Abstracts
BACKGROUND: Ultrasound (US) is a widely used and studied resource for physical therapy treatments. Given the scarcity of studies on the effects of US on healthy tissue, many physical therapy professionals make unfounded decisions regarding its methods and parameters of application. OBJECTIVES: The possible histological and morphometric changes in the healthy tissue of Wistar rats in vivo irradiated with different intensities of US were evaluated. METHODS: Thirty Wistar rats, randomly distributed among five groups of six animals each, were treated on the right side of the dorsal region, over an area of 4 cm². The left side served as a control. The treatment was applied over a four-day period, with two mins. of daily irradiation. The output intensity was checked using a precision dosimeter before the applications. Histological and morphometric analyses were performed using the Image Tool software. RESULTS: There were slight inflammatory infiltration and thinning of the dermis fibers, particularly in the groups irradiated with 1.5 and 2 W/cm². There was also thickening of the epidermis in the samples from the irradiated animals. To evaluate the quantitative results, the statistical analyses consisted of one-way ANOVAs with the post-hoc Tukey tests. There were significant differences in epidermis thicknesses between the control group and the groups irradiated with 1.0, 1.5 and 2.0 W/cm². CONCLUSIONS: Higher doses of US produced changes in the epidermis and dermis, i.e. increased thickness and collagen fiber thinning and proliferation, respectively. These results serve as a warning of the possible implications of therapeutic ultrasound use in esthetics.
Therapeutic ultrasound; dosimetry; side effects; tissue damage
CONTEXTUALIZAÇÃO: O Ultrassom (US) é um dos recursos físicos amplamente utilizado e pesquisado nos tratamentos de fisioterapia. Sabe-se que diante de uma escassa literatura sobre efeitos do US em tecidos sadios, muitos profissionais fisioterapeutas acabam realizando aplicações infundadas de métodos e parâmetros. OBJETIVO: Avaliar possíveis alterações histológicas e morfométricas do tecido sadio in vivo de ratos Wistar irradiados com diferentes intensidades de US. MÉTODOS: Trinta ratos da linhagem Wistar, distribuídos aleatoriamente em cinco grupos de seis animais cada foram tratados na região dorsal do lado direito numa área de 4cm². O lado esquerdo serviu como controle. O tratamento foi feito durante quatro dias com 2 minutos de irradiação. Verificou-se a intensidade de saída com dosímetro de precisão antes das aplicações. Analisou-se a histologia e a morfometria por meio do software Image Tool. RESULTADOS: Observou-se um discreto infiltrado inflamatório e adelgaçamento das fibras da derme, principalmente dos grupos irradiados com 1.5 e 2W/cm². Notou-se também um aumento na espessura da epiderme nas amostras dos animais irradiados. Para avaliar os resultados quantitativos, utilizou-se como análise estatística ANOVA one way e o teste post hoc de Tukey. Na espessura da epiderme, obtiveram-se diferenças significativas entre grupo controle e os grupos irradiados com 1.0, 1.5 e 2.0W/cm². CONCLUSÃO: Sob ação do US nas doses maiores houve alterações na epiderme e derme, respectivamente, o aumento da espessura e proliferação com adelgaçamento das fibras colágenas, o que alerta para possíveis implicações do uso do US em estética.
ultrassom terapêutico; dosimetria; efeitos colaterais; danos teciduais
ORIGINAL ARTICLE
IInter units Post Graduate Program in Bioengineering, Engineering School of São Carlos (EESC), Universidade de São Paulo (USP), São Carlos (SP), Brazil
IIDepartment of Electrical Engineering, EESC, USP
IIIDepartment of Patology, Dentistry School, Universidade Estadual Paulista (UNESP), Araraquara (SP), Brazil
IVDepartment of Physical Therapy, Universidade Federal de São Carlos (UFSCar), São Carlos (SP), Brazil
Correspondence to
ABSTRACT
BACKGROUND: Ultrasound (US) is a widely used and studied resource for physical therapy treatments. Given the scarcity of studies on the effects of US on healthy tissue, many physical therapy professionals make unfounded decisions regarding its methods and parameters of application.
OBJECTIVES: The possible histological and morphometric changes in the healthy tissue of Wistar rats in vivo irradiated with different intensities of US were evaluated.
METHODS: Thirty Wistar rats, randomly distributed among five groups of six animals each, were treated on the right side of the dorsal region, over an area of 4 cm2. The left side served as a control. The treatment was applied over a four-day period, with two mins. of daily irradiation. The output intensity was checked using a precision dosimeter before the applications. Histological and morphometric analyses were performed using the Image Tool software.
RESULTS: There were slight inflammatory infiltration and thinning of the dermis fibers, particularly in the groups irradiated with 1.5 and 2 W/cm2. There was also thickening of the epidermis in the samples from the irradiated animals. To evaluate the quantitative results, the statistical analyses consisted of one-way ANOVAs with the post-hoc Tukey tests. There were significant differences in epidermis thicknesses between the control group and the groups irradiated with 1.0, 1.5 and 2.0 W/cm2.
CONCLUSIONS: Higher doses of US produced changes in the epidermis and dermis, i.e. increased thickness and collagen fiber thinning and proliferation, respectively. These results serve as a warning of the possible implications of therapeutic ultrasound use in esthetics.
Key words: Therapeutic ultrasound; dosimetry; side effects; tissue damage.
Introduction
Ultrasonic energy is one of the physical resources widely used in physical therapy and aesthetic treatments and in medical diagnoses1. Several researchers have demonstrated the increase of collagen synthesis through ultrasound (US) applications, which accelerate the tissue healing in various types of injuries2. Using US stimulation with different intensities in cells which produce collagen type I and II, Tsai et al.3 demonstrated significant increases in the collagen synthesis of these cells when compared with the ones of the control group. Iwashina et al.4 studied the pulsed US stimulus with different intensities (ISATA, spatial and temporal mean intensities) in in vitro cells of the intervertebral discs of rabbits (7.5 up to 120 mW/cm2) and observed significant increases of collagen synthesis by the cells of the intervertebral discs. These results were also observed in groups irradiated at lower intensities. On the other hand, there is scarce literature about the US effects in healthy tissues, which is a basic condition for aesthetic treatments5. It is also unknown what are the action mechanisms behind many of the clinically applied therapeutic effects6.
Gonçalves et al.7 studied the US potential risks in therapies in dermatology, aesthetics and their implications in the cardiovascular system. They demonstrated changes of the hemodynamic parameters; lipid and glucose serum levels, besides producing attenuation of vasodilation induced by adenosine, a possible factor for the presence of cardiovascular risks. Studying the applications of US in the degranulation of the skin mast cells5, it was observed the occurrence of injury of the mast cells, when the US was applied at the intensity of 3,0W/cm2. In another approach, Valentini, Maciel and Parizotto8 studied the US calibrating, and highlighted the importance of compliance of the therapeutic US equipment with NBR-IEC 1689. In this study, it was reported that the US equipment in Brazil and the world do not consider the specifications of the parameters requested by the NBR-IEC 1689.
Thus, it is worrying regarding the popularity and increasing demand for the use of US in aesthetic treatments. It is known that in aesthetic treatments use higher intensities (2,5 to 3,0 W/cm2) during a greater number of applications. The combination of these factors may increase the risks of tissue injury. The main motivation of the present study was the hypothesis of the possible risks of US applications in aesthetic treatments. The aims of this research were to histologically evaluate possible changes of the healthy tissue of Wistar rats in vivo, irradiated with different intensities of US by analyzing the histological sections in a qualitative and quantitative way.
Integumentary system
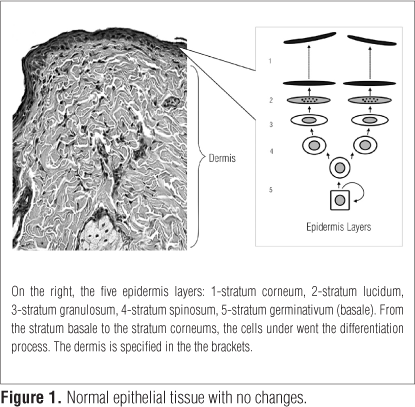
The integumentary system consists of the epidermis and dermis. The first consists of epithelial cells called keratinocytes which are produced in the basal layer and the dermis is responsible for skin resistance and elasticity9 (Figure 1). It is known that skin layers are avascular, thus, the only form of nutrition is by diffusion through the capillary beds of the dermis, which are well vascularized9.
In the basal stratum, occurs constant cell renewal, with high mitotic activity. These cells undergo differentiation, being pushed to the spinosum and lucidum stratum until they reach the epidermis. These cells form four to six overlapping layers of keratinocytes, providing mechanical strength to the epidermis. In the granulosum stratum, substances which ensure the cohesion to the corneum stratum are synthesized. The corneum stratum is the outer one, consisting of flattened and anucleate cells called corneocytes10 (Figure 1).
Ultrasound
US therapy is characterized by mechanical vibrations of high frequency above 20 KHz11. The main form of sound wave propagation in biological tissue is longitudinal, in which the particles vibrate parallel to the direction of the wave propagation12. According to Hedrick, Hykes and Starchman13, the wave propagates as it undergoes changes in pressure, occurring regions of higher compression and regions of low pressure (rarefaction). The sound field of US at the nearest part of the transducer, called the Fresnel zone, shows up as an uneven beam, with spatial and temporal peaks of power. As the beam moves away, from the transducer begins to show more regular behaviors, called the distant field or Fraunhofer zone13. Given this, it is known that physical therapy treatments are performed in the near field, making it difficult to measure the behaviors of the wave in the biological tissue.
Methods
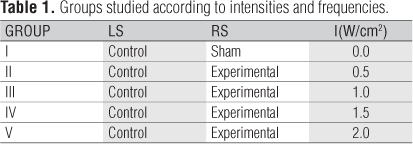
Thirty adult Wistar rats (Rattus norvegicus: var. albinus, Rodentia, mammalia) with a body mass of 195.06 g±20.9 were used in the present study. The animals were randomly divided into five groups of six animals. Each group was irradiated with different intensities of US, as shown in Table 1.
The locations chosen for the US applications were the dorsal region (Figure 2), which was divided into two parts; left side (LS) - control of the animal and right side (RS) - experimental. This study has been approved by the Ethics in Research Committee of the Universidade Federal de São Carlos (UFSCar), protocol nº 007/2008. Initially the animals went through the process of adaptation to the researcher for a week to avoid a high stress levels and ensuring the reliability of the results of the study. Soon after, the animals were placed in the vat, where they were anesthetized with ether for the performance of digital trichotomy of the region to be treated (Figure 2). For two days, it was simulated treatment - with the US off - in order to adapt the animals to the procedure.
The treatment was performed for four consecutive days, lasting two mins. of irradiation in an area of four cm2. The US equipment used was brand BIOSET, SONACEL Expert, with a frequency of three MHz (Figure 3). Before each application, measurements were carried out in each group to obtain greater precision of the different irradiated intensities. For this, a precision dosimeter (balance) was used, Ultrasound Power Metere, Model UPT-1 (Figure 3) to determine the spatial and temporal mean intensities.
After the fourth application of the treatment, the animals were anesthetized by a combination of Ketamine® (35 mg/kg) and xylazine® (4 mg/kg) intramuscular injections in their thighs. Then, the tissues of the dorsal, epidermis and dermis regions were withdrawn. While still anesthetized, the animals were euthanized by guillotine by a trained professional. After data collection, samples were placed in cassettes with filter paper and dipped in 10% formaldehyde, unbuffered for 24 hours. The samples went through the standard procedures for obtaining histological sections embedded in paraffin and afterwards were stained with hematoxylin-eosin (HE).
The histological sections were microscopically analyzed in a qualitative way to evaluate the structure of epithelial tissue in the control and experimental groups. These tests were carried out in the laboratory of Dentistry Physiology and Pathology of the Universidade Estadual de São Paulo (UNESP), Araraquara (SP), Brazil. To obtain the histological images, were used with the Olympus BX51 microscope and the digital camera Olympus Camedia C-5060, 5.1 mega pixels.
To obtain the measurements of the thickness of the epidermis for each sample, a quantitative analysis of images of the histological sections was performed using the software UTHSCSA Image Tool14. The software was calibrated using the image of a ruler photographed in the same size, and all images were analyzed by means of this calibration. There were four measures carried out of the thickness of the epidermis in random areas. This procedure was done for the six samples of each group, resulting in 120 measurements for each side (LS and RS). The results of each group were statistically analyzed by means of the one-way ANOVA with a significance level of 1% and supplemented by the Tukey post hoc test to verify the existence of significant differences between the groups means. The quantitative data analyses were carried out with the statistical procedures of Excel and the BioStat 3.0 programs.
Results
Qualitative results
The histological sections were observed under a microscope by an experienced pathologist, and tissue changes were analyzed in sections of the samples irradiated with the US. Figure 4 shows the histological sections of groups III, IV and V. The images on the left (A, C and E) were the control samples for each group. The images on the right (B, D and F) were the samples of the experimental groups. In group II, irradiated with 0.5 W/cm2, there was no changes. Changes were visible from the samples irradiated with 1 W/cm2, when compared with the controls. There were observed increases in the thickness of the epidermis, the presence of inflammatory infiltrates and a proliferation of collagen fibers, which were lower and thinner.
Quantitative Results
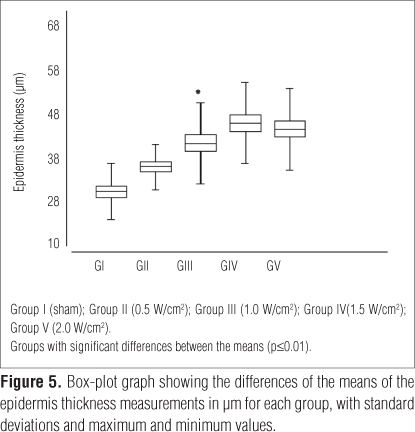
The ANOVA showed the presence of differences between the groups. The Tukey test demonstrated that group I had the lowest means for the thickness of the epidermis (29.25μm±6.53) compared with groups III (40.62μm±9.49), IV (45.32μm±9.43) and V (43.91μm±9.55), and the differences were significant (p<0.01). These data showed that the group irradiated with higher intensities demonstrated the highest means. A trend to increasing the thickness of the epidermis when correlated with the intensities used in each group was observed in Figure 5. It seemed that there was a saturation level of the increase after 1 W/cm2, leading to a plateau effect at higher dosages.
Discussion
The high intensities used in aesthetic treatments were a major concern in this study. It is well known that US is used very often in clinics for therapeutic and aesthetic treatments. However, studies have shown unfounded results regarding the intensity, exposure time and conditions for calibrating of the US15. To examine whether there were any differences between the intensities that the equipment panel records and the intensities transmitted by the transducer, Guirro et al.16 investigated the temporal and spatial mean acoustic intensities of the US equipment used in clinics in the city of Piracicaba. This study showed that most of the equipment emitted energy above 30%, exceeding the specific values proposed by the NBR-IEC 1689. In another study it was reported that one third of the US equipment were outside the standards, reducing its reliability in the application and the results17.
In an effort to evaluate the effects and potential risks of US at high intensities in the venous system, Araújo et al.18 carried out applications in the auricular veins of rabbits. It was observed that, with an intensity of 3W/cm2 in a continuous mode, there was the induction of venous thrombosis and significant increases in the lymphocytes, which could be observed macroscopically by edema, erythema and heat. The study's images showed injuries on the vessel walls with the extravasation of the cells.
Based upon the results obtained in this study, it could be suggested that the US application had also generated changes in epithelial tissue of healthy Wistar rats. There was a dramatic increase in the thickness of the epidermis. This probably occurred due to stimulating the mitosis of the basal stratum cells of the epidermis. The evidence observed in the present results showed a relationship with the study of Silva19, which evaluated the effects of pulsed US on the androgenic mitotic activity of the Leydig cells. It was reported that there were increases in the speed of the cellular cycle. Such evidence showed the role of the proliferative layer of the epidermis in the process of cellular renewal, which was susceptible to the influence of the US energy.
Boucaud et al.20 also reported changes in epithelial tissue with US. They investigated the biological effects that US can cause in phonophoresis. The in vitro skin samples which were irradiated above 2.5 W/cm2 showed injuries. These authors conducted in vivo studies in epithelial tissue of rats and observed deeper injuries, including capillary and muscle necrosis. These data corroborated the results of the present study.
According to the histological analyses of this study, there were also observed changes in the collagen fibers located in the dermis of the samples irradiated with 1W/cm2. Based upon these results, the thinning of the fibers could be considered as a proliferation mechanism of the collagen fibers, leading to changes in the local younger fibers and with a high probability of reducing the mechanical resistance of these tissues. Similar results were reported in the study of Visnardi21, which investigated the effects of low-intensity US on the collagen in the skin of healthy rats and showed, through the analysis of textural birefringence (intrinsic), that there was a disruption in the collagen fibers, mainly in deeper layers of the dermis.
Another tissue change observed in this study was the presence of a mild inflammatory infiltration beneath the epidermis of the samples irradiated above 1W/cm2, which might be related to the increases in local tissue temperatures. This temperature increases could be related to the behaviors of the US sound field which shows some irregularity. This irregularity could show peaks of intensity, resulting in greater heating in some regions of the tissue.
In this study, biopsies were carried out on the same day after the last US application. Thus, the changes obtained from epithelial tissue could be considered to have a more acute character. However, it is important to note that they occurred after only four days of application. In aesthetic treatments, generally, they are applied for more than 20 sessions with very high intensities (between 2,5 to 3,0 W/cm2), higher than the ones used in this study.
According to the morphometric results showed in Figure 5, there were linear increases regarding the epidermis thickness measures until the intensity of 1.0W/cm2, which could be observed. In the groups irradiated with 1.5 and 2.0W/cm2, these increases were maintained. Apparently, there was a maximum mitosis response in the cells of the basal stratum, resulting in similar thicknesses after the end of the four days of treatment. In a literature review about US dosimetry22, the authors reported that high dosages, such as 1.5 and 2.0W/cm2, could be less effective than the low doses. Moreover, the authors highlighted the fact that in the clinical applications, there was evidence that high intensities were not so efficient. Based on this, they suggested that professionals should preferentially use sub-doses instead of over-doses, because the first might be more effective and offer lower risks to cause injuries.
Besides showing the scarce literature about US effects in healthy tissue, it is not well known which factors caused these changes in the biological tissues. Most effects attributed to the US treatments were dependent on the chemical mediators whose action mechanisms in the biological tissues were not very well-known. There are many disagreements in the literature23, therefore, it is suggested, based on the results of this study, to use US with great caution, especially in aesthetic treatments, in which the doses are elevated. Elevated doses of US can cause injuries, some of them internal, whose consequences could appear later on.
Conclusions
The present experimental study demonstrated that continuous US (3MHz, with applications of two mins. of duration and with intensities above 1W/cm2 to 2W/cm2 on the epithelial tissues of healthy Wistar rats caused changes in their biological tissues. Such alterations were characterized by significant increases in the epidermis thickness, the presence of slight inflammatory infiltrations beneath the epidermis, and also changes in the collagen fibers, making them thinner and more numerous. Therefore, more studies about the applications of US to evaluate the possible morphological and biochemical changes on healthy tissues are important. Moreover, studies regarding the action mechanisms which would generate such modifications, by means of different techniques, could also be important. Care is important in US applications. Studies which observe the long-term effects of the applications of US, to verify if the changes persist and what is their results after a more prolonged period of treatments.
References
- 1. Associação Brasileira de Normas Técnicas ABNT. Ultra-som sistemas de fisioterapia: prescrições para desempenho e métodos de medição na faixa de freqüência de 0,5 MHz a 5 MHz. Rio de Janeiro: ABNT; 1998.
- 2. Dyson M. Mechanisms involved in therapeutic ultrasound. Physiotherapy. 1987;73:116-20.
- 3. Tsai WC, Pang JHS, Hsu CC, Chu NK, Lin MS, Hu CF. Ultrasound stimulation of types I and III collagen expression of tendon cell and upregulation of transforming growth factor β. J Orthop Res. 2006;24(6):1310-6.
- 4. Iwashina T, Mochida J, Miyazaki T, Watanabe T, Iwabuchi S, Ando K, et al. Low-intensity pulsed ultrasound stimulates cell proliferation and proteoglycan production in rabbit intervertebral disc cells cultured in alginate. Biomaterials. 2006;27(3):354-61.
- 5. Dyson M, Luke DA. Induction of mast cell degranulation in skin by ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 1986;33(2): 194-201.
- 6. ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93(1-3):111-29.
- 7. Gonçalves WLS, Cirqueira JP, Soares LS, Bissoli NS, Moysés MR. Utilização da terapia ultra-sônica de baixa intensidade na redução da lipodestrofia ginecóide: uma terapia segura ou risco cardiovascular transitório: Um estudo pré-clínico.. An Bras Dermatol. 2005;80(Suppl 3):S352-9.
- 8. Valentini EV, Maciel CD, Parizotto NA. Importância da conformidade dos equipamentos de ultra-som terapêutico com a NBR-IEC 1689. Fisioter Bras. 2006;7(1):59-65.
- 9. Spence AP. Anatomia humana sásica. São Paulo: Manole; 1991.
- 10. Gartner LP, Hiatt JL. Atlas colorido de histologia. 4Ş ed. Rio de Janeiro: Guanabara Kooogan; 2007.
- 11. Zagzebski JA. Essentials of ultrasound physics. Saint Louis: Mosby; 1996.
- 12. Leighton TG. What is ultrasound? Prog Biophys Mol Biol. 2007;93(1-3):3-83.
- 13. Hedrick WR, Hykes DL, Starchman DL. Ultrasound physics and instrumentation. Saint Louis: Mosby; 2005.
- 14. Dove SB. UTHSSA Image Tool [homepage na internet]. Texas: The University of Texas Health Science Center, Inc.; c1996 [atualizada em 2002/; acesso em _26/02/2007_]. Disponível em: http://ddsdx.uthscsa.edu/dig/itdesc.html
» link - 15. Speed CA. Therapeutic ultrasound in soft tissue lesions. Rheumatology (Oxford). 2001;40(12):1331-6.
- 16. Guirro R, Serrão F, Elias D, Bucalon AJ. Calibration of therapeutic ultrasound equipment. Physiotherapy. 1997;83(8):419-23.
- 17. Artho PA, Thyne JG, Warring BP, Wills CD, Brismee JM, Latman NS. A calibration study of therapeutic ultrasound units. Phys Ther. 2002;82(3):257-63.
- 18. Araujo M, Baptista-Silva JCC, Gomes PO, Campos HO, Novo NF, Juliano Y. Efeitos do ultra-som de baixa intensidade na veia auricular de coelhos. Acta Cir Bras. 2003;18(1):25-31.
- 19. Silva RF. Efeito da estimulação ultrasônica sobre a espermatogênese de ratos pré-púberes e adultos. Estudo experimental [dissertação]. São Carlos (SP): Universidade de São Paulo; 2007.
- 20. Boucaud A, Montharu J, Machet L, Arbeille B, Machet MC, Patat F, et al. Clinical, histologic and electron microscopy study of skin exposed to low-frequency ultrasound. Anat Rec. 2001;264(1):114-9.
- 21. Visnardi AR. Efeito do ultra-som de baixa intensidade no colágeno da pele sadia de ratos [dissertação]. São Carlos (SP): Universidade de São Paulo; 2007.
- 22. Blume K, Matsudo E, Lopes MS, Lopes LG. Dosimetria proposta para o tratamento por ultra-som uma revisão de literatura. Fisioter Mov. 2005;18(3):55-64.
- 23. Lopes LG, Bertolini SMMG, Martins ER, Gewehr P, Lopes MS. Análise morfométrica de tecido muscular de coelhos submetido a ultra-som pulsado e contínuo de 1 MHz. Fisioter Pesqui. 2005;12(3):15-21.
Histological analysis of healthy epithelium of Wistar rats in vivo irradiated with different intensities of therapeutic ultrasound
Publication Dates
-
Publication in this collection
07 May 2010 -
Date of issue
Apr 2010
History
-
Accepted
22 June 2009 -
Reviewed
25 Mar 2009 -
Received
28 Oct 2008