Abstracts
Bisphosphonates are potent inhibitors of bone resorption, and are used in the treatment of osteoporosis and other diseases that cause bone mass loss, such as Paget's disease, bone metastases, and multiple myeloma, to prevent pathological fractures. Since 2003, avascular osteonecrosis of the jaw has been associated with the use of bisphosphonates, mainly intravenous. According to the literature, the occurrence of osteonecrosis of the jaw has ranged from 0.8% to 12% of the patients on bisphosphonates, most of them on prolonged use. Physicians and odontologists should be aware of that potential complication in dental treatment.
osteonecrosis; maxillary diseases; osteoporosis
Os bisfosfonatos são potentes inibidores da reabsorção óssea e são utilizados no tratamento da osteoporose e de outras doenças que causam a perda de massa óssea, como doença de Paget, metástases ósseas e mieloma múltiplo, prevenindo fraturas patológicas. Desde 2003, estudos associam osteonecrose avascular dos ossos maxilares a seu uso, principalmente intravenoso. Na literatura, há relatos de ocorrência variando de 0,8% a 12%, dos pacientes, na sua maioria em uso prolongado. Médicos e dentistas devem estar cientes dessa potencial complicação no tratamento odontológico.
Osteonecrose; doenças maxilares; osteoporose
CASE REPORT
IFull professor of Oral and Maxillofacial Surgery, area of Plastic Surgery, Medical Sciences School - Unicamp
IIAssociate professor, area of Rheumatology, Department of Internal Medicine of the Medical Sciences School - Unicam
IIIDental surgeon; Specialist in Dental Radiology and Imaging at the Joinville City Hall, state of Santa Catarina
Correspondence to
ABSTRACT
Bisphosphonates are potent inhibitors of bone resorption, and are used in the treatment of osteoporosis and other diseases that cause bone mass loss, such as Paget's disease, bone metastases, and multiple myeloma, to prevent pathological fractures. Since 2003, avascular osteonecrosis of the jaw has been associated with the use of bisphosphonates, mainly intravenous. According to the literature, the occurrence of osteonecrosis of the jaw has ranged from 0.8% to 12% of the patients on bisphosphonates, most of them on prolonged use. Physicians and odontologists should be aware of that potential complication in dental treatment.
Keywords: osteonecrosis; maxillary diseases; osteoporosis.
INTRODUCTION
Bisphosphonates (BFT) are potent inhibitors of bone resorption, and are used in the treatment of osteoporosis and other diseases that cause bone mass loss, such as Paget's disease, bone metastases, and multiple myeloma, to prevent pathological fractures.1 Bisphosphonates increase bone mass and mineralization, with an increase in bone mineral density and resistance, and a reduction in the risk of bone fracture.
Bisphosphonates show selectivity for areas of bone resorption and inhibit the action of osteoclasts. In molecular terms, one of the major actions of BFT is the inhibition of the enzyme farnesyl diphosphate synthase, causing several cytoskeletal alterations, reducing the bone resorption capacity of the osteoclasts and inducing apoptosis of those cells.1-3 In general, BFT are systemically well tolerated. Their adverse effects are rare and consist in low and transient fever, fatigue, arthralgia, nausea, esophagitis, renal failure, hypocalcemia, and bone pain.4The use of BFT, however, has been associated with cases of avascular osteonecrosis of the jaw (Figure 1). Other skeletal bones have not been involved. The general symptoms include difficulty in eating and speaking, swelling, pain, bleeding, paresthesia of the lower lip, and tooth mobility and loss. The radiographic findings are not specific, thus, such lesions might require biopsy to rule out metastases.5
This study aimed at reviewing the literature regarding the risk of BFT-related avascular osteonecrosis of the jaws, in addition to providing guidance for prevention and treatment according to the stage of the disease.
REVIEW OF THE LITERATURE
Pamidronate and zoledronate belong to the drug class of bone resorption inhibitors, which have been used for years for the treatment of osteoporosis. The clinical efficacy of such drugs in the treatment of osteopenia/osteoporosis has been well established.6The presence of nitrogen in their formulation makes their metabolization difficult, leading to their accumulation in bones and prolonged action.7 Oral BFT have been more often indicated for the treatment of osteoporosis and osteopenia. They have also been indicated in other less common conditions, such as Paget's disease and osteogenesis imperfecta. Table 1 shows the characteristics of the BFT indicated for treating osteoporosis available in the Brazilian market.
The association of BFT with osteonecrosis of the jaws was first reported in 2003 by Marx.8The author reported 36 cases of avascular osteonecrosis, 29 of which in the mandible, five in the maxilla, and two in both bones. Of the 36 patients, 24 were on intravenous (IV) pamidronate, 90 mg monthly; six used initially pamidronate at the same dosage, and then changed to IV zoledronate, 4 mg monthly; and six received only IV zoledronate, 4 mg monthly. The indications for the use of those drugs were multiple myeloma-related hypercalcemia (18 patients), metastatic breast carcinoma-related hypercalcemia (17 patients), and treatment of osteoporosis (one patient).
After the first report in 2003,8 several studies by oral and maxillofacial surgeons have associated the use of BFT with avascular osteonecrosis of the jaws. In December 2004, Novartis, the manufacturer of pamidronate and zoledronate, called attention to the risk of osteonecrosis.9A search in the PubMed database (U.S. National Library of Medicine and the National Institutes of Health) (http://www.ncbi.nlm.nih.gov/sites/entrez) on February 2nd, 2011, with the descriptors (bisphosphonate) AND (osteonecrosis of the jaws) identified 928 articles.
To differentiate from other bone pathologies, the following working definition of avascular osteonecrosis of the jaw has been adopted by the American Association of Oral and Maxillofacial Surgeons (AAOMS):10 Patients may be considered to have BFT-related osteonecrosis of the jaw in the presence of all the following three characteristics: 1) previous or current treatment with a BFT; 2) exposed, necrotic bone in the maxillofacial region that has persisted for more than eight weeks; and 3) no history of radiation therapy in the region.
The cumulative incidence of avascular osteonecrosis of the jaw ranges from 0.8% to 12%. It is more often related to the intravenous, monthly administration and for a period longer than three years. The use of BFT in such conditions is more common in the treatment of hypercalcemia secondary to malignant tumors, to bone metastases from breast, prostate, and lung cancer, and to osteolytic diseases, such as multiple myeloma.10-12
The oral route is more common in the treatment of osteoporosis. Until 2006, according to IMS Health, over 190 million oral BFT prescriptions had been dispensed worldwide.10Patients under treatment with oral BFT are considered at low risk as compared with patients with cancer receiving intravenous monthly treatment with BFT, which significantly increases the risk of osteonecrosis.11 However, cases of osteonecrosis of the jaws have been reported in patients on oral BFT.13,14An Australian control has estimated that the incidence for patients treated weekly with alendronate ranges from 0.01% to 0.04%.15Among 13 thousand patients of an American health Bisphosphonate-related osteonecrosis of the jaws care insurance, receiving oral BFT for a long time, the prevalence was 0.06% or 1:1,700 patients.16
The occurrence of osteonecrosis of the jaw seems to be related to the use of intravenous BFT. However, data are inaccurate, and given the large number of patients being treated for osteoporosis, receiving oral BFT for a long period, it is likely that many cases may appear in that group of patients, since duration of therapy is one of the risk factors.17,18
Comorbidities, such as obesity and the chronic use of corticoids, can be associated, but that relation has not been clarified. The local risk factors include oral surgery, dental implant placement, extractions, and periodontal surgery involving bone lesions. Local factors also include the presence of bony exostoses (lingual and palatal tori) and poorly adapted prostheses. Regarding demographic and systemic risk factors, the studies are scarce. Gender does not seem to influence; however, the Caucasian race, advanced age and smoking seem to increase the risk, while alcohol consumption does not.18-20
Prior to treatment with intravenous BFT, the patient should have a thorough oral examination, all unsalvageable teeth should be removed, all invasive dental procedures should be completed, the prostheses should be well adapted, and optimal periodontal health should be achieved. Therefore, the risk for osteonecrosis of the jaw is reduced, but not eliminated.18,19Some instructions and protocols for patients at risk for osteoradionecrosis seem to be the same for patients under treatment with intravenous BFT. When previous surgery is needed, if systemic conditions permit, the beginning of the BFT therapy should be delayed until the operated area has mucosalized or until there is complete osseous healing.10-12
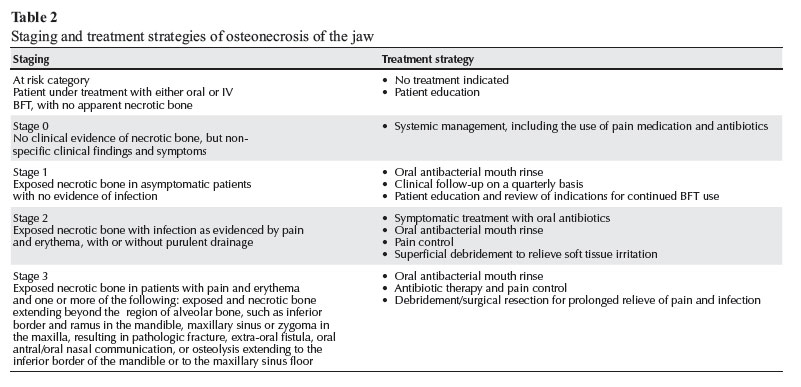
Table 2 shows staging of osteonecrosis of the jaw and the corresponding treatment strategies proposed by the AAOMS in 2009.10
The oral use of BFT is not that critical for the risk of avascular osteonecrosis of the jaw. Thus, elective oral surgeries are not contraindicated, but the patient should be aware of the risk. If systemic conditions permit, interruption of oral BFT should be considered for a period of three months before and three months after elective surgery to reduce the risk of osteonecrosis. That approach is justified based on extrapolated data, showing BFT-therapy-related fluctuations of osteoclast function, and on the conclusions of recent studies showing better results of the treatment of avascular osteonecrosis of the jaw with drug discontinuation.21-23
CONCLUSIONS
There is evidence that the use of bisphosphonates is associated with avascular osteonecrosis of the jaw, which is rare and affects the quality of life of patients already physically impaired. Patients under the treatment of BFT or who are going to initiate that treatment, particularly if intravenous, but also oral, for long periods, should be carefully assessed by a physician and dental surgeon to avoid osteonecrosis.
REFERENCES
-
1Rodan GA, Reszka AA. Bisphosphonate mechanism of action. Curr Mol Med 2002;2(6):571-7.
-
2Little DG, Peat RA, Mcevoy A, Williams PR, Smith EJ, Baldock PA. Zoledronic acid treatment results in retention of femoral head structure after traumatic osteonecrosis in young Wistar rats. J Bone Miner Res 2003;18(11):2016-22.
-
3Astrand J, Aspenberg P. Systemic alendronate prevents resorption of necrotic bone during revascularization. A bone chamber study in rats. BMC Musculoskelet Disord 2002;7(3):19.
-
4Conte P, Guarneri V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 2004;9(Suppl 4):28-37.
-
5Diego R, D'Orto O, Pagani D, Agazzi A, Marzano U, Derada Troletti G et al. Bisphosphonate-associated osteonecrosis of the jaws: a therapeutic dilemma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;103(3):e1-5.
-
6Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001;19(2):558-67.
-
7Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev 1998;19(1):80-100.
-
8Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003;61(9):1115-7.
-
9Hohnecker JA. Novartis "Dear Doctor" precautions added to label of Aredia and Zometa. September 24, 2004. Citado por Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws - 2009 update. J Oral Maxillofac Surg 2009;67(5 Suppl):2-12.
-
10Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg 2009;67(5 Suppl):2-12.
-
11Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D et al. Bisphosphonate-Associated Osteonecrosis of the Jaw: Report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007;22(10):1479-91.
-
12Yoneda T, Hagino H, Sugimoto T, Ohta H, Takahashi S, Soen S et al. Bisphosphonate-related osteonecrosis of the jaw: position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J Bone Miner Metab 2010;28:365-83.
-
13Wessel JH, Dodson TB, Zavras AI. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a casecontrol study. J Oral Maxillofac Surg 2008;66(4):625-31.
-
14Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff S. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004;62:527-34.
-
15Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis\osteopetrosis) of the jaws: risk factors, recognition, prevention and treatment. J Oral Maxillofac Surg 2005;63:1567-75.
-
16Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate- associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg 2007;65:415-23.
-
17Ault A. Jaw necrosis affects 1 in 1,700 on oral bisphosphonates. Internal Medicine News 2008;41(Aug 1):23.
-
18Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 2005;23(34):8580-7.
-
19Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 2008;23(6):826-36.
-
20Zervas K, Verrou E, Teleioudis Z, Vahtsevanos K, Banti A, Mihou D et al. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol 2006;134(6):620-3.
-
21Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 2005;23(34):8580-7.
-
22Dimopoulos MA, Kastritis E, Bamia C, Melakopoulos I, Gika D, Roussou M et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol 2009;20(1):117-20.
-
23Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumor patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol 2009;20(1):137-45.
Bisphosphonate-related osteonecrosis of the jaws
Publication Dates
-
Publication in this collection
11 July 2011 -
Date of issue
Aug 2011
History
-
Received
21 May 2010 -
Accepted
30 Apr 2011