG, Kyriaki D, Moutsopoulos HM. Anaemia in systemic lupus erythematosus: aetiological profile and the role of erythropoietin. Ann Rheum Dis 2000;59(3):217-22.
-
65Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol 2006;118(2-3):127-36.
-
66Miwa T, Maldonado MA, Zhou L, Sun X, Luo HY, Cai D et al Deletion of decay-accelerating factor (CD55) exacerbates autoimmune disease development in MRL/lpr mice. Am J Pathol 2002;161(3):1077-86.
-
67Ruiz-Arguelles A, Llorente L. The role of complement regulatory proteins (CD55 and CD59) in the pathogenesis of autoimmune hemocytopenias. Autoimmun Rev 2007;6(3):155-61.
-
68Richaud-Patin Y, Perez-Romano B, Carrillo-Maravilla E, Rodriguez AB, Simon AJ, Cabiedes J et al Deficiency of red cell bound CD55 and CD59 in patients with systemic lupus erythematosus. Immunology letters 2003;88(2):95-9.
-
69Tsunoda S, Kawano M, Koni I, Kasahara Y, Yachie A, Miyawaki T et al Diminished expression of CD59 on activated CD8+ T cells undergoing apoptosis in systemic lupus erythematosus and Sjogren's syndrome. Scand J Immunol 2000;51(3):293-9.
-
70Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol 2007;7(1):9-18.
The role of CD55/CD59 complement regulatory proteins on peripheral blood cells of systemic lupus erythematosus patients
Publication Dates
-
Publication in this collection
07 July 2009 -
Date of issue
June 2009
History
-
Accepted
18 Feb 2009 -
Received
14 Oct 2008