Abstracts
OBJECTIVES: To describe a population of children diagnosed with Kawasaki's disease (KD) in pediatric rheumatology centers of Rio de Janeiro, Brazil, defining the magnitude of the delay period in diagnosing KD and initiating treatment due to confusion with common childhood febrile illnesses and the impact of this delay on the frequency of coronary sequels. METHODS: Data analysis from hospital records summarized in a dedicated form, including name, gender, age, date of first recorded clinical signs, date of admission to the specialty service, information about symptoms, clinical evolution, intravenous immunoglobulin (IVIG) use and coronary sequels. RESULTS: Of 125 patients, 63% were males. 40% were under 2 years at diagnosis. Average lapse between earliest signs and KD diagnosis was 12 days (mean fever duration, 14 d). Only 22.4% had a diagnosis of KD before entering the specialty service. For the remainder, initial hipotheses included: bacterial (60%) and viral infections (12%), rheumatological diseases (4%) and adverse vaccination reactions (1.6%). Hence, prevalent febrile illnesses of childhood were major confounding factors. For records (85.6%) mentioning treatment, 46.7% reported IVIG treatment, beginning after day 10 in 23 cases (21.5%). 20 patients (16%) presented coronary sequels, 9 of which were diagnosed late, including 3 given IVIG after day 10, and 6 given no IVIG. We found no significant association between the frequency of coronary sequels and: a) sex; b) age; c) clinical criteria; d) initiation of IVIG treatment (before or after day 10). CONCLUSIONS: Common febrile illnesses of childhood often confound the diagnosis of KD.
vasculitis; mucocutaneous lymph node syndrome; coronary artery disease; antibiotic prophylaxis
OBJETIVOS: Descrever uma população de crianças com diagnóstico de doença de Kawasaki (DK) atendida em centros de reumatologia pediátrica do Rio de Janeiro. Analisar o período de atraso no diagnóstico e início do tratamento, devido à dificuldade de distinguir DK de outras doenças febris comuns da infância; e o impacto deste atraso na frequência de sequelas coronarianas. MÉTODOS: Os dados analisados incluíram: nome, sexo, idade, data do inicio dos sintomas e da admissão no serviço especializado, sintomatologia, evolução clínica, uso de Imunoglobulina Endovenosa (IGEV) e complicações coronarianas. RESULTADOS: Dos 125 casos estudados, 63% eram meninos. 40% tinham menos de 2 anos no momento do diagnóstico. O intervalo médio entre o inicio dos sintomas e o diagnóstico de DK foi de 12 dias (duração média da febre = 14 dias). Dos casos estudados, 22,4% receberam o diagnostico de DK antes do atendimento em serviço especializado; nos demais, as hipóteses diagnosticas iniciais incluíam: infecções bacterianas (60%), virais (12%), outras doenças reumatológicas (4%) e reações adversas à vacinação (1,6%). Em 85.6 % dos casos registrou-se o tratamento realizado, sendo administrada IGEV em 46,7%, e a partir do 10º dia em 21,5% dos casos. Dos 20 pacientes apresentando sequelas coronarianas, 9 tiveram diagnóstico tardio, incluindo 3 iniciando tratamento após o 10º dia e 6 sem tratamento. Não encontramos associação significativa entre a frequência de sequelas coronarianas e: sexo; idade; critérios clínicos; tratamento com IGEV antes ou depois do 10º dia de doença. CONCLUSÕES: O diagnóstico de DK pode ser atrasado pela dificuldade em diferenciá-lo de outras doenças febris da infância.
vasculite; síndrome do nódulo linfático mucocutâneo; doença das coronárias; antibioticoprofilaxia
ORIGINAL ARTICLE
IPediatric Reumatologist of SMS / RJ - MSc
IIPediatric Reumatologist of SMS/RJ - MSc
IIIPediatric Reumatologist of IPPMG-UFRJ e SMS / RJ - MSc
IVAssistent-Professor of UFRJ, Pediatric Reumatologist of UERJ - MSc
VMD, Titular researcher, Departament of Pediatrics IFF-FIOCRUZ - Titular researcher on Public Health
VIProfessor, Faculdade de Medicina, UFRJ, Pediatric Reumatologist - PhD
Correspondence to
ABSTRACT
OBJECTIVES: To describe a population of children diagnosed with Kawasaki's disease (KD) in pediatric rheumatology centers of Rio de Janeiro, Brazil, defining the magnitude of the delay period in diagnosing KD and initiating treatment due to confusion with common childhood febrile illnesses and the impact of this delay on the frequency of coronary sequels.
METHODS: Data analysis from hospital records summarized in a dedicated form, including name, gender, age, date of first recorded clinical signs, date of admission to the specialty service, information about symptoms, clinical evolution, intravenous immunoglobulin (IVIG) use and coronary sequels.
RESULTS: Of 125 patients, 63% were males. 40% were under 2 years at diagnosis. Average lapse between earliest signs and KD diagnosis was 12 days (mean fever duration, 14 d). Only 22.4% had a diagnosis of KD before entering the specialty service. For the remainder, initial hipotheses included: bacterial (60%) and viral infections (12%), rheumatological diseases (4%) and adverse vaccination reactions (1.6%). Hence, prevalent febrile illnesses of childhood were major confounding factors. For records (85.6%) mentioning treatment, 46.7% reported IVIG treatment, beginning after day 10 in 23 cases (21.5%). 20 patients (16%) presented coronary sequels, 9 of which were diagnosed late, including 3 given IVIG after day 10, and 6 given no IVIG. We found no significant association between the frequency of coronary sequels and: a) sex; b) age; c) clinical criteria; d) initiation of IVIG treatment (before or after day 10).
CONCLUSIONS: Common febrile illnesses of childhood often confound the diagnosis of KD.
Keywords: vasculitis, mucocutaneous lymph node syndrome, coronary artery disease, antibiotic prophylaxis.
INTRODUCTION
Kawasaki's disease (KD) is an acute systemic vasculitis of childhood, of unknown cause. The clinical manifestations result from injury to small and medium-sized blood vessels.1 KD was first described in Japan, where the incidence is highest (140 / 100.000 in children below 5 year),2,3 and further reported worldwide, with variable prevalence. Estimated annual incidence in children below 5 year is 17 / 100.000 in the USA4 and 3 / 100.000 in South America.5 Clinical and epidemiological patterns suggesting that KD follows exposure to infectious agent(s) include: a) the temporal and geographical distribution; b) a seasonal pattern; c) a low incidence during the first months of life, compatible with passive transfer of immunity from mother to child; d) aggregation of cases, compatible with an infectious outbreak; and e) rarely, a recurrent pattern, compatible with partial resistance to reinfection.6 From initial reports incriminating human coronaviruses,7 the list of suspected causative agents8 has grown to include bacteria, fungi and house mites.
If left untreated, 15%-25% of children with KD develop coronary sequels, ranging in severity from asymptomatic ectasia of coronary artery to giant coronary aneurisms that lead to thrombosis, myocardial infarction and sudden death.9-12 Although KD is uncommon, its coronary sequels have a major impact on pediatric care: in industrialized countries, KD is the major cause of acquired heart disease in childhood; in developing countries, it is second only to rheumatic fever.13-16 The prevalence of coronary aneurisms and overall mortality are effectively reduced by aspirin associated with intravenous immunoglobulin (IVIG). Timing of intervention, however, is reportedly critical to reduce coronary artery disease to 4%-5%.13-15 According to published studies, improved prognosis requires initiating treatment before the 10th day of fever.13
In Brazil, where systematic surveys of KD are insufficient,17 many infectious diseases, caused by bacterial, viral, protozoan and helminth agents, are considerably more common than KD, but have clinical presentations similar to the initial febrile period of KD, when proper diagnosis and institution of IVIG treatment are believed to be critical. These conditions represent a significant problem for diagnosing KD, especially when there is little awareness of the disease and its sequels.
Here we analysed the clinical evolution of KD in brazilian children, describing the frequency of KD diagnosis, and identifying the alternative hypotheses that were initially considered, as well as the extent to which the diagnosis of KD was delayed by confusion with more common infectious diseases, and the possible impact of these delays on the timing and effectiveness of IVIG therapy.
METHODS
Location and design of the study
We analysed retrospectively the hospital records of KD patients from the pediatric rheumatology services of Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG) and Hospital Municipal Jesus (HMJE), both from Rio de Janeiro, Brazil, with approval by the Ethics Committee of both institutions. These public tertiary care hospitals provide on-demand specialty care for residents of the state of Rio de Janeiro.
We have included children from 0 to 12 years, admitted from January 1992 to December 2005, who had a diagnosis of KD. Data from the respective records were summarized in a data collection form, including the following entries: name, gender, age, date of first recorded clinical signs, date of admission to the pediatric rheumatology service, information about symptoms, clinical evolution, IVIG use and coronary sequels.
Information related to diagnosis and treatment
The diagnosis of KD and its clinical evolution were established on the basis of the clinical history, date of beginning and cessation of fever, and physical examination. This included the evaluation of extremities, oral cavity, lips, lymph nodes, conjunctivae and rashes. Symptoms related to the gastrointestinal, genito-urinary, cardiovascular, musculoskeletal and respiratory systems, central nervous system, skin and fanera were also investigated. The following complications were detected: hepatitis, uveitis, facial paralysis, neurosensory hypoacusia. With respect to coronary artery injuries, the type of lesion was recorded (ectasia/aneurism). Data were also collected on the recurrence or persistence of fever after initiating treatment, and recurrence of the disease. The type and timing of medication used for treatment of KD were also determined. 14.4% of records did not contain sufficient information about treatment.
Case definition
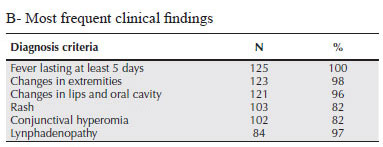
A case of KD was defined as occurring in a patient who had fever for at least 5 days, in association with at least 4 of the following clinical findings: alterations in the extremities, polymorphic rash, conjunctival hyperemia, typical changes in lips and/or oral mucosa and cervical lymphadenomegaly. Incomplete KD was defined as persistent fever, in association with 2 or 3 of the above findings, in the absence of other diseases which could account for the clinical presentation. Resistant KD was defined by persistent or recurrent fever, 36 h after IVIG infusion. The diagnosis of relapsing KD was made when the criteria for KD were fulfilled in a patient that had already undergone clinical remission. These criteria are detailed in Table 1A.
Statistical analyses
Pearsons Chi-square test with two degrees of freedom was used to evaluated the association between the frequency of coronary sequels and the following variables: a) sex; b) age (under 1 year or above 1 year); c) clinical criteria; d) initiation of IVIG treatment (before or after day 10).
RESULTS
Out of 125 cases (75 from IPPMG and 50 from HMJE) fulfilling criteria for inclusion, prevalence was highest between 2-5 years (37%) (Table 2A). Affected children were predominantly males (Table 2A).
Only 28 of the 125 patients (22.4%) had a diagnosis of KD before referral to specialty services. Before referral, alternative diagnostic hypotheses were entertained in most cases. These included bacterial infections (60%), viral infections (12%), other rheumatologic diseases (4%) and adverse reactions to vaccination (1.6%).
The duration of febrile illness before treatment initiation, one of the data most relevant to prognosis, was at least 5 days (range from 5 to 38 days, mean 14 days). Fever, in 36% of the patients (n = 45), lasted up to 10 days, and in 3% (n = 8), lasted beyond 30 days. However, the duration of febrile illness was not recorded for 23 patients. On average, diagnosis was made at the 12th day of fever.
Table 1B shows the frequency of different clinical manifestations that are accepted as diagnostic criteria for KD in patients with a fever lasting at least 5 days. All diagnostic criteria were found in a high frequency, and changes in extremities, oral cavity and lips were present in more than 95%. Even with this high frequency of diagnostic criteria, a diagnosis of incomplete KD was made in 7 patients (5.6 %), who had fever associated with 3 of the 5 criteria. In this group, only one case of incomplete KD occurred in a child over 5 years, all other cases being under 2 years.
In Table 2B, we show the frequency of manifestations of previously reported in studies of KD, but which do not qualify as diagnostic criteria by the current consensus. This included gastrointestinal (nausea, vomiting diarrhea, abdominal pain); genitourinary (sterile piuria); musculoskeletal (arthritis, arthralgia, myalgia, claudication); respiratory (cough, tachypnea, pulmonary infiltration, pleural effusion); central nervous (irritability, aseptic menigitis); skin and fanera (alopecia, Beau streaks).
Treatment and therapeutic response
All patients received aspirin, 80-100 mg/kg/day, until they were feverless apyearetic for at least 48 hours. The dose was then reduced to 3-5 mg/kg/day (dose that inhibits platelet aggregation), as a single dose, maintained for 6-8 weeks, except in cases that evolved with coronary artery injuries, in which treatment was continued as recommended. IVIG was administered as a continuous infusion for 10-12 hours at a dose of 2 g/kg to 46% of patients (n = 50) in a total of 107 patient with available treatment records. The timing of infusion with respect to the febrile period is detailed in Table 3A, distinguishing between cases where IVIG was instituted at the recommended period and those in which it was given with delay.
Complications
We found 3 cases of hepatitis, 1 of uveitis, 3 of facial paralysis due to injury of the 7th cranial nerve, and 3 cases of neurosensory hypoacusia. Thrombocytosis occurred in 71% of cases (6 cases had no laboratory test information). Only one child had thrombocytopenia.
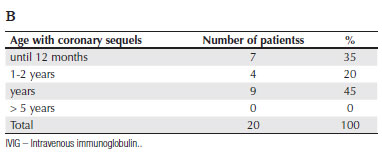
All patients had two-dimensional echocardiography at the moment of diagnosis and during the follow-up period. Coronary involvement in the subacute period, manifesting as aneurisms and coronary dilations, was found in a total of 20 patients, only 6 (5% of all cases) of which had been diagnosed and treated before day 10. For 5 additional patients presenting coronary involvement, we have no information about treatment. The remaining 9 patients diagnosed after day 10 included 3 receiving IVIG, and 6 more who could not be treated because of a nationwide shortage of supply. Table 3B shows the distribution of patients presenting coronary sequels as a function of the age group at the time of diagnosis.
No cases of acute myocardial infarction and no fatal outcomes were recorded during the follow-up period. Among patients presenting coronary involvement, 2 presented KD resistant to IVIG infusion, and 1 had incomplete KD.
Impact of different variables on the frequency of coronary sequels
We failed to detect a statistically significant association between the frequency of coronary sequels and any of the following: a) sex (chi-square = 0.05369), not significant/NS) ; b) age (under 1 year or above 1 year) (chi-square = 1.17557), NS); c) clinical criteria (lymphadenomegaly: chi-square = 0.82392, NS; Polymorphous rash: chi-square = 0.57721, NS; Changes in extremities: chi-square = 0.25818, NS; Changes in lips and oral cavity: chi-square = 0.70293, NS; Conjunctival hyperemia: chi-square = 0.01451, NS).
We also examined the effects of IVIG treatment. Treatment had a significant impact (chi-square = 33.65172, P < 0.001) on the frequency of coronary sequels. Administration of IVIG starting later than day 10 had no significant effect on the frequency of coronary sequels (chi-square = 2.63017, NS).
DISCUSSION
KD presents a challenge to the pediatrician working in a tropical country, because it is an uncommon rheumatologic affection that requires highly specific intervention to prevent serious or fatal sequels, but has a nonspecific clinical presentation18 in the critical period during which proper treatment should be instituted. Many infectious diseases more prevalent than KD, presenting as acute febrile illnesses of childhood, may confound the diagnosis of KD.
KD was first described in Japan in 1961,19 and further characterized in 19749 from the clinical, epidemiological and anatomopathological standpoints.20 Our data agreed with previous studies concerning the predominance of affected males and the age of diagnosis (under 5 years in 77% of cases).6,15 Incomplete KD is more common before 2 years old, and the affected children seem to be at greater risk of developing coronary disease.21 In our study, 6 of the 7 patients with incomplete KD were less than 2 years old, and 1 of these evolved with coronary lesions. Resistant KD, described in 10% to 15% of subjects, is associated to a greater risk of ectasias or aneurisms.22-24 This clinical evolution was observed in 7 of our patients (5%), with heart involvement in 2 of these cases (1.6%). Recurrent KD, which has been reported in 3%-5% of Japanese children,6 was observed in 2 of our subjects (1.6 %).
In our study, most delays in diagnosis were due to difficulties in distinguishing KD from bacterial and viral infections and adverse reactions to vaccines, which together constituted the initial diagnostic hypotheses in ~75% of cases. Hence, the impact of prevalent febrile illnesses of childhood on the timing of diagnosis and treatment of KD is considerable. Because these represent a problem common to many tropical countries, our findings should raise awareness in pediatricians elsewhere.
The diagnosis of KD relies on the observation of nonspecific signs and symptoms, such as high fever persisting for at least five days, in association with four out of five diagnostic criteria, which can be observed in the first few weeks of evolution.15 Most frequent findings are: changes in the oral cavity, conjunctival hyperemia, exantema, changes in extremities and cervical lymphadenopathy.12,25 These findings may be overlooked by parents or caretakers, or attributed to fever, and further go unreported, as they are often taken for irrelevant or obvious. Overall, the lack of an alarming initial presentation likely delays the search for medical care.
The mean time of duration of fever (14 days) in our study shows a marked delay in diagnosis, contrasting with 6.5 days in a canadian study.26 Importantly, KD can be diagnosed by the 4th day of fever, on the basis of 4 or more diagnostic criteria.15
Among the patients with properly information about treatment, only 54% of cases that used IGIV, received it in the recommended period. We could not find in our study a significant effect of initiating treatment after the recommended period.
In conclusion, delayed diagnosis of KD in Brazilian children led to initiating IVIG treatment after the recommended period in a considerable number of patients of our study, but this delay had no significant impact in the frequency of coronary sequels.
ACKNOWLEDGEMENTS
We thank professor Dulce Helena Orofino, for help in dealing with the literature on KD.
REFERENCES
-
1Gedalia A. Kawasaki disease: 40 years after the original report: Curr Rheumatol Rep 2007; 9:336-41.
-
2Senzaki H. Long-Term Outcome of Kawasaki Disease. Circulation 2008;118: 2763-72.
-
3Yanagawa H, Nakamura Y, Yashiro M, Oki I, Hirata S, Zhang T et al Incidence survey of Kawasaki disease in 1997 and 1998 in Japan: Pediatrics 2001; 107:e33. http://www.pediatrics.org/cgi/content/full/107/3/e33 [Acesso:15/12/2007]
-
4Holman RC, Curns AT, Belay ED, Steiner CA, Schonberger LB. Kawasaki Syndrome hospitalizations in the United States in 1997 and 2000. Pediatrics 2003; 112:495-501.
-
5Newburger JW, Taubert KA, Shulman ST, Rowley AH, Gewitz MH, Takahashi M et al Summary and abstracts of the Seventh International Kawasaki Disease Symposium. Pediatr Res 2003; 53:153-87.
-
6Burns JC, Glodé MP. Kawasaki syndrome. Lancet 2004; 364:533-44.
-
7Dominguez SR, Anderson MS, Glodé MP, Robinson CC, Holmes KV. Blinded Case-Control Study of the Relationship between Human Coronavirus NL63 and Kawasaki Syndrome. JID 2006, 94:1697-701.
-
8Burns JC, Taubert K, Rowley AH, Newburger JW, Gewitz M, Takahashi M et al Summary of the 8th International Kawasaki Disease Symposium [Presentation], 2006.
-
9Kawasaki T, Kasaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymphnode syndrome (MLNS) prevailing in Japan. Pediatrics 1974; 54:271-6.
-
10Burns JC, Kushner HI, Bastian JF, Shike H, Shimizu C, Matsubara T et al Kawasaki Disease: A Brief History. Pediatrics 2000; 106:e27. http://www.pediatrics.org/cgi/content/full/106/2/e27 [Acesso: 15/12/2007]
-
11Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y et al Long-term consequences of Kawasaki disease. A 10 to 21 year follow-up study of 594 patients. Circulation 1996; 94:1379-85.
-
12Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M et al Diagnosis and therapy of Kawasaki disease in children. Circulation 1993; 87:1776-80.
-
13Royle J, Burgner D, Curtis N. The Diagnosis and management of Kawasaki disease. J Pediatr 2005; 41:87-93.
-
14Taubert KA, Rowley AH, Shulman ST. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr 1991; 119:279-82.
-
15American Heart Association Scientific Statement. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease. Circulation 2004; 110:2747-71.
-
16Satou GM, Giamelli J, Gewitz MH. Kawasaki disease: diagnosis, management, and long-term implications. Cardiol Rev 2007; 15:163-9.
-
17Tomikawa SO, Sakamoto RA, Gonçalves AMF, Rodrigues Neto AJ, Sakane PT. A dificuldade diagnóstica na doença de Kawasaki: relato de caso. Pediatria 2003; 25:128-33.
-
18Maconochie IK. Kawasaki Disease. Arch dis Child Pract Ed 2004; 89:3-8.
-
19Kawasaki T. MCLS - Clinical observation of 50 cases [in Japanese]. Jap J Allerg 1967; 16:178-222.
-
20Chang LY, Chang IS, Lu CY, Chiang BL, Lee CY, Chen PJ et al Epidemiologic features of Kawasaki disease in Taiwan 1996-2002. Pediatrics 2004; 114:678-82.
-
21Rosenfeld EA, Corydon KE, Shulman ST. Kawasaki disease in infants less than one year of age. J Pediatr 1995; 126:524-9.
-
22Meissner HC, Leung DYM. Kawasaki Syndrome: Where are the answers? Pediatrics 2003; 112:672-6.
-
23Burns JC, Edmund VC, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatmen in Kawasaki disease. Pediatr Infect Dis J 1998; 17:1144-8.
-
24Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics 2000; 105:e78. http://www.pediatrics.org/cgi/content/full/105/6/e78 [Acesso: 15/12/2007]
-
25Han RK, Sinclair B, Newman A, Silverman ED, Taylor GW, Walsh P et al Recognition and management of Kawasaki disease. CMAJ 2000; 21:162-6.
-
26Ozen S, Ruperto N, Dillon MJ, Bagga A, Barron K, Davin JC et al EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis 2006; 65:936-41.
Profile of Kawasaki disease in children referred to two pediatric rheumatology services in Rio de Janeiro, Brazil
Publication Dates
-
Publication in this collection
12 Nov 2010 -
Date of issue
Oct 2010
History
-
Received
24 Apr 2010 -
Accepted
31 Aug 2010