Abstract
Introduction:
EpiFibro (Brazilian Epidemiological Study of Fibromyalgia) was created to study Fibromyalgia patients. Patients were included since 2011 according to the 1990 American College of Rheumatology Classification Criteria for Fibromyalgia (ACR1990).
Objectives:
To determine how many patients still fulfill the ACR1990 and the ACR2010 criteria in 2014; to determine the correlation between the impact of FM and to describe data on the follow-up evaluation.
Methods:
This is a cross sectional study in a multicenter cohort of patients. The data was collected between 2013 and 2015. Physician included patients that fulfilled the ACR1990 criteria on the date of entry. The follow-up data were considered only for patients with at least two evaluations. A minimally significant change was considered to be a 30% variation of parameters scores.
Results:
810 patients’ data were analyzed. Patients presented a mean age of 51.8 ± 11.5 years old. There were 786 female. Most patients met both criteria. There was a greater fulfilling of the ACR2010. There was a moderate correlation between Polysymptomatic Distress Scale and Fibromyalgia Impact Questionnaire. Three hundred fourteen patients with more than one assessment were found, but 88 patients were excluded. Thus, 226 patients with one follow-up monitoring parameter were considered (Fibromyalgia Impact Questionnaire: 222; Polysymptomatic Distress Scale: 199; both: 195). The mean follow-up time was 9.1 ± 7.5 months (1–44). Most patients became stable.
Conclusion:
InEpiFibro, most patients fulfill simultaneously the ACR1990 and ACR2010. A larger number of patients fulfill the ACR2010 at the time of the evaluation. There was a moderate correlation between the Polysymptomatic Distress Scale and the Fibromyalgia Impact Questionnaire. Most patients remained stable over time.
Keywords:
Fibromyalgia; Classification criteria; Diagnostic criteria; Follow-up
Resumo
Introdução:
O EpiFibro (Estudo Epidemiológico Brasileiro de Fibromialgia) foi criado para estudar pacientes com fibromialgia. Foram incluídos pacientes desde 2011 de acordo com os critérios de classificação para a fibromialgia do American College of Rheumatology de 1990 (ACR1990).
Objetivos:
Determinar quantos pacientes ainda atendem aos critérios ACR1990 e ACR2010 em 2014; determinar a correlação entre o impacto da FM medido pelo Questionário de Impacto da Fibromialgia (FIQ) e pela Polysymptomatic Distress Scale (PDS) e descrever dados sobre a avaliação de seguimento.
Métodos:
Estudo transversal em uma coorte multicêntrica de pacientes. Os dados foram coletados entre 2013 e 2015. O médico incluiu pacientes que atenderam aos critérios ACR1990 no momento da entrada. Consideraram-se os dados de seguimento apenas dos pacientes com pelo menos duas avaliações. Uma variação de 30% nos escores dos parâmetros foi considerada uma alteração minimamente significativa.
Resultados:
Analisaram-se os dados de 810 pacientes. Os pacientes apresentaram média de 51,8 ± 11,5 anos. Havia 786 mulheres. A maior parte dos pacientes atendeu a ambos os critérios. Houve um maior atendimento aos critérios ACR2010. Houve uma correlação moderada entre a PDS e o FIQ. Encontraram-se 314 pacientes com mais de uma avaliação, mas 88 pacientes foram excluídos. Assim, foram considerados 226 pacientes com um parâmetro de monitoramento no seguimento. (FIQ: 222; PDS: 199; ambos: 195). O tempo médio de seguimento foi de 9,1 ± 7,5 meses (1 a 44). A maior parte dos pacientes tornou-se estável.
Conclusão:
No EpiFibro, a maior parte dos pacientes atendia simultaneamente ao ACR1990 e ao ACR2010. Uma maior quantidade de pacientes atendia ao ACR2010 no momento da avaliação. Houve uma correlação moderada. A maior parte dos pacientes manteve-se estável ao longo do tempo.
Palavras-chave:
Fibromialgia; Critérios de classificação; Critérios diagnósticos; Seguimento
Significance and innovation
Brazilian Registry on Fibromyalgia. Fulfillment of ACR classification and diagnostic criteria. Follow-up data
Introduction
The Fibromyalgia Syndrome (FM) is a clinical condition characterized by chronic widespread pain usually associated to fatigue, sleep disturbances and cognitive symptoms. Its prevalence is high and in Brazil it is estimated at 2.5%.11 Senna ER, De Barros AL, Silva EO, Costa IF, Pereira LV, Ciconelli RM, et al. Prevalence of rheumatic diseases in Brazil: a study using the COPCORD approach. J Rheumatol. 2004;31:594-7. The influence of social, psychological and cultural aspects makes its clinical expression highly variable. There remains the need for epidemiological studies on FM in our country.
The EpiFibro (Brazilian Epidemiological Study of Fibromyalgia) was created in order to analyze the epidemiology of FM and its comorbidities across the country. It shall offer better conditions to diagnosis, treat and evaluate the impact of this disorder in Brazilian society through online questionnaires.
Patients were included since 2011 according to the1990 American College of Rheumatology Classification Criteria for Fibromyalgia (ACR1990).22 Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia – report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160-72. The clinical and epidemiological data was already published in 2013.33 Rezende MC, Paiva ES, Helfenstein M, Ranzolin A, Martinez JE, Provenza JR, et al. EpiFibro – a nationwide databank for fibromyalgia syndrome: the initial analysis of 500 women. Rev Bras Reumatol. 2013;53:382-7.
In 2010, an ACR committee presented the Preliminary Diagnostic Criteria for Fibromyalgia (ACR2010), after two decades of critics on the ACR1990. The new criteria abolished the tender points counting and emphasized the association of fatigue; sleep disorders, cognitive disorders and somatic symptoms to chronic widespread pain. It established 2 scores – an widespread pain index (WPI), composed by 19 potentially painful areas to be identified by the patients, and a symptom severity index (SSI) that results from the sum of fatigue, sleep disturbances, cognitive disorders and somatic symptoms scores (0–3 each). The total score ranges from 0 to 12.44 Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Katz R, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600-10. In 2011 the somatic symptoms item was modified, which allowed creating a self-report version in order to be used in epidemiological studies.55 Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz R, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38:1113-22.
The sum of these two indices, ranging from 0 to 31, named Polysymptomatic Distress Scale (PDS), can be used for patients’ clinical monitoring.66 Wolfe F, Walitt BT, Rasker JJ, Katz RS, Häuser W. The use of polysymptomatic distress categories in the evaluation of fibromyalgia (FM) and FM severity. J Rheumatol. 2015;42:1494-501. The follow-up measures for the FM patients usually are analogical symptoms scales, as for example for pain, and by quality of life or impact questionnaires such as the Fibromyalgia Impact Questionnaire (FIQ) and its revised form, whose versions have been translated and validated to Brazilian Portuguese.77 Marques A, Santos A, Assumpção A, Matsutani L, Lage L, Pereira C. Validation of the Brazilian version of the Fibromyalgia Impact Questionnaire (FIQ). Rev Bras Reumatol. 2006;46:24-31.,88 Paiva ES, Heymann RE, Rezende MC, Helfenstein M, Martinez JE, Provenza JR, et al. A Brazilian Portuguese version of the Revised Fibromyalgia Impact Questionnaire (FIQR): a validation study. Clin Rheum. 2013;32:1199-206.
Objectives
The main objectives of the present study are to determine how many patients still fulfill the ACR1990 and the ACR2010 criteria in 2014, to determine the correlation between the impact of FM measured by the FIQ and by the PDS and to describe data on the follow-up evaluation of the patients enrolled in the registry.
Patients and methods
This is a cross sectional study in a multicenter cohort of patients with FM. The data was collected between 2013 and 2015, including demographic, clinical information and follow-up parameters. Analogical symptom scales of pain and fatigue, FIQ and the PDS were considered clinical parameters.
Patients were included in EpiFibro by their physician, according to a tutorial by the Brazilian Rheumatology Society. It included patients that fulfilled the ACR1990 criteria on the date of the patient's entry in the registry. Patients with incomplete forms were excluded as that prevented the data analysis for the proposed objectives. The follow-up data were considered only for patients with at least two evaluation forms separated by three months interval as a minimum. A minimally significant clinical change was considered to be a 30% up or down variation of the follow-up parameters scores.
Descriptive statistics and the Pearson correlation coefficient were used.
Results
A total of 810 patients’ data were analyzed. Patients presented a mean age of 51.8 ± 11.5 years-old. There were 786 female patients (97.0%) and only 24 men (3%). Most patients were attended in public health care (648 patients – 80%) and 162 patients (20%) were treated in private services.
Most patients met both ACR criteria as shown in Table 1. There was a greater fulfilling of the ACR2010 compared to the ACR1990, suggesting that the new criteria are more sensitive and maybe less specific than the older one.
Fulfillment of the 1990 Classification Criteria for Fibromyalgia (ACR1990) and the 2010 Preliminary Diagnostic Criteria for Fibromyalgia (ACR2010).
Table 2 shows clinical data in patients’ self-scored reports. There was high intensity of pain and fatigue and a severe disease impact measured by PDS and FIQ.
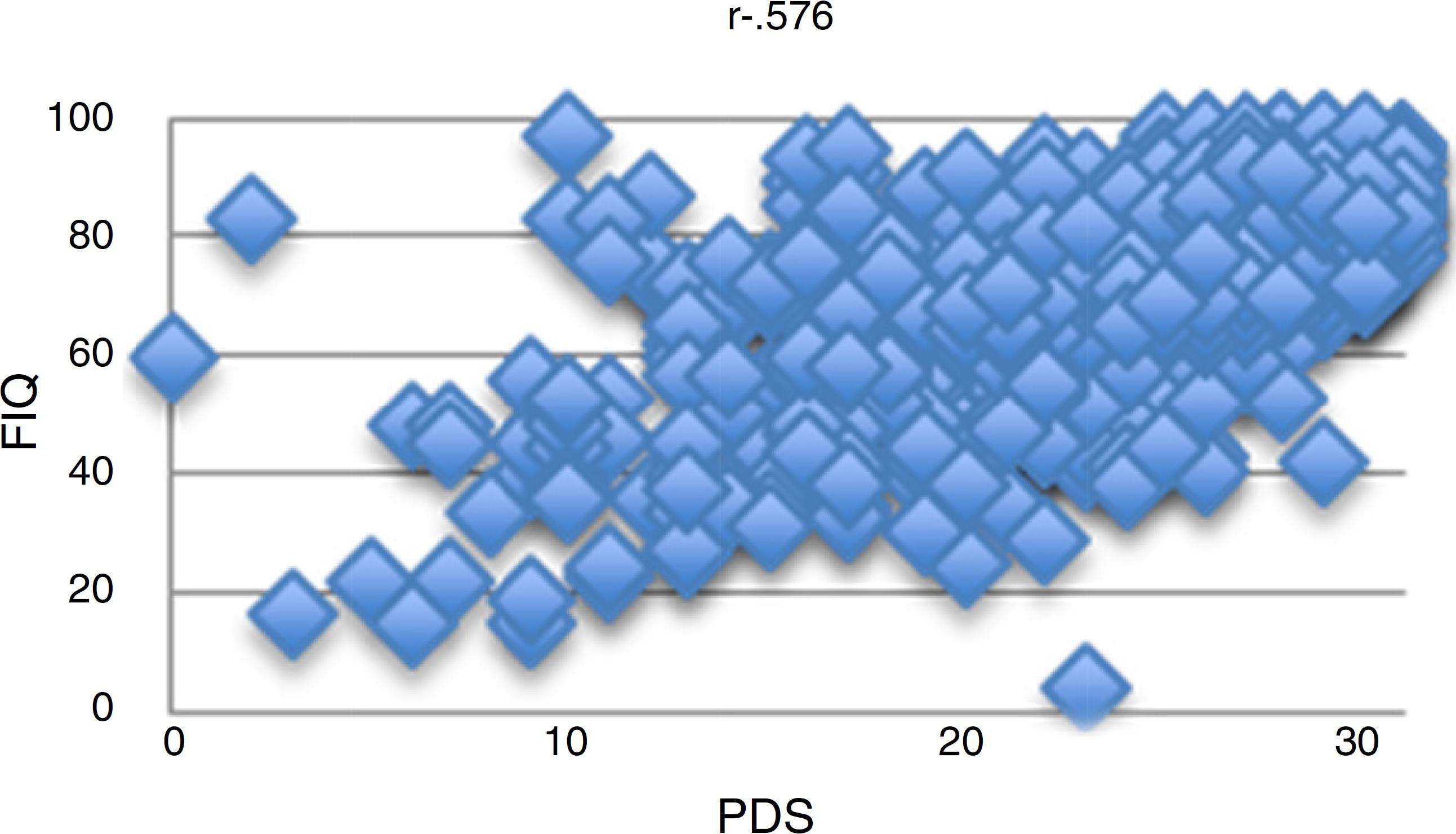
There was a moderate correlation between PDS and FIQ (r = 0.576), as shown in Fig. 1.
Three hundred fourteen patient's registries with more than one assessment were found, but 88 patients were excluded because an interval between evaluations under 3 months. Thus, 226 patients with one follow-up monitoring parameter registered were considered (FIQ: 222; PDS: 199; both: 195).
The mean follow-up time was 9.1 ± 7.5 months (1–44). FIQ and PDS measures are shown in Table 3.
Discussion
In this study, it was observed a female predominance, which is compatible with the literature.99 Henriksson CM, Liedberg GM, Gerdle B. Women with fibromyalgia: work and rehabilitation. Disabil Rehabil. 2005;27:685-94. Although there is a large majority of patients seen in the Brazilian Public Health System, the private network is also represented in this study.
The intensity of pain and fatigue and the impact clinical parameters shows that the reported cases are severe. Higher severity can be justified by the main data collection centers involved in this study are universities that composes the tertiary care health assistance level. So, this is a limitation of this research that prevents the data to be generalized to all Brazilian fibromyalgia patients. Anyway, it suggests a need for epidemiological studies involving community patients. The same argument may be used for the high male predominance. It is important to emphasize that this is not a prevalence study, but a registry data.
The moderate correlation between the two follow-up parameters (FIQ and PDS) suggests that both can be used in monitoring. The choice of the parameter should be based on the ease of use and the familiarity of the doctors or services. The FIQ has been used in clinical trials and in daily practice since 1993 with reliable results in our population even before being validated formally. In 2006, it was published its formal translation and validation for Portuguese in Brazil. The PDS was recently created and we still have not published data to assess its potential for clinical monitoring.77 Marques A, Santos A, Assumpção A, Matsutani L, Lage L, Pereira C. Validation of the Brazilian version of the Fibromyalgia Impact Questionnaire (FIQ). Rev Bras Reumatol. 2006;46:24-31.
The observed clinical stability is compatible with evolution studies present in the literature. Kennedy et al. studied 35 patients and showed that 100% continued to have symptoms after 10 years.1010 Kennedy M, Felson DT. A prospective long-term study of fibromyalgia syndrome. Arthritis Rheum. 1996;39:682-5. Other studies have reported improvements in pain over time, but the total resolution of symptoms seems rare.1111 Granges G, Zilko P, Littlejohn GO. Fibromyalgia syndrome: assessment of the severity of the condition 2 years after diagnosis. J Rheumatol. 1994;21:523-9.,1212 Bengtsson A, Backman E, Lindblom B, Skogh T. Long term follow-up of fibromyalgia patients: clinical symptoms, muscular function, laboratory tests – an eight year comparison study. J Musculoskeletal Pain. 1994;2:67-80. In 2011, Walitt et al. published a 5 year follow-up study with 1555 fibromyalgia patients and found a clinically significant improvement in the overall severity of symptoms, despite the pain improvement be only moderate in 25% of patients.1313 Walitt B, Fitzcharles MA, Hassett AL, Katz RS, Häuser W, Wolfe F. The longitudinal outcome of fibromyalgia: a study of 1555 patients. J Rheumatol. 2011;38:2238-46.
Conclusion
In the EpiFibro Cohort, most patients fulfill simultaneously the ACR1990 and ACR2010 criteria for FM. A larger number of patients fulfill the ACR2010 compared to ACR1990 at the time of the evaluation. There was a moderate correlation between the FIQ and the PDS monitoring and most patients remained stable over time.
References
-
1Senna ER, De Barros AL, Silva EO, Costa IF, Pereira LV, Ciconelli RM, et al. Prevalence of rheumatic diseases in Brazil: a study using the COPCORD approach. J Rheumatol. 2004;31:594-7.
-
2Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia – report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160-72.
-
3Rezende MC, Paiva ES, Helfenstein M, Ranzolin A, Martinez JE, Provenza JR, et al. EpiFibro – a nationwide databank for fibromyalgia syndrome: the initial analysis of 500 women. Rev Bras Reumatol. 2013;53:382-7.
-
4Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Katz R, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62:600-10.
-
5Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz R, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38:1113-22.
-
6Wolfe F, Walitt BT, Rasker JJ, Katz RS, Häuser W. The use of polysymptomatic distress categories in the evaluation of fibromyalgia (FM) and FM severity. J Rheumatol. 2015;42:1494-501.
-
7Marques A, Santos A, Assumpção A, Matsutani L, Lage L, Pereira C. Validation of the Brazilian version of the Fibromyalgia Impact Questionnaire (FIQ). Rev Bras Reumatol. 2006;46:24-31.
-
8Paiva ES, Heymann RE, Rezende MC, Helfenstein M, Martinez JE, Provenza JR, et al. A Brazilian Portuguese version of the Revised Fibromyalgia Impact Questionnaire (FIQR): a validation study. Clin Rheum. 2013;32:1199-206.
-
9Henriksson CM, Liedberg GM, Gerdle B. Women with fibromyalgia: work and rehabilitation. Disabil Rehabil. 2005;27:685-94.
-
10Kennedy M, Felson DT. A prospective long-term study of fibromyalgia syndrome. Arthritis Rheum. 1996;39:682-5.
-
11Granges G, Zilko P, Littlejohn GO. Fibromyalgia syndrome: assessment of the severity of the condition 2 years after diagnosis. J Rheumatol. 1994;21:523-9.
-
12Bengtsson A, Backman E, Lindblom B, Skogh T. Long term follow-up of fibromyalgia patients: clinical symptoms, muscular function, laboratory tests – an eight year comparison study. J Musculoskeletal Pain. 1994;2:67-80.
-
13Walitt B, Fitzcharles MA, Hassett AL, Katz RS, Häuser W, Wolfe F. The longitudinal outcome of fibromyalgia: a study of 1555 patients. J Rheumatol. 2011;38:2238-46.
Publication Dates
-
Publication in this collection
Mar-Apr 2017
History
-
Received
17 Mar 2016 -
Accepted
31 July 2016