Abstracts
OBJECTIVES: To investigate the implications of Dermosonic lipoclasis (DLC), i.e. lipolysis on subcutaneous white adipose tissue induced by ultrasound, for the energy metabolism and body composition of healthy rats. METHODS: Twenty four-month-old male Wistar rats weighting ±380g were randomly divided into two groups: 1) sham control (SC) and 2) low-intensity ultrasound therapy (LIUST). For 10 days, after sedation with 3% vaporized halothane, the animals underwent LIUST (I SATA =3MHz, 1 Wcm-2, pulsed mode 2:8ms, 30% cycles for 3 minutes) in the infra-abdominal and inguinal regions. Weight measurements, naso-anal length and metabolic parameters (food and water intake and excretion) were monitored during the study. At the end of the treatment, blood samples were collected for biochemical analyses. Retroperitoneal (RET), perirenal (PR), epididymal (EP) and inguinal (ING) adiposity was evaluated. HOMA-IR (homeostasis model assessment) was calculated to estimate insulin resistance. For statistical analyses, the Student t test, ANOVA and the Tukey test were used, and differences were established as p<0.05. RESULTS: Regarding body weight, the SC group (384±9g) did not show any changes, while the treated group (337±2g) showed reductions (p<0.01). This was also seen in relation to food intake: (25±1g) vs. (21±1g). There were also reductions (p<0.05) in the RET, PR and ING fat-pads, obesity index, triglyceride levels and plasma lipoprotein levels. Hyperinsulinemia without changes in glycemia characterized a state of insulin resistance, which was confirmed by HOMA-IR. CONCLUSIONS: DLC reduced the food intake and body weight and modified the fat deposition in the RET, PR and ING stores in rats. This changed the lipid profile to produce a significant state of insulin resistance.
ultrasound therapy; dermosonic lipoclasis; adipose tissue; energy metabolism; insulin resistance
OBJETIVOS: Investigar as implicações da lipoclasia dermossônica (LCD), lipólise do tecido adiposo branco subcutâneo induzido por ultrassom (US), no metabolismo energético e na composição corporal de ratos saudáveis. MÉTODOS: Utilizaram-se 20 ratos Wistar saudáveis, com 4 meses de idade, pesando ±380g, divididos aleatoriamente em 2 grupos: 1) controle-sham (CS), 2) terapia ultrassônica de baixa intensidade (TUSBI). Durante 10 dias, após sedação (halotano-3% vaporizado), os animais eram submetidos à TUSBI (I SATA=3MHz, 1W.cm-2, modo pulsado 2:8ms, ciclo de 30% por 3 minutos) em região infra-abdominal e inguinal. Medidas de peso, comprimento naso-anal e parâmetros metabólicos (ingestão e excreção) foram controlados durante o estudo. Ao final do tratamento, amostras de sangue foram coletadas para dosagens bioquímicas, e então avaliadas adiposidades retroperitoneal (RET), perirenal (PR), epididimal (EP) e inguinal (ING). O HOMA-IR (homeostasis model assessment) foi calculado para estimar resistência insulínica (RI). Para análise estatística, utilizou-se ANOVA, teste de Tukey e teste t de Student, com diferenças estabelecidas em p<0,05. RESULTADOS: No peso corporal, não houve alteração nos animais CS (384±9g), no entanto reduziu (p<0,01) no grupo TUSBI (337±2g), assim como a ingestão de comida (25±1g) vs.(21±1g). Houve ainda reduções (p<0,05) nos coxins RET, PR e ING, índice de obesidade, níveis de triglicerídeos e lipoproteínas plasmáticas. A hiperinsulinemia, sem alterações da glicemia, caracterizou estado de RI confirmado pelo HOMA-IR. CONCLUSÕES: A LDC reduziu a ingestão de comida e o peso corporal, além de modificar a deposição de gordura nos depósitos RET, PR e ING em ratos, o que alterou o perfil lipoprotéico produzindo importante quadro de RI.
terapia ultrassônica; lipoclasia dermossônica; tecido adiposo; metabolismo energético; resistência insulínica
ORIGINAL ARTICLE
Implications of dermosonic lipoclasis for energy metabolism and body composition of healthy Wistar rats
Gonçalves WLS; Cirqueira JP; Abreu GR; Moysés MR
Department of Physiological Sciences, Center of Health Sciences, Universidade Federal do Espírito Santo (UFES), Vitória (ES), Brazil
Correspondence to
ABSTRACT
OBJECTIVES: To investigate the implications of Dermosonic lipoclasis (DLC), i.e. lipolysis on subcutaneous white adipose tissue induced by ultrasound, for the energy metabolism and body composition of healthy rats.
METHODS: Twenty four-month-old male Wistar rats weighting ±380g were randomly divided into two groups: 1) sham control (SC) and 2) low-intensity ultrasound therapy (LIUST). For 10 days, after sedation with 3% vaporized halothane, the animals underwent LIUST (ISATA =3MHz, 1 Wcm-2, pulsed mode 2:8ms, 30% cycles for 3 minutes) in the infra-abdominal and inguinal regions. Weight measurements, naso-anal length and metabolic parameters (food and water intake and excretion) were monitored during the study. At the end of the treatment, blood samples were collected for biochemical analyses. Retroperitoneal (RET), perirenal (PR), epididymal (EP) and inguinal (ING) adiposity was evaluated. HOMA-IR (homeostasis model assessment) was calculated to estimate insulin resistance. For statistical analyses, the Student t test, ANOVA and the Tukey test were used, and differences were established as p<0.05.
RESULTS: Regarding body weight, the SC group (384±9g) did not show any changes, while the treated group (337±2g) showed reductions (p<0.01). This was also seen in relation to food intake: (25±1g) vs. (21±1g). There were also reductions (p<0.05) in the RET, PR and ING fat-pads, obesity index, triglyceride levels and plasma lipoprotein levels. Hyperinsulinemia without changes in glycemia characterized a state of insulin resistance, which was confirmed by HOMA-IR.
CONCLUSIONS: DLC reduced the food intake and body weight and modified the fat deposition in the RET, PR and ING stores in rats. This changed the lipid profile to produce a significant state of insulin resistance.
Key words: ultrasound therapy; dermosonic lipoclasis; adipose tissue; energy metabolism; insulin resistance.
Introduction
The broad use of ultrasound therapy in several health branches made it possible to identify several different biological effects of low-intensity ultrasound (US) in a number of tissues1-14, namely fibrinolytic effects9, as well as thrombolytic10, lipolytic6,8, angiogenic13, enzymatic5,14, and oxidative effects13, as well as synergic in vasoactive drugs4,5, to name but a few3,11. All of these effects are directly associated with the dosages used (power, frequency, time-length), and also with the responsiveness of the biological tissue exposed, i.e. specific US dosages directly imply certain cell actions and tissue responses.
Current studies show that US applications in usual dosages (1 and 3 MHz) in physical therapy3,8 and in clinical diagnosis1, although not unsafe for daily practice, should not be taken as a risk-free tool, because it only uses small variations in frequency, power, and time i.e. dosages and ways to apply them to bring about local changes in the biological tissue exposed1-14.
Miwa et al.6 have recently showed that the US application on the white adipose tissue (WAT) of rats, in different frequencies, promotes an increase in the local secretion of noradrenaline by the sympathetic nervous system, causing local lipolysis and the mobilization of fat by the release of free fatty acids (FFA). Besides, Kogure et al.7 reported that the stimulation through US not only produced the lipolysis of the subcutaneous WAT with FFA release, but also boosted the synthesis of the family of type-2 and type-3 mitochondrial uncoupling proteins (UCPs) in the gastrocnemius muscle of rats by means of a mechanism other than exercise.
Moreover, other investigations on energetic metabolism have evidenced that high levels of FFAs regulate the gene expression of the UCP-3 in the skeletal muscle, liver, pancreas, and other tissues, and that this protein family is in close association with obesity, fasting, and exercise15,16. These and other effects of low-intensity ultrasound therapy (LIUST) on the lipid and glycemic metabolism fostered several new possibilities for therapy applications in the treatment of obesity and type-II diabetes mellitus (DM2). However, recents studies on thermogenesis, obesity, and physical activity revealed that the increase in plasma FFA is involved with the conditions of insulin pre-resistance (IR), obesity, and DM2, and have several implications in the pathogenesis of metabolic syndrome (MS)17-24.
Paradoxically, the beneficial findings of low-intensity ultrasound therapy in various fields2,5,7,10,11 are rarely found in clinical studies or in robust experimental research dealing with the local and systemic metabolic risks of fat mobilization and release triggered by the use of dermosonic lipoclasis (DLC), lysis of subcutaneous adipose cells by LIUST, seeing that this is broadly used in surgical, aesthetic, and cosmeceutical treatments4,8,10,23,25. Therefore, the goal of this study was to evaluate the implications of DLC on the energetic metabolism and body fat distribution of healthy rats, by means of measurements such as weight, length, plasma biochemistry, body composition (obesity index)26 and bilateral lipectomy of the fat depositions27.
Methods
This investigation was conducted according to the directives set out by the Guide for the Care and Use of Laboratory Animals and approved by the Animal Research Ethics Committee of Universidade Federal do Espírito Santo (UFES), under protocol number 014/2008.
Experimental groups and metabolic control
Twenty 4-month-old Wistar rats, obtained from the Center of Health Sciences of UFES vivarium were used in this study. Weighing ±380 grams, they were randomly divided into two groups (n=10 each): 1) sham-control (CS) and 2) treated with DLC. The rats were placed in metabolic individual cages for control and adjustment of food (g) and water (mL) intake, and kept in a well-ventilated environment under controlled temperature (±22ºC) and artificial lightning, considering a photo-period of 12h (light)/ 12h (dark).
The study started off with a period of adaptation (5 days), during which the animals followed the procedural routines and returned to their cages, thus minimizing the handling stress and the effects of isolation. Next, measurements such as weight and naso-anal (N-A) length were taken, and the rectal thermal control was carried out during the period of adaptation and treatment. On day 0, after sedating the animals through the inhalation of halotane-3%, the infra-abdominal and bilateral inguinal areas were shaved, covering an area of 5 cm2. There was no sample loss, i.e. all animals survived until the end of the study (when they were euthanized).
Ultrasound therapy (3MHz)
In order to mimic the effects of clinical DLC, as well as reduce the thermal effects, the following parameters were set for ultrasound irradiation: ISATA (spatial average temporal average Intensity)=3MHz, 1 W.cm2, pulsed mode (2 ms ON: 8 ms OFF), cycle of 30% for 3 minutes, during 10 consecutive days, in the morning. Every day, the animals under induced sedation (halotane-3% vaporized in a saturation chamber) were placed in dorsal decubitus and the hind paws were held in abduction with an elastic band. Sonogel (H2O) was applied to the pre-established area (infra-abdominal and bilateral inguinal regions), and a 3-MHz transducer (US AVATAT II® [KLD - Biosystems - São Paulo, Brazil]) was placed 1 mm from the skin by using a universal support. After the LIUST began, the transducer was moved bilaterally and systematically from the right to the left throughout all of the irradiation area. The CS and LIUST groups were submitted to the same procedures. However, in the CS group the transducer was disconnected from the US-generating equipment, which was switched off.
The ultrasound transducer was calibrated in a precision scale in the Department of Physics of UFES. The level of US irradiation used in this study was the one recommended for US therapy use by the World Federation for Ultrasound in Medicine and Biology (WFUMB) and by the Food and Drug Administration (FDA)1,2.
Plasma biochemistry and homeostatis model assessment (HOMA)
At the end of the DLC, the animals received an intraperitoneal injection of chloral hydrate (10% - 0.4mL.100g-1). Under induced anesthesia, they were decapitated for blood collection and subsequent analysis of the plasma biochemistry. Next, the blood was centrifuged at 3000 rpm. for 10 min. under refrigeration (4°C) and the plasma was stored at -20°C. Plasma dosages were carried out by the conventional methods of laboratorial analysis, namely total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), very-low density lipoprotein (VLDL), triglycerides (TG), glucose and insulin and lipoprotein lipase enzyme (LPL).
The HOMA testing method was used to estimate the insulin resistance (in which the HOMA-IR=insulinemia after fasting [mU/L] x glycemia after fasting [mg/dL]) and to determine the functional capacity of the beta cells (βcC) (in which HOMA-βcC = 20 x insulinemia after fasting [mmol/L] / glycemia after fasting [mmol/L]-3.5). For the calculation itself, we used the software HOMA Calculator© 2.2, Diabetes Trial Unit, University of Oxford©.
Body composition and bilateral lipectomy
The obesity index for rodents (Lee Index) was calculated by means of the equation 3√Δg/m-1 (cube root [3√] of delta [difference between final and initial weight] of body weight in grams [g] divided by the N-A length in meters [m])26.
The measures for right and left retroperitoneal abdominal adiposity (RET), perirenal (PR), epididymal (EP), and inguinal (ING) adiposities were determined by means of bilateral lipectomy, which consists of the surgical extraction of fat deposits (fat pads). A longitudinal incision of ± 6cm was made on the abdominal skin, using the Alba line as a reference. Next, the ING compartments were collected and measured, the peritoneum was cut open and the RET and PR fat pads were taken out. The peritoneal incision extended up to the epididymus, where the EP27 fat pads were collected.
Data analysis
The data were input into and analyzed by a statistical software (Graph-Pad-Prism4®) using the t test as well as one-way and two-way analyses of variance (ANOVA), followed by the Tukey test for multiple comparisons. The values were expressed as mean ±SEM (standard error of the mean), and the differences set at p<0.05.
Results
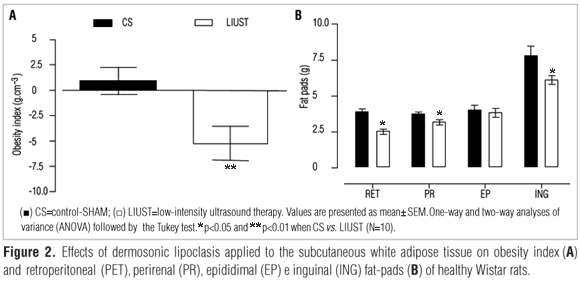
As shown in Figure 1A, there was a reduction (p<0.01) in the body weight of the LIUST group (341±13g) vs. CS group (380±11g) from the 5th day of DLC onwards, which was maintained until the tenth day of treatment (337±12g) vs. (384±9g), respectively. The LIUST group also reduced its food intake as the DLC treatment drew to a close (21.0±0.7g), compared to the CS group (25.6±0.7g) (Figure 1B). There was no reduction in water intake (Figure 1C) or body temperature (Figure 1D). Nevertheless, there was a reduction in the obesity index (p<0.01) of the LIUST group (-5.28±1.68) compared to that of the CS group (0.58±1.47) (Figure 2A).
Figure 2B displays the values for the RET (3.8±0.2g), PR (3.7±0,1g) EP (3.8±0.3g) and ING (7.4±0.6g) fat pads in the CS group, which had a reduction (p<0.05) in the RET (2.5±0.1g), PR (2.8±0.2g) and ING (5.8±0.4g) after being treated with the DLC. The EP values, however, did not change (3.9±0.2g).
Table 2 displays the values for the glycolipoprotein and enzymatic profile of both the CS and LIUST groups, which showed no differences concerning the CT, LDL, or glycemia after fasting values. In contrast, the values for VLDL, triglycerides, HDL and LPL, and insulinemia diverged considerably. The HOMA-IR was high (p<0.01) in the LIUST group compared to the CS group, a result that is compatible with an acute IR condition.
Discussion
This study focused on analyzing the effects of DLC on the energetic metabolism and body composition of healthy rats. As observed in clinical practice25 and also based on these results, DLC (3-MHz LIUST) on the subcutaneous WAT promoted lipolysis with a significant reduction in the weight of the rats from the 5th application onwards, and these effects remained unchanged up to the end of the treatment (Figure 1A). In addition to the weight loss, there was also a gradual reduction in food intake from the 5th day of DLC, which was significantly different by the 10th day compared to the CS group (Figure 1B).
This weight loss combined with the reduction in food intake in the DLC-treated group suggests an increase in the local and systemic energy metabolism, with the probable secretion and release of several adipokines by the subcutaneous WAT. Some evidence and several reports in the literature20,21,25 show that, in this compartment of the WAT (i.e. the subcutaneous fat pad) of humans and rodents, there is a greater capacity for leptin expression, synthesis, and secretion, as well as other pro-inflammatory adipokines, namely the interleukin-6 (IL-6) and the Tumor Necrosis Factor- alpha (TNF-α)4-7. In addition, they make it clear that high levels of these adipokines lead to expressive cardiovascular repercussions, and also modulate important neuropeptide functions and anorexigen hormones in the hypothalamic arcuate nucleus17,28,29. They also act directly on it, interfering with the energetic metabolism and with the regulation of appetite18,21,23, which may have caused the reduction in food intake and the weight loss observed in the DLC-treated rats (Figures 1A and B).
Likewise, in the evaluation of body composition, there was a significant reduction in the obesity index (Lee's Index) among the DLC-treated rats, possibly due to the reduction of inguinal subcutaneous fat (lipolysis), effects already described in non-obese animals6,8. Nevertheless, it was interesting to observe the reductions in the RET and PR fat deposits (fat pads) without any changes in the EP fat pad in healthy males, considering the distance between the epididymal and perirenal fat deposits, as the former is anatomically closer to the US-stimulated areas than the perirenal fat pad. Moreover, a low level of high-frequency ultrasound irradiation was used, which prevented the spreading effect of the mechanic wave, a fact that may imply a remarkable sonochemical effect of the LIUST on the WAT (Figures 2A and B). Considering the lipolytic effects of the DLC on the subcutaneous WAT6-8, the RET and PR fat pad reductions are believed to be related with the endocrine, paracrine, and autocrine actions of the adipokines liberated by the subcutaneous WAT17,18-21,24,28 during the DLC; or they could have occurred because of an increase in the local enzymatic activity (LPL-lipoprotein and LHS-hormone-sensitive lipases) stimulated by the noradrenalin released in the sympathetic nerve endings of the subcutaneous WAT6,7,9,30 after the DLC. Such effects, which corroborate the high levels of LPL obtained in this study (Table 2) are indicative of noteworthy sonochemical actions of the 3MHz US in the subcutaneous inguinal and abdominal WAT17,18,24.
As far as the energy (lipoprotein) metabolism is concerned, the implications produced by the DLC were as follows: hypertriglyceridemia, elevation of the VLDL and reduction of the plasma LDL, characterizing an increase in the risk of dyslipidemia18,24,28 due to the daily changes in the mobilization and storage of body fat in the RET, PR, and ING fat pads, not to mention the increase in the plasma LPL (Table 2).
When comparing the plasma biochemical change to the reduction in the RET, PR, and ING fat pads after the treatment with DLC, one can identify a worrying effect of massive lipolysis and body fat redistribution through the bloodstream, as countless studies have shown that constant rises in the plasma lipid levels are the main risk factor for dyslipidemia and SM17-18 among overweight and obese individuals24,28,30.
Studies carried out by Lewis et al.18 and other authors18-23 describe that the maintenance of high levels of circulating, non-esterified FFA give rise to deleterious effects in the energetic biochemistry and in the cellular metabolism. They also report that the increased FFA flow originating from adipose tissues to non-adipose ones, in general worsens the signaling of the insulin receptor substrate 1 (IRS-1)21-27, suggesting that the IR observed in the animals treated with DLC might have occurred due to the lipotoxic effects of the FFA in the IRS-1 signaling, bringing about dysfunctions in the activation cascade of this receptor18-21, which was indicated by the high values of HOMA-IR. Other studies show that the inhibition and/or loss of sensibility of the IRS-1 may facilitate the accumulation of tissue fat through the reduction of the lipolytic activity of the insulin, leading to the resterification of the FFA in the muscles and other tissues, a critical effect which is currently being discussed as lipotoxicity17-24.
Although the levels of glycemia were similarly elevated in the control and DLC-treated groups, the insulinemia was higher among the animals treated than the basal values found in the control, indicating an acute IR condition, confirmed by the HOMA-IR, which suggested secondary (systemic) actions of the DLC.
These findings suggest that the DLC holds a great potential as an exogenous modulator of the WAT metabolism, as it effectively interferes with the distribution of body fat, reduces body weight and food intake. The consequence is an important lipolysis with the mobilization of the fat deposited in the right and left RET, PR, and inguinal fat pads without altering the EP fat, thus demonstrating a high potential for the treatment of gynoid obesity. Nevertheless, it also provokes significant changes in the lipoprotein and enzymatic plasma profile, implying a serious dyslipidemic and dysmetabolic condition, which induces to the onset of acute IR.
Therefore, more experimental and clinical investigations must be conducted for a better characterization of the cell mechanisms and molecular signal transducers activated by the DLC in the subcutaneous WAT. The purpose of this is to attenuate or abolish the harmful effects and perfect the beneficial effects of the DLC on this essential endocrine organ28.
Acknowledgements
The authors would like to thank Dr. M. Borsoi and Dr. F. Souza, coordinators of the Clinical Pathology Laboratory of Hospital Universitário Cassiano Antônio de Moraes - UFES, for the invaluable cooperation in the dosage and analysis of the biochemical profile for this study. This research was supported by Fundo de Apoio à Ciência e Tecnologia de Vitória (Facitec) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES (Brasília).
References
- 1. Barnett SB, ter Haar GR, Ziskin MC, Rott HD, Duck FA, Maeda K. International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound Med Biol. 2000;26(3):355-66.
- 2. ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93(1-3):111-29.
- 3. Koeke PU, Parizotto NA, Carrinho PM, Salate AC. Comparative study of the efficacy of the topical application of hydrocortisone, therapeutic ultrasound and phonophoresis on the tissue repair process in rat tendons. Ultrasound Med Biol. 2005;31(3):345-50.
- 4. Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy - a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11(6):349-63.
- 5. Li JK, Chang WH, Lin JC, Ruaan RC, Liu HC, Sun JS. Cytokine release from osteoblasts in response to ultrasound stimulation. Biomaterials. 2003;24(13):2379-85.
- 6. Miwa H, Kino M, Han LK, Takaoka K, Tsujita T, Furuhata H, et al. Effects of ultrasound application on fat mobilization. Pathophysiology. 2002;9(1):13-9.
- 7. Kogure A, Yoshida T, Takakura Y, Umekawa T, Hioki C, Yoshioka K, et al. Effect of ultrasonic stimulation on mRNA abundance of uncoupling protein (UCP) 2 and UCP 3 in gastrocnemius muscle of rats. Clin Exp Pharmacol Physiol. 2005;32(1-2):91-3.
- 8. Gonçalves WLS, Cirqueira JP, Soares LS, Bissoli NS, Moysés MR. Utilização da terapia ultra-sônica de baixa intensidade na lipodistrofia ginecóide: uma terapia segura ou risco cardiovascular transitório? um estudo pré-clínico. An Bras Dermatol. 2005;80 Supl 3:S352-9.
- 9. Hardig BM, Persson HW, Olsson SB. Direct action on the molecule is one of several mechanisms by which ultrasound enhance the fibrinolytic effects of reteplase. Blood Coagul Fibrinolysis. 2006;1(2):105-12.
- 10. Nedelmann M, Eicke BM, Lierke EG, Heimann A, Kempski O, Hopf HC. Low-frequency ultrasound induces nonenzymatic thrombolysis in vitro. J Ultrasound Med. 2002;21(6):649-56.
- 11. Noble JG, Lee V, Griffith-Noble F. Therapeutic ultrasound: the effects upon cutaneous blood flow in humans. Ultrasound Med Biol. 2007;33(2):279-85.
- 12. O'Brien WRJr. Ultrasoundbiophysics mechanisms. Prog Biophys Mol Biol. 2007;93(1-3):212-55.
- 13. Wu J. Shear stress in cells generated by ultrasound. Prog Biophys Mol Biol. 2007;93(1-3):363-73.
- 14. Araújo M, Baptista-Silva JCC, Gomes PO, Campos HO, Novo NF, Juliano Y. Efeitos do ultra-som de baixa intensidade na veia auricular de coelhos. Acta Cir Bras. 2003;18(1):25-31.
- 15. Depieri TZ, Pinto RR, Catarin JK, de Carli MCL, Garcia-Junior JR. UCP-3: regulação da expressão gênica no músculo esquelético e possível relação com o controle do peso corporal. Arq Bras Endocrinol Metab. 2004;48(3):337-43.
- 16. Tunstall RJ, Mehan KA, Hargreaves M, Spriet LL, Cameron-Smith D. Fasting activates the gene expression of UCP3 independent of genes necessary for lipid transport and oxidation in skeletal muscle. Biochem Biophys Res Commun. 2002;294(2):301-8.
- 17. Hermsdorff HHM, Monteiro JBR. Gordura visceral, subcutânea ou intramuscular: onde está o problema? Arq Bras Endocrinol Metabol. 2004;48(6):803-11.
- 18. Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23(2):201-29.
- 19. Sarafidis PA, Bakris GL. Non-esterified fatty acids and blood pressure elevation: a mechanism for hypertension in subjects with obesity/insulin resistance? J Hum Hypertens. 2007;21(1):12-9.
- 20. Bajaj M, Berria R, Pratipanawatr T, Kashyap S, Pratipanawatr W, Belfort R, et al. Free fatty acid-induced peripheral insulin resistance augments splanchnic glucose uptake in healthy humans. Am J Physiol Endocrinol Metab. 2002;283(2):E346-52.
- 21. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697-738.
- 22. Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes. 2004;5(6)3:1412-7.
- 23. Rasouli N, Molavi B, Elbein SC, Kern PA. Ectopic fat accumulation and metabolic syndrome. Diabetes Obes Metab. 2007;9(1):1-10.
- 24. Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19(4):471-82.
- 25. Rawlings AV. Cellulite and its treatment. Int J Cosmet Science. 2006;28(3):175-90.
- 26. Stephens DN. Does the Lee obesity index measure general obesity? Physiol Behav. 1980;25(2):313-5.
- 27. Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am J Physiol Endocrinol Metab. 2007;293(4):E1012-20.
- 28. Kershew EE. Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548-56.
- 29. Sivitz WI, Walsh SA, Morgan DA, Thomas MJ, Haynes WG. Effects of leptin on insulin sensitivity in normal rats. Endocrinology. 1997;138(8):3395-01.
- 30. Lagin D. Control of fatty acid and glycerol release in adipose tissue lipolysis. C R Biol. 2006;329(8):598-607.
Correspondência para:
Publication Dates
-
Publication in this collection
06 Feb 2009 -
Date of issue
Feb 2009
History
-
Accepted
06 Oct 2008 -
Reviewed
05 July 2008 -
Received
16 Feb 2008